
SEISMOCARDIOGRAPHY: A NOVEL APPLICATION
FOR THE NON-INVASIVE ASSESSMENT OF THE FIRST
MAXIMAL DERIVATIVE OF LEFT VENTRICULAR PRESSURE
Melonie Burrows
1
, Graeme Jahns
1
, Geoffrey Houlton
1
, Berry van Gelder
2
and Frank Marcus
3
1
Heart Force Medical Inc., Vancouver, British Columbia, Canada
2
Catharina Hospital, Eindhoven, Netherlands
3
The University Medical Centre, Tucson, Arizona, U.S.A.
Keywords: Cardiac Resynchronization Therapy, Seismocardiography, Ballistocardiography, Biventricular pacing, Heart
failure.
Abstract: Cardiac resynchronization therapy (CRT) results in improved clinical status in patients with heart failure
and left ventricular dyssynchrony. One third of CRT patients fail to respond due to the inability to 1)
identify non-responders prior to treatment, 2) optimize coronary sinus lead placement for left ventricular
pacing and 3) optimize the atrio-ventricular (A-V) and inter-ventricular (V-V) intervals. Although invasive
measurements of first maximal derivative of left ventricular pressure (dP/dt
max
) are used to optimize lead
placement and A-V and V-V intervals in CRT, it would be preferable to have a non-invasive assessment of
dP/dt
max
. Echocardiographic dyssynchrony and left ventricular function are current parameters for non-
invasively evaluating responders to CRT, but they are not recommended due to their poor reproducibility.
We applied recent advances in technology to develop a device called the digital ballistocardiograph (dBG
®
),
which assesses the mechanical function of the heart using triaxial accelerometry. We show that dBG
®
cardiac events are valid in comparison to 2D transthoracic echocardiography and reliable in comparison to
cardiac magnetic resonance imaging. We present preliminary data to support our position that the dBG
®
could be used as a non-invasive assessment of dP/dt
max
in heart failure patients to identify responders and
optimize CRT.
1 INTRODUCTION
The science of ballistocardiography (BCG) was con-
ceived over a century ago (Starr et al., 1955, Starr et
al., 1953) as the study of body motion resulting from
myocardial contraction and blood flow from the
heart to the periphery (Noordergraff, 1961). During
the 1930s to 1960s, there was a surge of studies
showing the importance of BCG measurements in
clinical cardiology (Starr et al., 1950, Starr and
Hildreth, 1952, Starr, 1964), especially in relation to
identifying patients with coronary heart disease
(Baker, 1950, Scarborough, 1952, Baker, 1968) and
development of circulatory abnormalities such as co-
arctation of the aorta (Brown et al., 1949, Starr,
1964). Although conceptually attractive, BCG was
limited in practice, as the devices were cumbersome
requiring fixed installation. In addition, the ability
to analyze the signals electronically was not yet
available. As such, BCG was impractical to use on a
large scale and was abandoned in the 1970s.
Bayevski and colleagues developed seismo-
cardiography (SCG) in 1964. The technique con-
sisted of an accelerometer attached over the sternum
area of the chest, which recorded compression
waves transmitted through the chest wall from heart
contractions during each cardiac cycle. Over the
years, SCG has been refined as a technique for
cardiac stress monitoring (Jerosch-Herold et al.,
1999), left ventricular monitoring during ischemia
(Salerno and Zanetti, 1991, Korzeniowska-Kubacka
et al., 2005), estimation of left ventricular function
(Korzeniowska-Kubacka and Piotrowicz, 2002), and
detection of coronary artery disease (Salerno et al.,
1991). However, SCG devices were not imple-
mented on a large scale due to the emergence and
sudden interest in echocardiography.
251
Burrows M., Jahns G., Houlton G., van Gelder B. and Marcus F..
SEISMOCARDIOGRAPHY: A NOVEL APPLICATION FOR THE NON-INVASIVE ASSESSMENT OF THE FIRST MAXIMAL DERIVATIVE OF LEFT
VENTRICULAR PRESSURE.
