
Locally Oriented Anisotropic Image Diffusion:
Application to Phenotyping of Seedlings
Landry Benoit
1
, David Rousseau
2
,
´
Etienne Belin
1
, Didier Demilly
3
,
Franc¸ois Chapeau-Blondeau
1
and Carolyne Durr
4
1
Laboratoire d’Ing
´
enierie des Syst
`
emes Automatis
´
es (LISA),
Universit
´
e d’Angers, 62 avenue Notre Dame du Lac, 49000 Angers, France
2
Universit
´
e de Lyon, CREATIS, CNRS UMR 5220, INSERM U1044,
Universit
´
e Lyon 1, INSA-Lyon, 69621 Villeurbanne, France
3
GEVES, Station Nationale d’Essais de Semences (SNES),
rue George Morel, 49071 Beaucouz
´
e, France
4
INRA, UMR 1345, IRHS, 42 rue Georges Morel, 49071 Beaucouz
´
e, France
Abstract. A variant of the Perona-Malik anisotropic diffusion equation is in-
troduced for the segmentation of multiple objects with crossings. The diffusion is
automatically applied at the crossings locations along the main orientations of the
objects. Application of this process is given for illustration on an original prob-
lem of plant imaging with the monitoring of the elongation of multiple crossing
seedlings.
1 Introduction
Since its introduction some 3 decades ago by Perona and Malik [12] partial differen-
tial equations (PDE) as a mathematical framework for image processing have raised a
considerable attention [14]. This interest is mathematically motivated by the possibility
of a variational interpretation of the action of the PDE. Also, PDE inspired by physi-
cal modeling constitute a vast field of investigation to be considered for its own sake
or for new approaches to various image processing applications (restoration, segmen-
tation, in-painting, to name few areas where PDE have been competitively applied). In
this report, we revisit the recently introduced oriented diffusion equation [5] where a
diffusion process is applied to an input image with a selective function of the angle
between the local gradient and a global orientation of reference. For gradient along
the reference, there is no diffusion, while gradients perpendicular to the reference are
associated to maximal diffusion. As such, the oriented diffusion equation of [5] is in-
teresting when a single direction is taken as reference for the whole image. This is the
case in the tagged grid MRI sequence considered for illustration in [5]. However, there
are practical situations where a selective diffusion process would be useful if applied
with a local orientation of reference. This is the case when the targeted information
task is the individual restoration of multiple objects crossing with various angles. As an
extension of [5] we therefore propose a locally oriented anisotropic diffusion process
with a local orientation of reference at the scale of an imagette. We demonstrate the
usefulness of this approach with an original bioimaging application. The report is orga-
nized as follows. We first describe the orientation diffusion equation of [5] and discuss
Benoit L., Rousseau D., Belin É., Demilly D., Chapeau-Blondeau F. and Dürr C..
Locally Oriented Anisotropic Image Diffusion: Application to Phenotyping of Seedlings.
DOI: 10.5220/0004330100030009
In Proceedings of GEODIFF 2013 (GEODIFF-2013), pages 3-9
ISBN: 978-989-8565-49-5
Copyright
c
2013 SCITEPRESS (Science and Technology Publications, Lda.)

the limitation of this approach in the case of our plant imaging application. We then
introduce our new anisotropic diffusion equation and illustrate its performance on our
application.
2 Locally Oriented Anisotropic Diffusion
Anisotropic diffusion applied in image processing has been introduced in [12]. It is a
process inspired from the physics of temperature diffusion in which an input image
ψ
0
is transformed in an output image ψ taken as the solution of the partial differential
equation given by
∂ψ
∂t
= div(g(k∇ψk)∇ψ), ψ(x, y, t = 0) = ψ
0
. (1)
The anisotropy of this diffusion process is governed by g(.) a nonlinear decreasing func-
tion of the norm of the gradient ∇ψ. Function g(.) is a nonlinear decreasing function
like
g(u) = exp
−
u
2
k
2
, (2)
in the original work of [12] where parameter k can be seen as a soft threshold controlling
the decrease of g(.) and the amplitude of the gradients to be preserved from the diffusion
process. Many variants of the diffusion process of Eq. (1) have been proposed (see [14]
for a review). The benefit of such diffusion processes lies in the ability to smooth data
in a nonlinear way, while preserving important image features (contours, corners, . . . ).
