
ASARM
A System for CFS/ME Monitoring and Treatment
Philip McDermott
1
, Steve Pettifer
1
and Paul Abeles
2
1
School of Computer Science, The University of Manchester, Manchester, U.K.
2
Royal Manchester Children’s Hospital, Manchester, U.K.
Keywords:
Chronic fatigue syndrome, Myalgic encephalomyelitis, Actigraphy, CBT, Mobile computing.
Abstract:
CFS/ME (Chronic Fatigue Syndrome/Myalgic Encephalomyelitis) affects up to 2.5% of adults in the UK and
USA and between 1% and 2% of children in the UK. Sufferers report that they are low on energy, and find
performing everyday activities difficult. The illness is commonly treated using Cognitive Behavioural Therapy
(CBT), which aims to help patients learn how to build up their energy levels in a gradual way, and how best to
spend and preserve their energy. A crucial aspect of this treatment is for the health care professional to monitor
and record how the patient spends time on a day-to-day basis, then prescribe appropriate and precise baseline
levels for periods of rest, sleep and activity. These levels are then gradually adjusted as the patient’s condition
improves. Current methods typically rely on paper diaries, however, these offer little guidance to the patient
and are time consuming for the health care professional to analyse. The ASARM (Advanced Sleep, Activity
and Rest Monitoring) system combines an electronic diary with automated recording of actigraphy data, with
the aim of improving the process of assessing, monitoring, prescribing for, and then treating patients with the
condition.
1 INTRODUCTION
CFS/ME (Chronic Fatigue Syndrome/Myalgic En-
cephalomyelitis) is a condition characterised by a de-
bilitating fatigue that is not relieved by rest, and per-
sists over at least six months (Reeves et al., 2005;
Royal College of Paediatrics and Child Health, 2004;
Fukuda et al., 1994). It typically co-occurs with other
symptoms such as muscle pain, headache, sore throat
and memory or sleep problems. While the preva-
lence of the illness can be difficult to define, since
this depends on the diagnostic criteria used, recent
studies suggest up to 2.5% of the population in the
UK and USA may be affected (Avellaneda Fernandez
et al., 2009; Reyes et al., 2003; Jason et al., 1999),
and between 1% and 2% of children in the UK. Ap-
proximately one in ten of these will be of a severity
that means the illness is disabling, leaving the patient
housebound (Farmer et al., 2004). The most recently
published UK guidelines (National Institute of Clin-
ical Excellence (NICE), 2007) recommend immedi-
ate assessment by a specialist, and (Hinds and Mc-
Cluskey, 1993) found that children and young people
with the condition had a significantly better prognosis
than adults if diagnosed early.
Patients with the condition find it difficult to reg-
ulate their energy expenditure. They can frequently
become very low on energy, which then makes per-
forming everyday activities challenging. A critical
aspect of CFS/ME is that an activity can be costly to
the energy levels of the person with the condition by
being either physically, emotionally or cognitively de-
manding. This means that a thorough understanding
of patients’ lifestyles is vital to prescribing effective
treatment.
We present a technology-based solution for both
monitoring and then treating CFS/ME by collecting
and analysing data around patients’ everyday activ-
ities, making use of subjective metrics recorded by
the patients themselves and objective data describing
their movements.
2 CLINICAL NEED
Currently, the most prominent and successful treat-
ment of CFS/ME is the use of Cognitive Behavioural
Therapy (CBT) to help people with the condition
learn how to build up their energy levels in a gradual
way, and inform them how best to spend and preserve
160
McDermott P., Pettifer S. and Abeles P..
ASARM - A System for CFS/ME Monitoring and Treatment.
DOI: 10.5220/0003765701600166
In Proceedings of the International Conference on Biomedical Electronics and Devices (BIODEVICES-2012), pages 160-166
ISBN: 978-989-8425-91-1
Copyright
c
2012 SCITEPRESS (Science and Technology Publications, Lda.)

the right amount of their energy (Stulemeijer et al.,
2005). This approach is applicable to both young
people and adults, and a recent randomised control
trial (the PACE Trial
1
) using CBT for adults with
CFS/ME has further reinforced its clinical effective-
ness for treating this condition (White et al., 2011).