DOI: 10.5220/0003845202510256
In Proceedings of the International Conference on Biomedical Electronics and Devices (BIODEVICES-2012), pages 251-256
ISBN: 978-989-8425-91-1
Copyright
c
2012 SCITEPRESS (Science and Technology Publications, Lda.)
At present, modern accelerometer-based tech-
nology is revitalizing the science of BCG and SCG,
allowing the motion of the heart to be recorded and
analyzed quickly and efficiently for the assessment
of cardiac function (Alametsa et al., 2009). We have
applied recent advances in hardware and software
technologies to develop a new medical device called
the digital ballistocardiograph (dBG
®
), which allows
rapid, non-invasive assessment of cardiac events and
the force of the heart’s contraction, lending itself to
patient monitoring and assessment.
Clinical trials have demonstrated that cardiac
resynchronization therapy (CRT) results in improved
clinical status and lower mortality in selected
patients (Abraham et al., 2002, Bristow et al., 2004).
However, approximately one third of CRT patients
fail to respond due to the inability to accurately
1) identify non-responders prior to treatment,
2) optimize coronary sinus lead placement during
the procedure, and 3) optimize the atrio-ventricular
(A-V) and inter-ventricular (V-V) intervals
(Abraham et al., 2002, van Gelder et al., 2004,
Cleland et al., 2005, Jansen et al., 2006). Although
invasive measurements of the first maximal
derivative of left ventricular pressure (dP/dt
max
) can
be used to increase the number of CRT responders
via optimization of lead placement, A-V and V-V
intervals, (Kurzidim et al., 2005, van Gelder et al.,
2008, van Gelder et al., 2009), it would be preferable
to have a non-invasive assessment of dP/dt
max
(Houthuizen et al., 2011). Echocardiographic dys-
synchrony and left ventricular function are current
parameters for non-invasively evaluating responders
to CRT (Altman et al., 2011, Bai et al., 2011).
However, even though echocardiographic variables
