
PROPOSAL FOR A FILTERLESS FLUORESCENCE SENSOR
FOR SNP GENOTYPING
K. Yamasaki
1
, H. Nakazawa
1,2
, N. Misawa
1,3
, M. Ishida
1,3
and K. Sawada
1,3,4
1
Toyohashi University of Technology (TUT), 1-1 Hibarigaoka, Tempaku-cho, Toyohashi, Aichi 441-8580, Japan
2
JSPS Research Fellow, Chiyoda, Tokyo 102-8472, Japan
3
Electronics-Inspired Interdisciplinary Research Institute (EIIRIS), Toyohashi, Aichi 441-8580, Japan
4
JST-CREST, Chiyoda, Tokyo 102-8666, Japan
Keywords: SNP genotyping, Fluorescence, Filterless, Multiwavelength.
Abstract: This study describes a biosensor for single nucleotide polymorphism (SNP) genotyping based on the
filterless fluorescence detection methods. The filterless fluorescence sensor is able to distinguish lights with
more than two different wavelengths without optical filters, mirrors, and gratings. From the final results, we
observed that emission lights form the “fluorescein isothiocyanate (i.e., FITC)” and the “sulforhodamine
101 acid chloride (i.e., Texas Red)”, which are kinds of fluorescent dyes commonly used in SNP genotyping,
were detected with less interference using the filterless fluorescence sensor. Thus, our approach is effective
for SNP genotyping with low cost and high portability.
1 INTRODUCTION
Micro-total analysis systems (μ-TAS) are highly
desirable for the detection of various types of bio-
chemical information with low cost and high porta-
bility. Furthermore, μ-TAS offers the potential for
highly efficient, simultaneous analysis of a large
number of biologically important molecules in ge-
nomic, proteomic and metabolic studies.
μ-TAS compounds pumps, valves, reactors, hea-
ters, micro-fluidic channels, and sensors. The vari-
ous types of sensors that have functionality for bio-
chemical analyses have recently been studied, with
research aimed at compounding with μ-TAS.
One of the most useful sensing methods in bio-
chemistry is the fluorescence detection method. We
have previously devised a filterless fluorescence
sensor which can be applied to the detection of fluo-
rescence (Maruyama et al., 2006); (Maruyama et al.,
2006); (Nakazawa et al., 2011). This sensor detected
several signals with different wavelengths simulta-
neously, without the need for optical filters, mirrors,
and gratings. In this paper, we purpose an ability of
a filterless fluorescence sensor to apply to single
nucleotide polymorphism (SNP) genotyping based
on the filterless fluorescence detection method.
2 FILTERLESS FLUORESCENCE
SENSOR
2.1 Sensor Structure
Figure 1 shows a photomicrograph of the devised
sensor. The devised sensor was fabricated in our
laboratory using 5-μm-rule, N-substrate, 1P1M (1-
poly and 1-metal), and single well modified com-
plementary metal-oxide semiconductor fabrication
technology. The size of the sensing area is 300 × 300
µm
2
, and the depth of the p-n junction is about 4 µm
beneath the sensing area.
Figure 1: Photomicrograph of the fabricated filterless
fluorescence sensor.
185
Yamasaki K., Nakazawa H., Misawa N., Ishida M. and Sawada K..
PROPOSAL FOR A FILTERLESS FLUORESCENCE SENSOR FOR SNP GENOTYPING.
DOI: 10.5220/0003774201850189
In Proceedings of the International Conference on Biomedical Electronics and Devices (BIODEVICES-2012), pages 185-189