Generally, the CBT approach to managing and
treating CFS/ME is to monitor and record, with the
patient’s help, how his or her time is divided between
activities of varying energy-expenditure, specifically
periods spent sleeping, resting and being active. Av-
eraged over a period of days, these amounts serve as
baselines, which are then rebalanced to more healthy
and sustainable proportions. An important aspect of
this treatment is the method by which the patient’s
time is monitored and recorded, since this is the in-
formation that setting appropriate and precise base-
line levels relies on. In current clinical practice (for
example, in (Royal College of Paediatrics and Child
Health, 2004)), the process is as follows. In phase
one of treatment, baseline levels are established by
analysing data recorded by the patient in a paper di-
ary over a set period of time (typically a fortnight). In
these diaries, the patient records the amount of time
spent doing daily activities (including periods sleep-
ing or resting). This data informs the clinician who
establishes, by averaging the figures to remove daily
fluctuations, the exact amounts of sleep, rest and ac-
tivity that the patient is capable of achieving without
detrimental effects on his or her health. In phase two,
the patient aims to follow these set ‘budgets’, and
over the course of the treatment they are gradually
adapted—by the clinician—to achieve a more healthy
balance. As part of a more detailed assessment, the
patient supplements activity diary information with
subjective ratings: a record of reactions to, and ap-
praisals of, the activities being performed. These sub-
jective ratings may be, for example, the sense of mas-
tery achieved or the level of fatigue felt while carrying
out a task, or the feeling of refreshment following rest
or sleep. These ratings contribute in two ways: they
provide additional information for the clinician to aid
prescribing a patient’s daily schedule, and also help
the patient by adding a degree of reflection to his or
her daily routine.
The collection of data through paper diaries, and
the data itself, can be problematic in three ways. First,
the data can be highly subjective, reflecting patients
own opinions on how they feel. Second, the diary
information relies upon the patient’s diligence for its
accuracy and completeness. Third, the actual act of
data collection places additional strains on the patient,
in particular on memory, and especially in the early
1
http://www.pacetrial.org/
stages of care which are often characterised by feel-
ings of hopelessness and disempowerment.
In the following sections, we describe how each
of these problems may be addressed, and a system we
have built to put these improvements into practice.
3 METHOD
Our method for addressing the shortcomings of the
current clinical practice (described in the previous
section) is to apply cost-effective, robust, technolog-
ical solutions to key aspects of the existing method-
ology, and to integrate and automate processes where
possible to provide a complete integrated system for
monitoring and treating the condition. Existing work
by (Abeles et al., 2009) involving the application of
computer-assisted CBT to treat a not dissimilar con-
dition (adolescent depression) has proved successful
(also see (Robinson et al., 2011)).
To compliment the subjective diary data used in
current practice, more objective data in the form
of gross-motor movement measurements can be col-
lected alongside it. This can be captured through an
actigraphy device: a small device worn on the body to
measure and record the patient’s movement through-
out the day. A variety of small, lightweight and low-
cost devices exist for this purpose, and have been tri-
alled in limited ways as part of clinical research. For
example, the Philips Respironics Actiwatch
2
has been
used as a secondary outcome measure while monitor-
ing CFS/ME patients (Kop et al., 2005). Prior to our
work, however, actigraphy data has not been used di-
rectly in treatment settings.
Data gathered using such devices is invaluable in
understanding patients’ energy expenditure, but does
not replace the existing diary system, since not all ac-
tivities will necessarily afford movement on the part
of the patient. A fundamental aspect of the condi-
tion is that physical, emotional and cognitive effort af-
fect the overall energy expenditure associated with an
activity (Afari and Buchwald, 2003). A cognitively-
demanding activity without physical movement, for
example, would not be detected by a system rely-
ing solely on actigraphy. If diary information is still
to be collected, however, the issues of accuracy and
completeness must be addressed. Our system uses an
electronic diary that can record information, speed up
the process of entering, and also serve as a visual aid.