have been proposed as surrogates for left ventricular
dP/dt
max
, they are not highly recommended due to
their poor reproducibility (Thomas et al., 2009).
Cardiac timings recorded non-invasively by BCG
and SCG have been shown to provide valuable
insight into the heart’s function (Starr, 1964, Crow,
1994, Lyseggen et al., 2005). As such, if the dBG
®
could non-invasively predict dP/dt
max
across a range
of heart rates, it would have potential to be a
valuable tool for the non-invasive assessment of
dP/dt
max
for identification of CRT responders and
optimization of CRT.
In this position paper, we present preliminary
data from animal studies to support our position that
the dBG
®
could be used as a non-invasive
assessment of dP/dt
max
in heart failure patients to
identify responders and optimize CRT.
2 METHODS
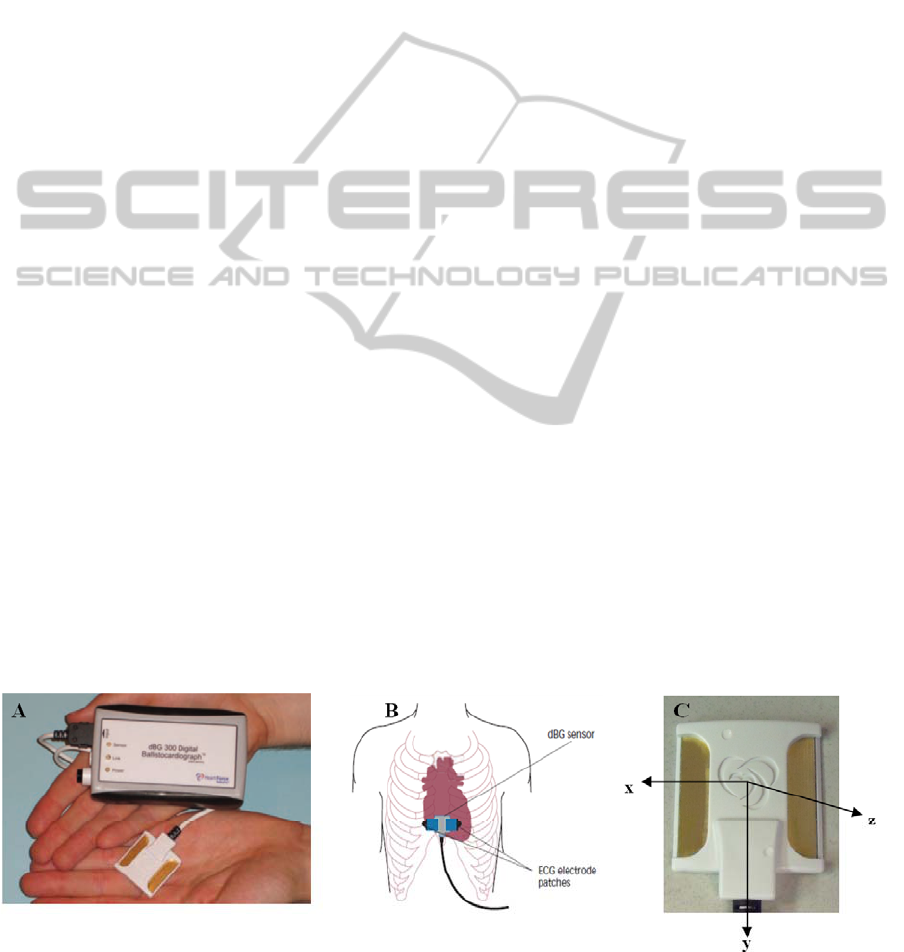
2.1 The Digital Ballistocardiograph
®
The dBG
®
(Heart Force Medical Inc., Vancouver,
Canada) consists of three main components: the
sensor containing the triaxial accelerometer and two
exposed pads for electrocardiograph (ECG) elec-
trodes, the digitizing transceiver unit which
conditions and samples the signals and transmits the
data to a PC via Bluetooth™, and the software
application used for device control and manual data
analysis (Figure 1A). The sensor is placed on the
sternum in the midline, with its lower edge ap-
proximately 3 cm above the xiphoid process (Figure
1B). The triaxial accelerometer (Figure 1C) detects
the SCG vibrations generated by the heart’s motion.
A single, non-diagnostic ECG similar to a lead 1 is
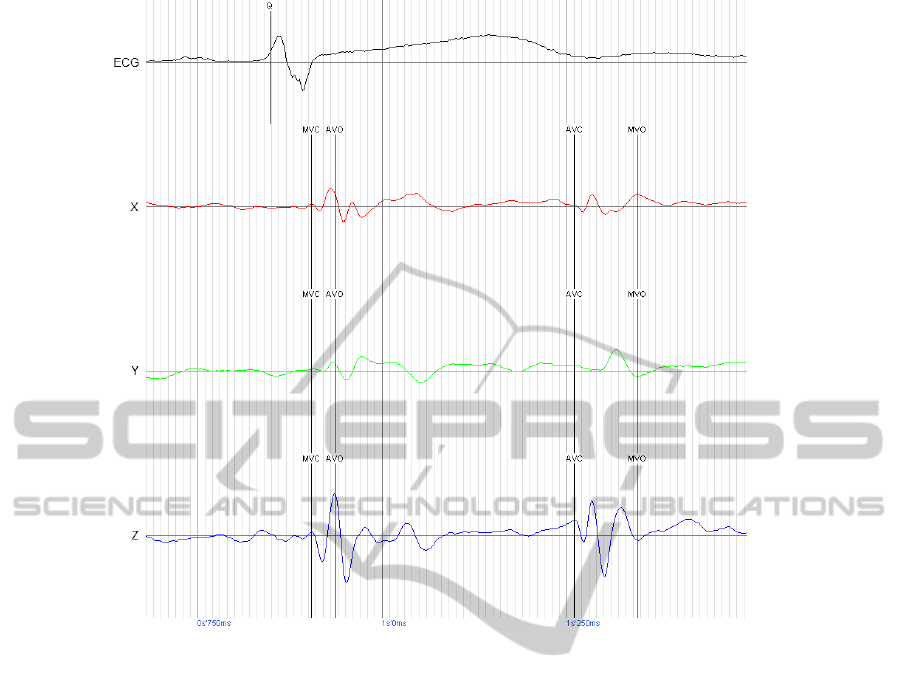
also recorded. From specific peaks on the SCG, the
following cardiac events can be determined: mitral
valve closure (MVC), aortic valve opening (AVO),
aortic valve closure (AVC) and mitral valve opening
(MVO). Isovolumetric contraction time (IVCT; t
MVC
– t
AVO
) and isovolumetric relaxation time (IVRT;
t
AVC
– t
MVO
) are derived variables (Figure 2).