Recently an oriented variant of the anisotropic diffusion process of Eq. (1) has been
proposed in [5] to control the diffusion process with the direction of the gradient instead
of only the norm of the gradient. The partial differential equation of [5] reads
∂ψ
∂t
= div(g(A.∇ψ)∇ψ), ψ(x, y, t = 0) = ψ
0
, (3)
where A is a vector field defining the particular direction to preserve from the diffu-
sion process. In [5] application of Eq. (3) was given in a case where the vector field
A was selecting a single direction in a whole single image. In certain situations the di-
rections to be preserved can spatially change in the image. This is for instance the case
with the separation of multiple crossing objects in image. Crossing objects appear in
various fields of science like for instance vessel or muscle fibers crossing in biomed-
ical imaging [4,8,2], crossings roads in remote sensing [9], or assemblies of crossing
nano-objects with microscopes in physics [3]. The restoration of each object in such
images of crossing objects is an important problem if one is interested in performing
individual measurements on each object. In this work, we consider this crossing objects
problem with the oriented anisotropic diffusion of Eq. (3). In [5] illustration is given
with a unique direction in the vector field. To deal with multiple crossing objects, we
have implemented a locally oriented anisotropic diffusion process that preserves a local
orientation of reference at the scale of an imagette. To this purpose, we propose to per-
form a crossing detection before applying diffusion. We then apply the diffusion process
of Eq. (3) locally only in the vicinity of the crossing objects. The crossing is character-
ized by two directions. The idea is to diffuse in one of these directions to restore and
4
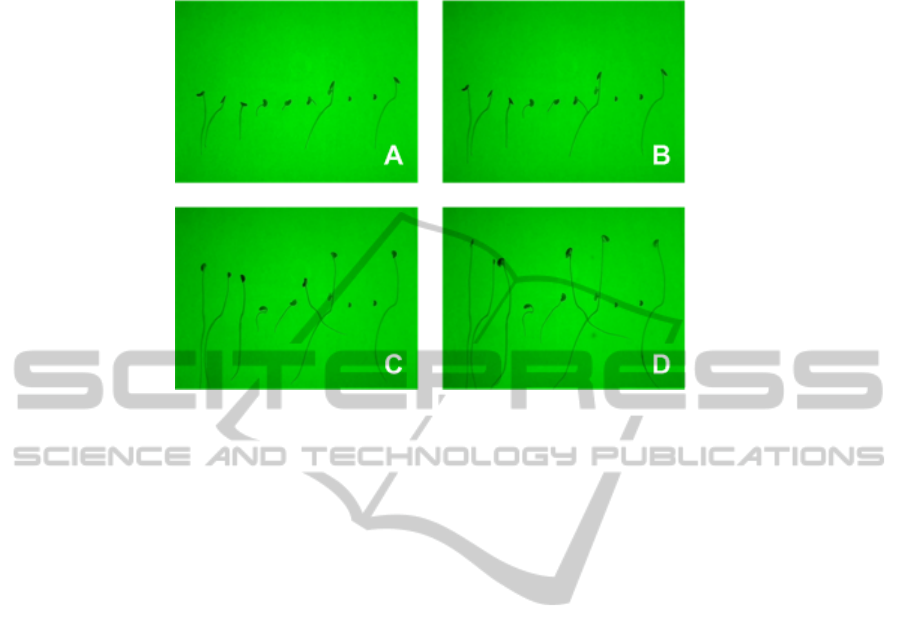
Fig. 1. Sequence of acquired images with green backlight during seedling elongation with a 2
hour time step between each snapshot A, B, C and D. The typical duration of the elongation
process can be several days.
segment one object and then to repeat this process in the other direction to restore the
other object. We apply this strategy to a real world problem from plant science in the
next section. For illustration in this work we have taken as in [6] the nonlinear function
g(.) in Eq. (3) as a hard threshold
g(u) =
0 for u ≥ k
1 for u ≤ k .