Such a diary system can guide patients as they plan
and record their activities, helping to account for their
2
http://www.healthcare.philips.com/main/homehealth/sl
eep/actiwatch/default.wpd
ASARM - A System for CFS/ME Monitoring and Treatment
161

time and to classify it accordingly. Further, the di-
ary can address the third issue described, the cognitive
strain on the patient, by being more accessible than a
paper system, and by prompting the user where neces-
sary (for example, to record subjective feelings along-
side activities). During the first phase of treatment,
where the patient is simply recording all activity, the
device can assist data entry, reducing the amount of
cognitive workload. In the second phase of treatment,
where the patient is following a prescription, the de-
vice can instruct the user as to what type of activity
should be carried out, and when, further assisting the
patient in his or her scheduling.
In addition to addressing these issues, an elec-
tronic system can improve communication between
patient and clinician. Both devices—the actigraphy
device and diary—communicate with a central server,
so that the data can be synchronised and viewed by the
clinician. The clinician can view both diary and actig-
raphy data daily via a web interface, unlike the tradi-
tional system whereby access to this data was limited
to scheduled appointments, typically occurring only
fortnightly. This allows actigraphy data to be used
as a primary treatment measure, to inform the clin-
ician at the point at which they are creating a pre-
scription for the patient. Not only can the clinician
monitor much more closely the progress of the pa-
tient, changes to the prescription can be made much
more rapidly: the clinician can revise and then ‘push’
a new prescription to the patient at any time. It is
these small but incremental updates to a prescription
that help the patient’s condition improve. Not hav-
ing to wait for more costly and less-frequent face-to-
face appointments for each adjustment can potentially
speed up the recovery of the patient.
4 RESULTS
The system developed in this work comprises: a cen-
tral server, used for data storage and communication;
a web front-end for the clinician; and a patient pack-
age. The patient package comprises: a wrist-worn ac-
celerometer device to measure sleep, activity and rest;
a docking station for the device; and an electronic di-
ary (as a smartphone application). An overview of the
architecture is shown in Figure 1.
4.1 Server and Web Application
The server combines a Pylons
3
web application and
a PostgreSQL
4
database. The database stores infor-
3
http://www.pylonsproject.org
4
http://www.postgresql.org
Figure 1: Architecture of the ASARM system, showing the
relationship between the clinician’s interface, the patient’s
interface and the central server.
mation about registered devices, activities, subjective
activity data, actigraphy data, prescriptions and al-
lowances. The web application (Figure 2) lets the
clinician view each registered ASARM device, its ac-
tivity information, and also create, view and amend
prescriptions for the patients associated with each de-
vice.
The primary role of the server is to provide Repre-
sentational State Transfer (Fielding, 2000) end-points
for the diary application to communicate with. New
data, and requests for existing data, are sent asyn-
chronously via HTTP from the mobile device to the
server, which in turn communicates with the database
and responds appropriately. The web application
communicates with the server in the same fashion.