Figure 1: A) The digital ballistocardiograph
®
sensor and transceiver; B) Digital ballistocardiograph
®
sensor placement, C)
Digital ballistocardiograph
®
sensor axes from the perspective of the observer; x – from right to left, y – from head to toe, z
– from back to chest. Abbreviations: dBG - digital ballistocardiograph; ECG – electrocardiograph.
BIODEVICES 2012 - International Conference on Biomedical Electronics and Devices
252
We investigated the accuracy of cardiac events
using the dBG
®
against the clinical reference
standard of 2D transthoracic Doppler echocardio-
graphy, and found that the cardiac events measured
by dBG
®
were equivalent to cardiac events measured
by 2D echocardiography (95% of dBG
®
cardiac
events fell within ±2SD of echocardiography cardiac
events). We have also determined within-device,
intra- and inter-operator reliability for the dBG
®
measurements. Precision was calculated as the root
mean square error coefficient of variation
(RMSECV, %) and was less than 4% (within), 8%
(intra) and 10% (inter) for all cardiac events, which
is comparable to the gold standard technique of
cardiac magnetic resonance imaging whose
reliability is known to be between 2.9–9% (Chuang
et al., 2000). As such, dBG
®
cardiac events are both
valid and reliable for clinical use.
2.2 The Digital Ballistocardiograph
®
in Cardiac Resynchronization
Therapy
We used a swine model to investigate if the dBG
®
variables were predictive of dP/dt
max
across various
heart rate conditions as well as following induced
changes in blood volume. We studied 10 hybrid
farm pigs (10–16 weeks old), utilizing the dBG
®
device to measure cardiac timings (MVC, AVO,
AVC, MVO), and catheterization to measure left
ventricular pressure for computation of dP/dt
max
.
A guiding catheter (Medtronic, MN, USA) and
sensor-tipped PressureWire
®
(St. Jude Medical Inc.,
MN, USA) were inserted into the left femoral artery
and placed in the apex of the left ventricle under
fluoroscopic guidance (HICOR/ACOM-TOP, Sie-
mens, Erlangen, Germany). The PressureWire
®
was
connected to the RadiAnalyser
®
Xpress (St. Jude
Medical Inc., MN, USA) for the measurement of left
ventricular pressure. The RadiAnalyser
®
Xpress
Figure 2: Example digital ballistocardiograph
®
waveform annotated for four valve timings. Abbreviations: Mitral valve
closure – MVC; Aortic valve opening – AVO; Aortic valve closure – AVC; Mitral valve opening – MVO; ECG –
Electrocardiogram; X – dBG
®
sensor axis from right to left, Y – dBG
®
sensor axis from head to toe, Z – dBG
®
sensor
axis from back to chest.
SEISMOCARDIOGRAPHY: A NOVEL APPLICATION FOR THE NON-INVASIVE ASSESSMENT OF THE FIRST
MAXIMAL DERIVATIVE OF LEFT VENTRICULAR PRESSURE
253

system utilized the PhysioMon™ software (Version
2.02, St. Jude Medical Inc.) for computation of
dP/dt
max
. The output from the RadiAnalyser
®
Xpress
was routed to a signal processing unit (CMS,
Module M1006A, Phillips Medical Systems, MA,
USA) for simultaneous monitoring of left ventricular
pressure. A catheter was inserted into the right
femoral artery and placed into the aortic arch under
fluoroscopic guidance (HICOR/ACOM-TOP,
Siemens). The catheter output was routed to the
signal-processing unit (CMS, Module M1006A,
Phillips Medical Systems) for simultaneous
monitoring of aortic blood pressure. All data output
from the CMS were routed to a Biopac MP150
(Biopac Systems Inc., CA, USA). A dBG
®
pro-
prietary sensor (Heart Force Medical Inc.,
Vancouver, Canada) was placed on the midline of
the sternum with the lower edge of the sensor placed
approximately 3 cm above the xiphoid process. A
pacing wire (Medtronic) was inserted into the left
femoral vein, advanced into the right atrium, and
connected to an external, single chamber pacemaker
(Medtronic) for pacing of the heart at specific heart
rate (HR) conditions. We paced animals via the right
atrium for 10 counterbalanced heart rate conditions:
90, 100, 110, 120, 130, 140, 150, 160, 170 and 180
bpm. At each HR, we observed a period of 5
minutes for normalization of hemodynamics, after
which we collected all pressure and dBG
®
data
simultaneously for 1 minute. After the pacing
protocol was completed, each animal had blood
withdrawn equivalent to 10% of body weight, which
was subsequently reperfused. After each blood
withdrawal/reperfusion, all pressure and dBG
®
data
were collected simultaneously at 1 minute
immediately after withdrawal/reperfusion, and 3
minutes after withdrawal/reperfusion.