ISBN: 978-989-8425-91-1
Copyright
c
2012 SCITEPRESS (Science and Technology Publications, Lda.)
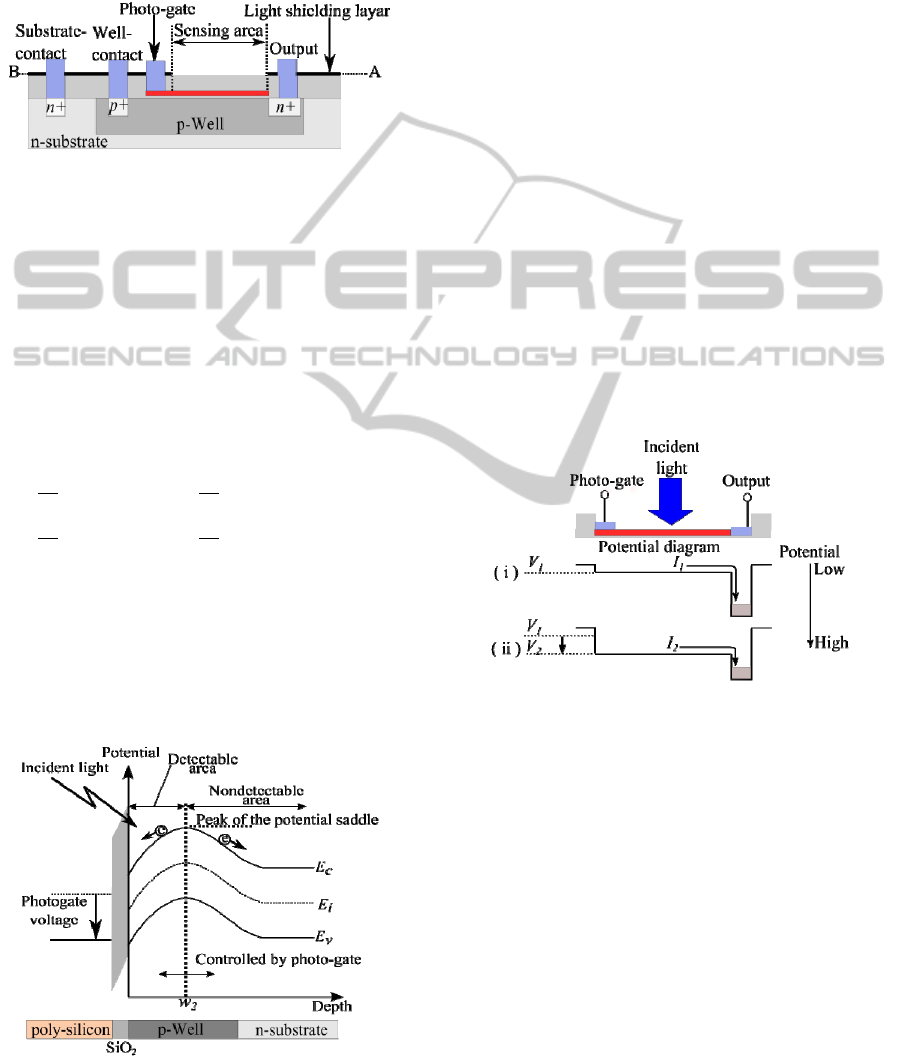
Figure 2 shows the concept and configuration of
the devised sensor. The devised sensor is based on a
photo-gate structure. The sensing area of the device
consists of four layers: n-type poly-Silicon (500
nm)/SiO
2
(90 nm)/p-well (4 µm)/n-type silicon sub-
strate.
Figure 2: Schematic diagram of section A-B shown in Fig.
1. The cross-section structure is based on a photo-gate
structure.
2.2 Basic Principle
The principle of the filterless fluorescence detection
is based on the variation of optical absorption depth
with wavelength (Mckelvey, 1996). When lights
with two different wavelengths (e.g., fluorescence
and the excitation light) are incident simultaneously,
the currents I generated at absorption depths w
1
and
w
2
are given by the following equations (1):
I
1
=
qS
hc
λ
1
߮
1
ሺ
1-e
-α
1
w
1
ሻ
+
qS
hc
λ
2
߮
2
ሺ
1-e
-α
2
w
1
ሻ
,
I
2
=
qS
hc
λ
1
߮
1
ሺ
1-e
-α
1
w
2
ሻ
+
qS
hc
λ
2
߮
2
ሺ
1-e
-α
2
w
2
ሻ
.
(1)
where φ
1
and φ
2
are the intensities at wavelengths λ
1
and λ
2
with absorption coefficients α
1
and α
2
, respec-
tively, S is the size of the sensing region, q is the
elementary charge, h is the Planck constant, and c is
the speed of light in a vacuum. Both illumination
intensities (φ
1
and φ
2
) are obtained by solving these
simultaneous equations.