4.2 Diary Application
The primary interaction with the ASARM system for
the patient is through the diary application, imple-
mented as an app for the iPhone (Figure 3). The di-
ary app’s main purpose is to provide a quick and easy
method for the patient to record day-to-day tasks. It
can be in one of two modes: data collection mode
or prescription mode. Data collection mode is used
to learn how the patient’s time is spent. Prescrip-
tion mode is used once a prescription has been cre-
BIODEVICES 2012 - International Conference on Biomedical Electronics and Devices
162
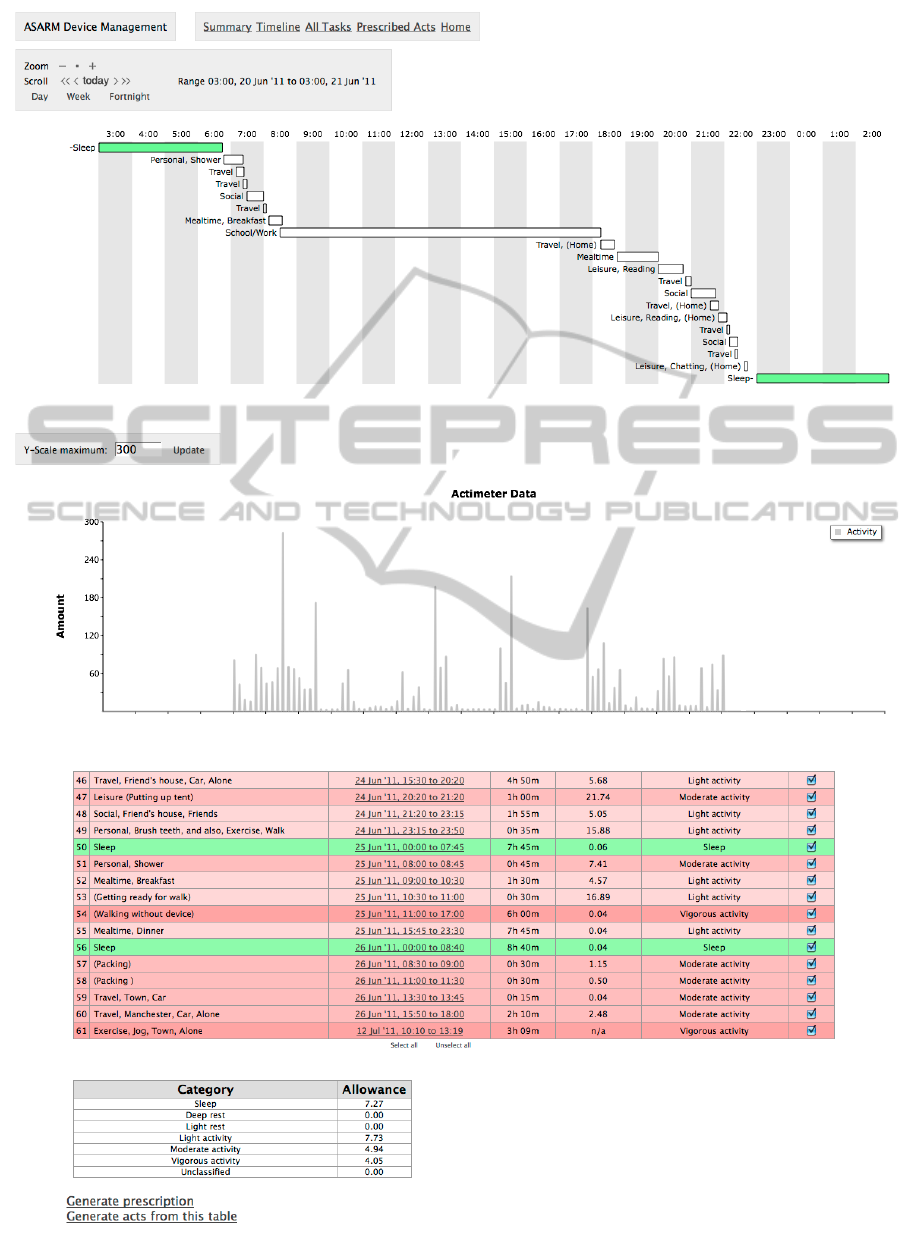
Figure 2: The clinician’s web application, showing task timeline and actigraphy data, activities and calculated baselines.
ASARM - A System for CFS/ME Monitoring and Treatment
163

Figure 3: The diary app, in prescription mode.
Figure 4: The diary app, showing the task builder.
ated by the clinician and delivered to the patient: this
mode is designed to help patients follow their pre-
scription as closely as possible. Data collection gen-
erally lasts around two weeks, during which time the
patient uses the Task Builder to quickly describe the
activity he or she is engaged in. The Task Builder
presents high-level types of activity to the user (i.e.