The left ventricular pressure, dP/dt
max
and dBG
®
data collected during the counterbalanced HR
conditions were used to assess the relation between
dP/dt
max
and dBG
®
variables and devise a regression
equation to predict dP/dt
max
non-invasively. The left
ventricular pressure, dP/dt
max
and dBG
®
data
collected during the blood volume conditions were
used only to assess if changing blood volumes
affected the relation between dP/dt
max
and dBG
®
variables. The aortic blood pressure signals were
captured only to facilitate time alignment of the
dBG
®
waveforms to the dP/dt waveforms.
We created a proprietary software tool (Heart
Force Medical Inc.) to manually annotate the
merged data files. We also used the tool to offset the
data streams to achieve heart beat synchronization
for all data. We manually annotated MVC, AVO,
AVC, MVO and a dBG
®
amplitude measure (dBGv)
calculated from the measured cardiac timings on the
dBG
®
waveform, and dP/dt
max
on the dP/dt
waveform. We used a repeated measure regression
model to assess if dBGv could predict catheter-based
measurement of dP/dt
max
. Heart rate, HR order and
pig weight were used as covariates, and the within
pig (between HR and within HR) and between pig
variation were assessed. Alpha was set at P <0.05.
All statistical analyses were performed by an
independent statistician using R (www.r-
project.org).
3 RESULTS AND DISCUSSION
dBGv was a significant predictor of dP/dt
max
(P
<0.0001), with a one unit change in dBGv equiv-
alent to 4.01 unit changes in dP/dt
max
(95% CI 3.00–
5.05 units). The relation between dBGv and dP/dt
max
was consistent across HRs (P >0.8), but varied
across pigs (P <0.0001). dBGv remained a sig-
nificant predictor of dP/dt
max
(P <0.0001) even when
blood volumes were decreasing and increasing.
These preliminary data show a strong predictive
capability of dBG
v for dP/dt
max
across heart rates,
suggesting that dBGv is a non-invasive surrogate of
dP/dt
max
and is able to detect the force-frequency
relation between HR and dP/dt
max
. The potential of a
cardiac amplitude variable to predict dP/dt
max
has
been suggested previously, with peak endocardial
acceleration (PEA) amplitude (measured using a
micro-accelerometer located on the tip of a trans-
venous pacing lead), shown to be related to global
ventricular contractility independent of recording
site and atrial rhythm (Bongiorni et al., 1996). Acute
variations in PEA, measured using endocardial leads
and implantable device, have also been shown to
closely parallel changes in dP/dt
max
(Rickards et al.,
1996).
Despite the remarkable clinical results associated
with CRT (Houthuizen et al., 2011), the percentage
of non-responders is still 25–35% (Bristow et al.,
2004, Cleland et al., 2005). Acute measurements of
left ventricular dP/dt
max
have shown to be necessary
to identify non-responders prior to treatment, to
optimize coronary sinus lead placement, and to
optimize A-V and V-V intervals during CRT to
obtain maximal hemodynamic benefit in patients
(van Gelder et al., 2004, Jansen et al., 2006, van
Gelder et al., 2008, van Gelder et al., 2009).