(4)
With this choice the diffusion process simply corresponds to a threshold on the gradients
oriented in the direction of the vector field A. Gradients oriented in the correct direction
are preserved while other direction are erased. This is obtained in one iteration in the
diffusion equation.
3 Application to Seedling Elongation
Seedling elongation is an early stage of the development of plants. During this stage,
the seed is in the soil. Following a geotropism, the upper part of the seedling grows
to reach the light and activates photosynthesis while the lower part of the seedling is
going deeper to anchor in the soil and access to water and nutrients. In field conditions
this seedling elongation is not accessible to plant analysts. However, non invasive mon-
itoring of seedling growth is accessible in vitro with computer vision machines [11, 7,
13,15], French et al., 2011, [1]. A set of seedlings can for instance be positioned on
an horizontal row in a gelose box. A backlight system associated with a camera then
produces sequences of images like in Fig. 1. From such image sequences, the temporal
evolution of the length of the radicle of the seedling is measurable with classical binary
5
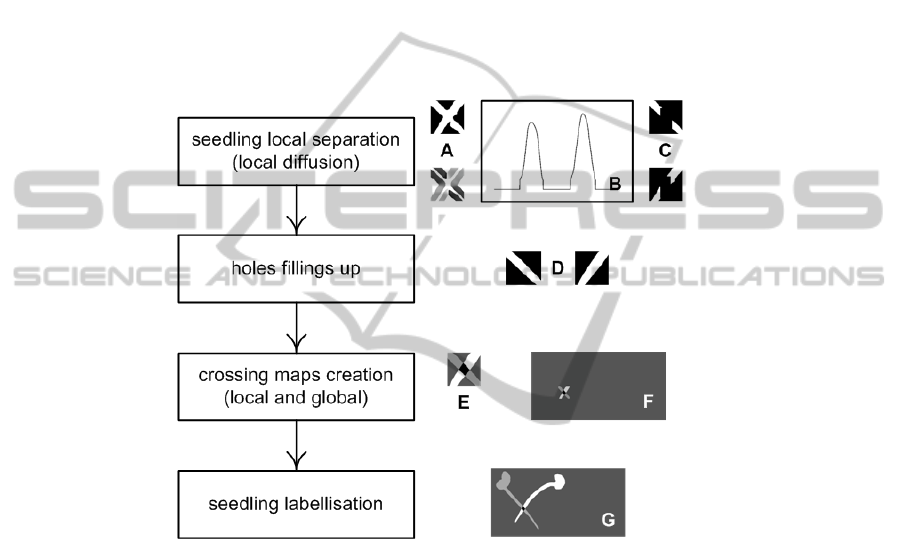
image skeletonisation [11, 7, 13,15], French et al., 2011, [1]. However, other traits like
the respective size of the organs of the seedling are not accessible from the skeleton and
require more elaborated image processing approaches. A difficulty visible in the images
of Fig. 1 is that the radicle of the seedlings can cross. To overcome this difficulty we
propose the following pipeline including the diffusion process of Eq. (3) implemented
in the algorithm of Fig. 2. In the images of Fig. 1 the seedlings are well-contrasted from
the background. The segmentation of the seedlings can thus be done after a simple
thresholding. The resulting binary image is skeletonized. Crossings are detected in this
skeleton around points of the skeleton having connectivity higher than two. Regions of
interest including a single crossing are then defined. Such a region of interest serves as
input to the algorithm detailed in Fig. 2.
Fig. 2. Four steps seedling crossing segmentation algorithm. In a first step the algorithm load
(A) an input binary image corresponding to a crossing in the input image. (B) Histograms of
the orientation of the gradients are calculated. Two main directions of the crossing are present
in this histogram. The diffusion equation of Eq. (3) is consecutively applied in each one of these
two main directions to produce two images preserving the respective other direction (C). The
threshold k in Eq. (4) is consecutively chosen equal to the main directions of each modes in the
histogram in (B). In the second step, the holes in the binary images are filled (D). A seedling
crossing map is created in (E-F) in a third step and the seedlings are labeled (G) in the fourth
step.