the patient), such as leisure, household, travel, etc.,
followed by additional types of information, such as
location, company, and so on, to quickly and accu-
rately describe an activity (see Figure 4). For exam-
ple, the user could create the activity ‘Doing home-
work at home with a friend’ in 5 touches, or the ac-
tivity ‘travelling to school on the bus, alone’ in 4
touches. This input mechanism is designed to min-
imise the interaction with the diary as much as pos-
sible: it is vital that the monitoring and treatment of
the patient do not increase the workload—either phys-
ically or cognitively—unnecessarily, and become a
significant activity in itself. For instances where the
use of the task builder do not enable the user to de-
scribe the activity fully, manual entry using the de-
vice’s keyboard is possible. When the diary is in pre-
scription mode, instead of the Task Builder the user
chooses an action from a list of prescribed activities,
grouped by energy-expenditure. This encourages pa-
tients to follow the prescription set for them, and al-
lows the application to keep track of their time more
accurately. When an activity is not in the prescribed
list, the patient can use the Task Builder or manual
entry to add the new activity.
The diary application communicates with the
server using standard HTTP requests over the cellular
phone network (or a wireless broadband connection if
available). When a connection cannot be established,
the diary application queues requests to be sent when
a connection becomes available.
4.3 Actigraphy Device and Dock
The actigraphy device used is a 3-axis accelerome-
ter unit
5
. The device is small and lightweight, worn
on the wrist in a small pouch, and can record data
for up to 60 hours. It charges and transfers data via
USB. For this system the device is set to sample at
a rate of 10Hz in +/-2g range. When uploaded to
the ASARM server, these raw values are processed to
take the change in magnitude of the three-dimensional
vector each 1/10th of a second, and then the mean is
taken over each minute for display purposes.
A netbook is used as a ‘docking station’ for the
actigraphy device: when it is plugged into the docking
station (using a standard USB connection), all new
data on the device is backed up and uploaded to the
ASARM server. The netbook is configured to use
the iPhone as an internet ‘hotspot’ and so can auto-
matically upload data to the server without additional
configuration at the patient’s home. This obviates the
need for the user to provide an internet connection for
the system to work. Unlike tele-medicine systems that
may need to optimise the use of a narrow-band phone
5
specifically, a Gulf Coast Data Concepts X6-2mini
http://www.gcdataconcepts.com/x6-2mini.html
BIODEVICES 2012 - International Conference on Biomedical Electronics and Devices
164

line (for example, (Lai et al., 2005)), or make use of
an existing broadband connection (for example, (Lai
et al., 2007), high-throughput is not a requirement of
this system, and so ease of use can be given a higher
priority.
The netbook additionally acts as a power source
and charges the actigraphy devices while they are
docked. In operation, a pair of these devices is ac-
tually used: one is worn throughout the day, while
the other is worn at night. The night time device is
plugged into the docking station during the day, and at
bedtime (when the user of the diary selects ‘bedtime’
as the current activity), the two devices are swapped,
so that night time movement is recorded whilst the
day time device uploads data and recharges.
Following the initial period of activity monitor-
ing, using the data from the web application (the di-
ary and actigraphy data in Figure 2) the clinician cat-
egorises each activity into sleep, rest, or activity, or
even more precisely (deep or light rest; light, moder-
ate or vigorous activity). The rating of activity will
depend on both the actigraphy data and the descrip-
tion of the task, thereby allowing both physical and
cognitive aspects to be taken into account. For ex-
ample, watching a favourite television programme,
whilst being physically undemanding, is cognitively
much more demanding than spending the time just
sitting. When the clinician has completed the cat-
egorisation, the application automatically generates
starting baselines using the mean values of these cat-
egories over the monitoring period, as well as a set
of rated activities to be sent to the diary application
(Figure 2). This prescription—the baselines and set
of rated activities—is sent to the diary app, which
then changes mode from data collection to prescrip-
tion mode. From this point, the patient has a prede-
fined set of rated activities and daily baseline amounts
to follow. The diary app interface changes accord-
ingly as described above, to show the patient his or
her activity choices, baseline amounts and ‘budgets’
of time for each category. The clinician continues to
monitor the patient’s activities, and can change and
update the prescription—either by altering the base-
lines or by adding or removing rated activities—-at
any time.
5 PRELIMINARY TESTS
The system as described has been ‘field-tested’ over
a number of weeks to ensure each component works
as expected and reliably. This testing was successful,
and led to minor user interface improvements in the
diary application and docking station. The output of
the actigraphy device was compared with a commer-
cially available actigraphy device, and found to be of
comparable precision. It has not yet been used clini-
cally: this will be the next stage of the work.