Although catheterization is the gold standard for the
assessment of ventricular function, it is invasive,
costly and time consuming, and is therefore limited
BIODEVICES 2012 - International Conference on Biomedical Electronics and Devices
254

in its clinical utility. The dBGv measured in this
study is a non-invasive surrogate for dP/dt
max
as it
describes the rate of change of contractile force,
while being measured from an accelerometer placed
on the sternum. During CRT, heart rate is a known
variable that, in conjunction with a measured dBG
v,
could be inserted into an equation to derive a pre-
dicted dP/dt
max
without left ventricular catheter-
ization. An Electrophysiologist could use the pre-
dictive equation during CRT to 1) identify patients
who experience an increase in dP/dt
max
at time of
coronary sinus lead insertion and therefore likely to
be CRT responders, and 2) optimize coronary sinus
lead placement and A-V and V-V intervals. The fact
that the relation between dP/dt
max
and dBGv remains
strong across changes in blood volume suggests that
the predictive relation between dBG
v and dP/dt
max
is
robust. The use of the dBG
®
during CRT could help
prevent implantation of CRT devices in the one third
of patients who do not currently benefit from this
therapy (van Gelder et al., 2004).
4 CONCLUSIONS
In this position paper, we presented a brief history of
BCG and SCG and noted that modern technology
has revitalized the science of BCG and SCG,
allowing the motion of the heart to be recorded,
captured and used in the assessment of cardiac
function. A new medical device called the dBG
®
was
introduced. The use of the dBG
®
in CRT
optimization was discussed and preliminary data
presented showing the strong predictive capability of
the dBG
®
to track dP/dt
max
across a wide variety of
heart rates in swine. The dBG
®
is small and
relatively inexpensive. It appears to have potential to
assist in identifying CRT responders and optimizing
lead position and A-V and V-V intervals. Clinical
studies in heart failure patients are required to
document the ability to assist in selecting patients
who will benefit from CRT, as well as its use in
optimizing A-V and V-V intervals.
ACKNOWLEDGEMENTS
The authors would like to thank Dr Rick White
(University of British Columbia, Canada) for
conducting the independent statistical analysis
herein; Brandon Ngai (Heart Force Medical Inc.) for
his help in developing the position paper; and
Heather Braybrook (Heart Force Medical Inc.) for
her help in editing and formatting this manuscript.
The authors would also like to acknowledge the
work of Heather Braybrook, Hayley Corbett, Dorel
Dumencu and Gonzalo Portacio (Heart Force
Medical Inc.) in data collection for the dBG
®
studies
mentioned herein.
REFERENCES
Abraham, W. T., Fisher, W. G., Smith, A. L., Delurgio, D.
B., Leon, A. R., Loh, E., Kocovic, D. Z., Packer, M.,
Clavell, A. L., Hayes, D. L., Ellestad, M., Trupp, R. J.,
Underwood, J., Pickering, F., Truex, C., McAtee, P. &
Messenger, J. 2002. Cardiac Resynchronization in
Chronic Heart Failure. New England Journal of
Medicine, 346, 1845-1853.
Alametsa, J., Varri, A., Viik, J., Hyttinen, J. & Palomaki,
A. 2009. Ballistocardiogaphic studies with accel-
eration and electromechanical film sensors. Med Eng
Phys, 31, 1154-65.
Altman, R. K., McCarty, D., Chen-Tournoux, A. A.,
Tournoux, F. B., Riedl, L., Orencole, M., Park, M. Y.,
Picard, M. H. & Singh, J. P. 2011. Usefulness of low-
dose dobutamine echocardiography to predict response
and outcome in patients undergoing cardiac resynch-
ronization therapy. Am J Cardiol, 108, 252-7.
Bai, R., Biase, L. D., Mohanty, P., Hesselson, A.B., Ruvo,
E. D., Gallagher, P. L., Elayi, C. S., Mohanty, S.,
Sanchez, J. E., David Burkhardt, J., Horton, R., Joseph
Gallinghouse, G., Bailey, S. M., Zagrodzky, J. D.,
Canby, R., Minati, M., Price, L. D., Lynn Hutchins,
C., Muir, M. A., Calo, L., Natale, A. & Tomassoni, G.
F. 2011. Positioning of Left Ventricular Pacing Lead
Guided by Intracardiac Echocardiography With Vector
Velocity Imaging During Cardiac Resynchronization
Therapy Procedure. J Cardiovasc Electrophysiol.