Figure 3: Potential distribution beneath the sensing area.
The energy band structure beneath the sensing
region shown in Fig. 3 is essential for fluorescence
sensing. A potential well is formed at the surface of
the silicon substrate beneath the sensing region. The
most important consideration for fluorescence sens-
ing with the devised sensor is that the absorption
depth w is tunable, because there is a variation in the
absorption depth w for different wavelengths. In the
devised sensor, the absorption depth w can be con-
trolled by changing the photo-gate voltage, and
electrons generated within the selected absorption
depth w are collected for read out.
2.3 Sensing Procedure
Figure 4 shows a potential diagram for the intensity
measurements of fluorescence and excitation light. If
the photo-gate voltage is set to V
1
then the absorp-
tion depth changes to w
1
. When light is incident on
the sensing area, photons are absorbed in the deple-
tion layer below the sensing area. Finally, the cur-
rents, I
1
, generated at absorption depth w
1
are read
out via the output node. The currents, I
2
, generated
at absorption depth w
2
are similarly obtained by
changing the photo-gate voltage from V
1
to V
2
.
Figure 4: Potential diagram for the intensity measurements
of fluorescence and excitation light.
3 EXPERIMENTS
3.1 Sensor Property
3.1.1 Wavelength Dependency
We investigated output current of the devised sensor
for several wavelengths. The sensor was exposed to
a range of wavelength lights from approx. 430 nm to
610 nm using programmable light source equipment
(OSVIS-500, Tokyo Instruments, Inc.) in a dark
room. Each power of the incident light was prelimi-
narily measured using an external power meter
BIODEVICES 2012 - International Conference on Biomedical Electronics and Devices
186
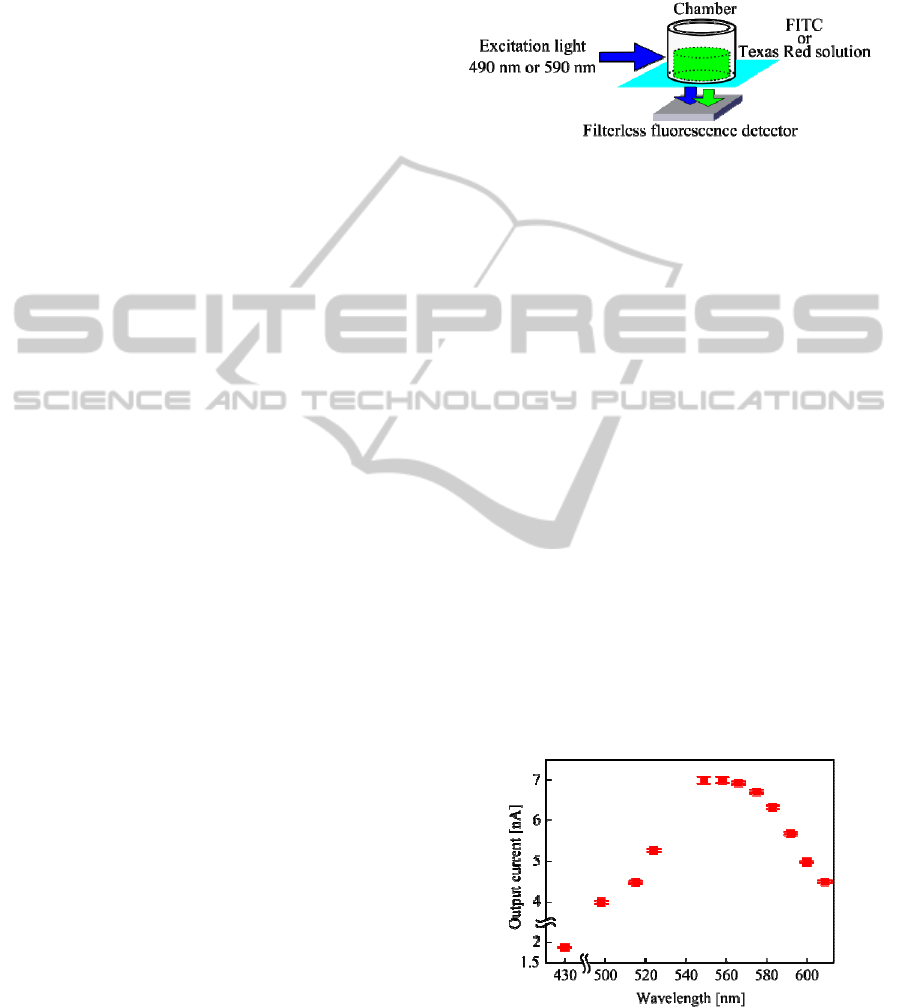
(8230E, ADC Corporation) to keep fixed light pow-
er of 100 μW/cm
2
for the reference.