6 CONCLUSIONS
The ASARM system described herein was developed
to address issues identified in the current best prac-
tice methods to treat CFS/ME. The current method
is to use paper diaries to collect data on the patient’s
day-to-day activities, then prescribe (and periodically
update) baseline targets for levels of energy expendi-
ture. Using this method, it is difficult for the clini-
cian to accurately analyse and categorise the patient’s
activities, since there is a lack of objective move-
ment information to compliment the diary informa-
tion. Additionally, the paper diary system is reliant
on the patient’s diligence, and can increase cognitive
strain. The ASARM system combines the collection
of objective movement data with diary information
and subjective ratings, presenting them together to the
clinician so that a more informed judgement can be
made on the patient’s condition. This data is available
remotely, and is updated much more frequently then
in current practice. These features allows the clinician
to monitor the patient’s progress much more closely
without disrupting the patient’s schedule with formal
appointments.
The ASARM system aids the treatment process in
additional ways. The electronic diary provides finer-
grained timetabling than the existing paper system,
increasing the accuracy of recorded periods of rest,
sleep and activity. The diary app also automatically
prompts patients to answer questions about their cur-
rent state of wellbeing at the appropriate time, rather
than the patient recording this information at a later
stage (with the paper diary system this is often only
at the end of each day). The task builder in the di-
ary application reduces the physical and cognitive de-
mands of filling in a paper diary—and reduces mem-
ory load—by speeding up diary entry and by auto-
matically recording start and end times of activities.
Finally, the application allows a regular bedtime to be
prescribed and suggested to the patient, and can be
used to inform the patient of sleep periods. This can
be useful where the patient’s regular sleeping pattern
is inverted, a regular feature of CFS/ME.
7 FUTURE WORK
The next clinically relevant stage in the ASARM pro-
ASARM - A System for CFS/ME Monitoring and Treatment
165

ject is to use the system in a formal pilot trial, with a
select group of CFS/ME patients. The planned trial
would be performed in two stages as follows. In
stage one, an initial sample of five patients with the
condition will be offered traditional CBT, but will
use the ASARM system alongside the CBT and the
completion of paper diaries. Qualitative responses
from the use of the devices will also be captured.
This stage will lead to the development of a clini-
cal ASARM protocol which will subsequently be fol-
lowed for stage two, with a further 25 patients, as
well as allowing iterative refinement of the ASARM
system and software. All participants will use the
ASARM devices at the various stages of treatment:
assessment (establishment of baseline activity level),
early treatment phase and late treatment phase. Rele-
vant clinical outcome measures will also be collected,
pretreatment and post-treatment, as well as informa-
tion on the patient experience of the ASARM devices.
ACKNOWLEDGEMENTS
This work was funded by The Manchester Centre
for Integrating Medicine & Innovative Technology
(http://www.mimit.org.uk/).
REFERENCES
Abeles, P., Verduyn, C., Robinson, A., Smith, P., Yule, W.,
and Proudfoot, J. (2009). Computerized CBT for ado-
lescent depression (”stressbusters”) and its initial eval-
uation through an extended case series. Behavioural
and Cognitive Psychotherapy, 37(2):151–165.
Afari, N. and Buchwald, D. (2003). Chronic fatigue syn-
drome: a review. American Journal of Psychiatry,
60:221–226.
Avellaneda Fernandez, A., Perez Martin, A., Izquierdo Mar-
tinez, M., Arruti Bustillo, M., Barbado Hernandez,
F. J., de la Cruz Labrado, J., Diaz-Delgado Penas, R.,
Gutierrez Rivas, E., Palacin Delgado, C., Rivera Re-
dondo, J., and Ramon Gimenez, J. R. (2009). Chronic
Fatigue Syndrome: aetiology, diagnosis and treat-
ment. BMC Psychiatry, 9 Suppl 1:S1.
Farmer, A., Fowler, T., Scourfield, J., and Thapar, A.