Baker, B., Scarborough WMR, Mason RE, Singewald ML
1950. Coronary heart disease studied by ballisto-
cardiography. Transaction of the American Clinical
and Climatological Association, 62, 191-201.
Baker, B. M. 1968. Ballistocardiography: predictor of
coronary heart disease. Circulation, 37, 1-3.
Bongiorni, M. G., Soldati, E., Arena, G., Quirino, G.,
Vernazza, F., Bernasconi, A. & Garberoglio, B. 1996.
Is Local Myocardial Contractility Related to Endo-
cardial Acceleration Signals Detected by a Trans-
venous Pacing Lead? Pacing and Clinical Electro-
physiology, 19, 1682-1688.
Bristow, M. R., Saxon, L. A., Boehmer, J., Krueger, S.,
Kass, D. A., De Marco, T., Carson, P., DiCarlo, L.,
DeMets, D., White, B. G., DeVries, D. W. & Feldman,
A. M. 2004. Cardiac-resynchronization therapy with
or without an implantable defibrillator in advanced
chronic heart failure. N Engl J Med, 350, 2140-50.
Brown, H. R., Jr., Hoffman, M. J. & De, L. V., Jr. 1949.
Ballistocardiograms in coarctation of the aorta; obser-
vations before and after operation. N Engl J Med, 240,
715-8.
SEISMOCARDIOGRAPHY: A NOVEL APPLICATION FOR THE NON-INVASIVE ASSESSMENT OF THE FIRST
MAXIMAL DERIVATIVE OF LEFT VENTRICULAR PRESSURE
255

Chuang, M. L., Hibberd, M. G., Salton, C. J., Beaudin, R.
A., Riley, M. F., Parker, R. A., Douglas, P. S. &
Manning, W. J. 2000. Importance of imaging method
over imaging modality in noninvasive determination
of left ventricular volumes and ejection fraction:
Assessment by two- and three-dimensional echo-
cardiography and magnetic resonance imaging. J Am
Coll Cardiol, 35, 477-484.
Cleland, J. G. F., Daubert, J.-C., Erdmann, E., Freemantle,
N., Gras, D., Kappenberger, L. & Tavazzi, L. 2005.
The Effect of Cardiac Resynchronization on Morbidity
and Mortality in Heart Failure. New England Journal
of Medicine, 352, 1539-1549.
Crow, R. S., Hannan P, Jacobs D, Hedquist L, Salerno
DM 1994. Relationship between seismocardiogram
and echocardiogram for events in the cardiac cycle.
American Journal of Noninvasive Cardiology, 39-46.
Houthuizen, P., Bracke, F. & van Gelder, B. 2011.
Atrioventricular and interventricular delay optimi-
zation in cardiac resynchronization therapy: physio-
logical principles and overview of available methods.
Heart Failure Reviews, 16, 263-276.
Jansen, A.H., Bracke, F.A., van Dantzig, J.M., Meijer, A.,
van der Voort, P.H., Aarnoudse, W., van Gelder, B.M.
and Peels, K.H. 2006. Correlation of echo-Doppler
optimization of atrioventricular delay in cardiac re-
synchronization therapy with invasive hemodynamics
in patients with heart failure secondary to ischemic or
idiopathic dilated cardiomyopathy. Am J Cardiol, 97,
552-7.
Jerosch-Herold, M., Zanetti, J., Merkle, H., Poliac, L.,
Huang, H., Mansoor, A., Zhao, F. & Wilke, N. 1999.
The seismocardiogram as magnetic-field-compatible
alternative to the electrocardiogram for cardiac stress
monitoring. Int J Card Imaging, 15, 523-31.
Korzeniowska-Kubacka, I., Bilinska, M. & Piotrowicz, R.
2005. Usefulness of seismocardiography for the diag-
nosis of ischemia in patients with coronary artery
disease. Ann Noninvasive Electrocardiol, 10, 281-7.
Korzeniowska-Kubacka, I. & Piotrowicz, R. 2002. Seis-
mocardiography--a non-invasive technique for estim-
ating left ventricular function: preliminary results.