3.1.2 Detection Limit
On the assumption of FITC use, the sensor was
irradiated by commercial light emitting diode (LED)
(L-7113VGC-H, Kingbright Elec. Co., Ltd.) that had
the dominate wavelength of 525 nm and the output
current was measured at 27 °C. Incident light power
was changed from 0.1 to 100 µW/cm
2
confirmed by
an external power meter (8230E, ADC Corporation).
3.1.3 Temperature Dependency
We investigated the influence of dark current which
comes from thermal excitation. The surrounding
temperature of the sensor was set 21, 27, and 35 °C
independently. Then, output currents were measured.
3.2 Fluorescence Detection
3.2.1 Measurements of FITC and Texas Red
To confirm the sensor’s performance of the quantita-
tive measurements for fluorescent dyes, fluorescein
isothiocyanate (FITC) and sulforhodamine 101 acid
chloride (Texas Red) which are typically used bio-
analysis including SNP genotyping, we measured
the excited fluorescence intensity 525 nm of FITC
(Wako Pure Chemical Industries, Ltd.) or 615 nm of
Texas Red (Life Technologies Corporation) 200 μl
solution of 0, 1, 10 μM individually. Each fluores-
cent solution was prepared using diluted ethanol
(50v/v%). And the each sample solution was set in a
cylindrical plastic chamber of a 7 mm inner diameter
and a 10 mm height as shown in Fig. 5. The excita-
tion lights, 490 nm and 590 nm, for FITC and Texas
Red were independently applied by light source
equipment (LAX-C100, Asahi Spectra Co., Ltd.).
The absorption depth of the sensor was varied by
changing of photo-gate voltage. The intensity of the
fluorescence light and excitation light can be calcu-
lated by using equations (1) where λ
1
= 490 nm, α
1
=
20.0×10
5
m
-1
, w
1
= 0.761 µm, and λ
2
= 525 nm, α
2
=
12.7×10
5
m
-1
, w
2
= 1.135 µm were substituted in the
case of FITC use. In the same way, λ
1
= 590 nm, α
1
=
6.39×10
5
m
-1
, w
1
= 0.803 µm, and λ
2
= 615 nm, α
2
=
4.82×10
5
m
-1
, w
2
= 1.135 µm were substituted in the
case of Texas Red use. The results of detected the
light intensities were normalized assuming the inten-
sity was 1.0 that was a value in the case of measur-
ing 10 μM fluorescent solutions. Furthermore, we
evaluated the possibilities of cross talks of two exci-
tation lights, 590 nm and 490 nm, to FITC and Tex-
as Red. Each fluorescent solution was additionally
exposed by ineffective light for excitation (i.e., 590
nm light for FITC and 490 nm for Texas Red).
Figure 5: Schematic view of the measurement setup.
3.2.2 Detection of FITC and Texas Red Mix
As a model sample of a heterozygote in SNP varia-
tions, we additionally measured the 200 μl mixture
solution of equal parts of FITC and Texas Red (net
0.5 μM for each). The results of detected light inten-
sities were also normalized as same as above section
experiment.
4 RESULTS AND DISCUSSION
Figure 6 shows that the correlation between wave-
length and output current of the sensor. The result
means that the sensor has high sensitivity near the
center of 550 nm at least in this wavelength region.
And also, the sensor has almost the same sensitivity
level for emission wavelengths of FITC (525 nm)
and Texas Red (615 nm).
We think that the similarity of the sensor’s out-
put current level of FITC and Texas Red will enable
us to compare them without any other complicated
calibrations in this study.