(2004). Prevalence of chronic disabling fatigue in
children and adolescents. British Journal of Psychi-
atry, pages 477–481.
Fielding, R. T. (2000). Architectural Styles and the Design
of Network-based Software Architectures. PhD thesis,
University of California, Irvine.
Fukuda, K., Straus, S. E., Hickie, I., Sharpe, M. C., Dob-
bins, J. G., and Komaroff, A. (1994). The Chronic
Fatigue Syndrome; A Comprehensive Approach to its
Definition and Study. Annals of Internal Medicine,
121:953–959.
Hinds, G. M. E. and McCluskey, D. R. (1993). A restrospec-
tive study of chronic fatigue syndrome. Proceedings
of the Royal College of Physicians, pages 10–14.
Jason, L., Richman, J. A., Rademaker, A., Jordan, K. M.,
Plioplys, A. V., Taylor, R. R., McCready, W., Huang,
C., and Plioplys, S. (1999). A community-based study
of Chronic Fatigue Syndrome. Archive of Internal
Medicine, 159:2129–2137.
Kop, W., Lyden, A., Berlin, A., Ambrose, K., Olsen, C.,
Gracely, R., Williams, D., and D.J., C. (2005). Ambu-
latory monitoring of physical activity and symptoms
in fibromyalgia and chronic fatigue syndrome. Arthri-
tis and Rheumatism, 52(1):296–303.
Lai, A. M., Nieh, J., and Starren, J. B. (2007). REPETE2:
A next generation home telemedicine architecture.
AMIA Annual Symposium Proceedings, 1020.
Lai, A. M., Starren, J. B., and Shea, S. (2005). Architec-
ture for remote training of home telemedicine patients.
AMIA Annual Symposium Proceedings, 1015.
National Institute of Clinical Excellence (NICE)
(2007). Chronic Fatigue Syndrome/Myalgic En-
cephalomyelitis (or Encephalopathy): diagnosis and
management of CFS/ME in adults and children.
http://www.nice.org.uk/CG53.
Reeves, W. C., Wagner, D., Nisenbaum, R., Jones, J. F.,
Gurbaxani, B., Solomon, L., Papanicolaou, D. A.,
Unger, E. R., Vernon, S. D., and Heim, C. (2005).
Chronic fatigue syndrome – a clinically empirical ap-
proach to its definition and study. BMC Medicine,
3(19).
Reyes, M., Nisenbaum, R., Hoaglin, D. C., Unger, E. R.,
Emmons, C., Randall, B., Stewart, J. A., Abbey, S.,
Jones, J. F., Gantz, N., Minden, S., and Reeves, W. C.
(2003). Prevalence and incidence of Chronic Fatigue
Syndrome in wichita, kansas. Archive of Internal
Medicine, 163:1530–1536.
Robinson, A., Yule, W., Verduyn, C., Smith, P., and Abeles,
J. P. P. (2011). Stressbusters: The development of a
computerised CBT programme for adolescent depres-
sion. In Yule, W. and Udwin, O., editors, Increasing
Access to CAMHS, volume 30, pages 45–50. ACAMH
Occasional Papers, Association For Child and Adoles-
cent Mental Health.
Royal College of Paediatrics and Child Health (2004).
Evidence based guidelines for the manage-
ment of CFS/ME in children and young people.
http://www.ayme.org.uk/article.php?sid=10&id=137.
Stulemeijer, M., de Jong, L. W. A. M., Fiselier, T. J. W.,
Hoogveld, S. W. B., and Bleijenberg, G. (2005). Cog-
nitive behaviour therapy for adolescents with chronic
fatigue syndrome: randomised controlled trial. Brit-
sish Medical Journal, pages 14–17.
White, P., Goldsmith, K. A., Johnson, A., and et al. (2011).
Comparison of adaptive pacing therapy, cognitive be-
haviour therapy, graded exercise therapy, and special-
ist medical care for chronic fatigue syndrome (pace):
a randomised trial. Lancet, pages 611–690.
BIODEVICES 2012 - International Conference on Biomedical Electronics and Devices
166