Acta Cardiol, 57, 51-2.
Kurzidim, K., Reinke, H., Sperzel, J., Schneider, H. J.,
Danilovic, D., Siemon, G., Neumann, T., Hamm, C.
W. and Pitschner, H.-F. 2005. Invasive Optimization
of Cardiac Resynchronization Therapy: Role of
Sequential Biventricular and Left Ventricular Pacing.
Pacing and Clinical Electrophysiology, 28, 754-761.
Lyseggen, E., Rabben, S. I., Skulstad, H., Urheim, S.,
Risoe, C. & Smiseth, O. A. 2005. Myocardial
acceleration during isovolumic contraction: relation-
ship to contractility. Circulation, 111, 1362-9.
Noordergraff, A. 1961. Further studies on a theory of the
ballistocardiogram. Circulation, 23, 413-425.
Rickards, A. F., Bombardini, T., Corbucci, G., Plicchi, G.
& on behalf of, T. M. P. S. G. 1996. An Implantable
Intracardiac Accelerometer for Monitoring Myocardial
Contractility. Pacing and Clinical Electrophysiology,
19, 2066-2071.
Salerno, D. M. & Zanetti, J. 1991. Seismocardiography for
monitoring changes in left ventricular function during
ischemia. Chest, 100, 991-3.
Salerno, D. M., Zanetti, J. M., Green, L. A., Mooney, M.
R., Madison, J. D. & Van Tassel, R. A. 1991. Seismo-
cardiographic changes associated with obstruction of
coronary blood flow during balloon angioplasty. Am J
Cardiol, 68, 201-7.
Scarborough, W., Mason RE, Davis FW, Singewald ML,
Baker BM, Lore SA 1952. A ballistocardiographic and
electrocardiographic study of 328 patient with
coronary artery disease: Comparison with results from
a similar study of apparently normal persons.
American Heart Journal, 44, 645-655.
Starr, I. 1964. Prognostic Value of Ballistocardiograms.
Studies on Evaluation of the Doctor's Experience.
JAMA, 187, 511-7.
Starr, I. & Hildreth, E. A. 1952. The effect of aging and of
the development of disease on the ballistocardiogram;
a study of eighty subjects, originally healthy, followed
from ten to fourteen years. Circulation, 5, 481-95.
Starr, I., Horwitz, O., Mayock, R. L. & Krumbhaar, E. B.
1950. Standardization of the ballistocardiogram by
simulation of the heart's function at necropsy; with a
clinical method for the estimation of cardiac strength
and normal standards for it. Circulation, 1, 1073-96.
Starr, I., Pedersen, E. & Corbascio, A. N. 1955. The effect
of nitroglycerine on the ballistocardiogram of persons
with and without clinical evidence of coronary heart
disease. Circulation, 12, 588-603.
Starr, I., Schnabel, T. G., Jr. & Mayock, R. L. 1953.
Studies made by simulating systole at necropsy. II.
Experiments on the relation of cardiac and peripheral
factors to the genesis of the pulse wave and the
ballistocardiogram. Circulation, 8, 44-61.
Thomas, D. E., Yousef, Z. R. & Fraser, A. G. 2009. A
critical comparison of echocardiographic measure-
ments used for optimizing cardiac resynchronization
therapy: stroke distance is best. European Journal of
Heart Failure, 11, 779-788.
van Gelder, B., FA, B., Meijer A, LJM, L. & NHJ, P.
2004. Effect of optimizing the VV interval on left
ventricular contractility in cardiac resynchronization
therapy. The American journal of cardiology, 93,
1500-1503.
van Gelder, B. M., Meijer, A. & Bracke, F. A. 2008. The
optimized V-V interval determined by interventricular
conduction times versus invasive measurement by LV
dP/dtMAX. J Cardiovasc Electrophysiol, 19, 939-44.
van Gelder, B. M., Meijer, A. & Bracke, F. A. 2009.
Timing of the left ventricular electrogram and acute
hemodynamic changes during implant of cardiac
resynchronization therapy devices. Pacing Clin
Electrophysiol, 32 Suppl 1, S94-7.
BIODEVICES 2012 - International Conference on Biomedical Electronics and Devices
256