Figure 6: Wavelength dependency of the sensor's output.
Figure 7 shows the detection current versus the
incident light intensity. Each data point was obtained
by 10 times experiments and the each error bar was
PROPOSAL FOR A FILTERLESS FLUORESCENCE SENSOR FOR SNP GENOTYPING
187
standard deviation. Fig. 7(a) and (b) show the inci-
dent light intensity in the range of 2-100 µW/cm
2
and 0.1-2 µW/cm
2
, respectively. The detection cur-
rent is proportional to the incident light power in the
range at least above about 0.1 µW/cm
2
. The detec-
tion current was nearly equal to the dark current less
than 0.1 µW/cm
2
. This result implies that the sensor
can almost linearly detect incident light in the range
of 0.1-100 µW/cm
2
.
(a)
(b)
Figure 7: Detection current versus the incident light inten-
sity in the range of 2-100 µW/cm
2
(a) and 0.1-2 µW/cm
2
(b).
Figure 8 shows the dark current level depending
on the temperature. The result shows that dark cur-
rent increases with increasing of surrounding tem-
perature. According to the dark current level, we
found that our sensor is able to detect the fluores-
cence intensity quantitatively in the range of more
than about 0.1 µW/cm
2
. And it is presumed that the
dark current level is a few pA at room temperature.
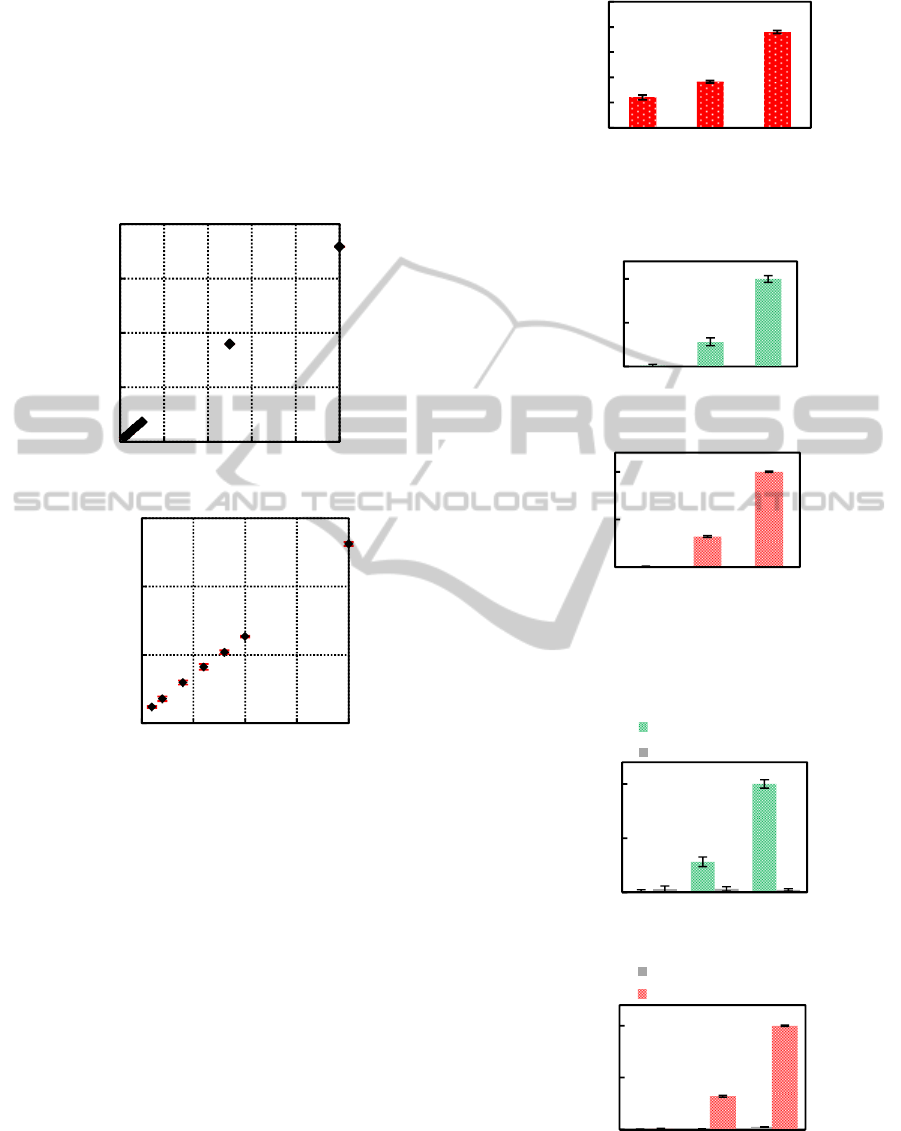
The results of individual measurements of FITC
and Texas Red solution are shown in Fig. 9. Each of
them can be quantitatively detected in these rough
differences in concentration. It implies that the sen-
sor will be able to use for SNP genotyping because a
typical concentration of polymerase chain reaction
product is about 10 µM.
Figure 8: Temperature dependence of the dark currents of
the devised sensor.
(a) FITC solution.
(b) Texas Red solution.
Figure 9: Concentration dependency of detected fluores-
cent intensities.
(a) FITC solution.
(b) Texas Red solution.
Figure 10: Differences of fluorescence intensity by the two
different lights excitation.
0
1
2
3
4
0 20406080100
Output current [nA]
Incident light intensity [µW/cm
2
]
0
0.03
0.06
0.09
00.511.52
Output current [nA]
Incident light intensitey [µW/cm
2
]
0
0.002
0.004
0.006
0.008
0.01
21 27 35
Dark current [nA]
Temperature [°C]
0.0
0.5
1.0
0110
Light intensity
(525 nm) [arb. unit]
Concentration of FITC [μM]
0.0
0.5
1.0
0110
Light intensity
(615 nm) [arb. unit]
Concentration of Texas Red [μM]
0.0
0.5
1.0
0110
Light intensity
(525 nm) [arb. unit]
Concentration of FITC [μM]
Excitation = 490 nm
Excitation = 590 nm
0.0
0.5
1.0
0110
Light intensity
(615 nm) [arb. unit]
Concentration of Texas Red [μM]
Excitation = 490 nm
Excitation = 590 nm
BIODEVICES 2012 - International Conference on Biomedical Electronics and Devices
188

Figure 11: Distributions of fluorescence intensities for
FITC solutions, Texas Red solutions and their mixtures.
Ideally, those mixtures should be measured to the
center of both axes. However, they slightly leaned to
FITC side. We suppose that this result comes from
FITC appears large seemingly due to fluorescence of
Texas Red which was excited by 490 nm light is
mixed. According to these results, we think that the
devised sensor could potentially be applied to real
SNP genotyping for individual determination of
homozygote or heterozygote.
5 CONCLUSIONS
We showed that our devised sensor could detect
quantitatively fluorescent dyes; FITC and Texas Red
without the filters. And, we found that the sensor
successfully distinguished FITC and Texas Red
solution and their mixture. We believe that the sen-
sor enable us to SNP genotyping in compact system.
ACKNOWLEDGEMENTS
This work was partially supported by the Global
COE Program titled Frontiers of Intelligent Sensing,
the projects of the Hamamatsu Optoelectronics
Knowledge Cluster Initiative, a grant-in-aid for
scientific research (A) (No. 21246051) from the
Ministry of Education, Culture, Sports, Science and
Technology (MEXT) of Japan, and a grant-in-aid for
JSPS Fellows from Japan Society for the Promotion
of Science (JSPS).
REFERENCES
Y. Maruyama et al., IEEE Trans. Electron Devices, 53(3)
(2006) 553–558.
Y. Maruyama et al., Sens. Actuators A 128 (2006) 66-70.
H. Nakazawa et al., Proc. Transducers 2011 (2011) 100-
103.
J.P. Mckelvey, Solid State and Semiconductor Physics,
Harper and Row, New York, 1966, p. 463.
0
0.3
0.6
0.9
1.2
00.30.60.91.2
Texas Red fluorescence light
intensity rate [arb. unit]
FITC fluorescence light
intensity rate [arb. unit]
▲ Only Texas Red
■ Mixture
◆ Only FITC
PROPOSAL FOR A FILTERLESS FLUORESCENCE SENSOR FOR SNP GENOTYPING
189