As illustrated in Fig. 3, seedlings crossing in the sequence are correctly separated.
We have tested successfully the algorithm of Fig. 2 on various species including species
with several seminal roots like wheat. In these cases crossings are very common and
separating the different roots is crucial. Finally, the good performance of our algorithm
in this study is important for two reasons. First it enables to perform the elongation
monitoring at more advanced stages of development. Second this enables to concentrate
6
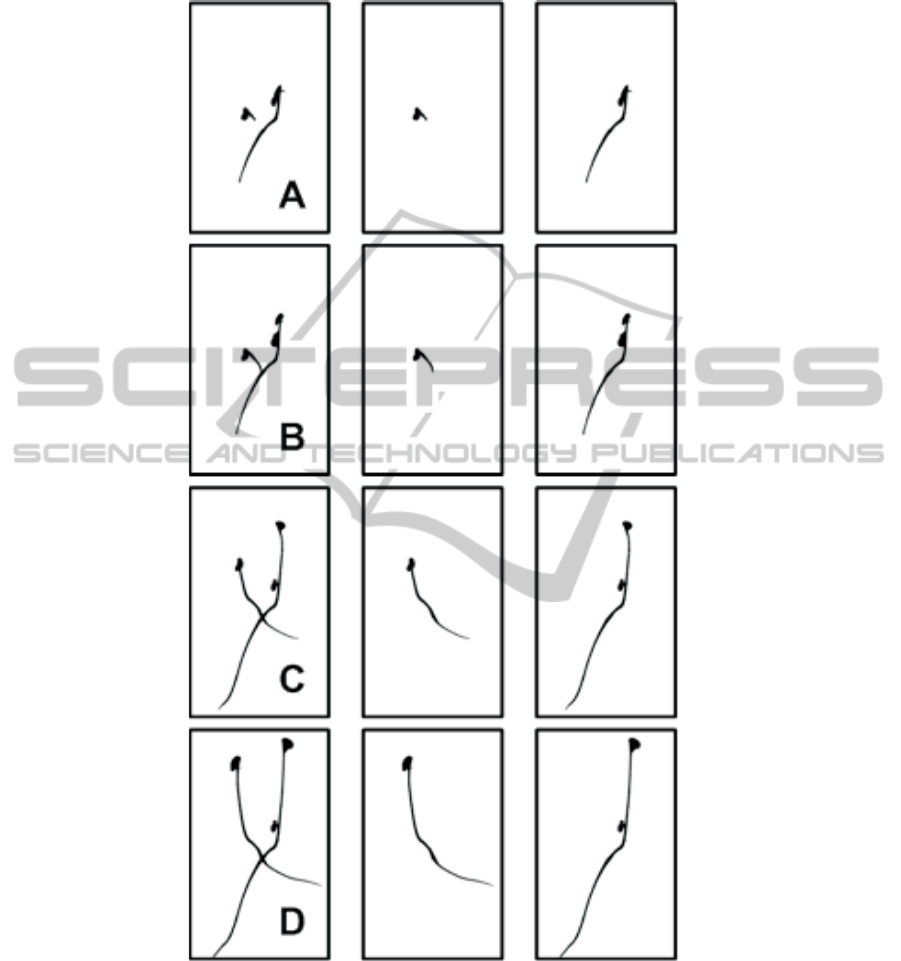
Fig. 3. Results of our crossing seedlings segmentation on two crossing seedlings of the image
sequence of Fig. 1. In the left column a binarized version of a crop form Fig. 1 at instant A, B C
and D. The two other columns represent the output of the algorithm of Fig. 2 with the two labeled
seedlings.
7

higher number of seeds of different types of species in the field of view of the camera
and therefore contributes to increase the throughput of the monitoring.
4 Conclusions
In this work we have presented an extension of the partial differential equation of [5].
In our case, a detection of the part which requires to be diffused is first performed and
the partial differential equation is then applied locally only in these parts. This presents
the interest to speed up the diffusion process by comparison to a global approach. This
also avoids diffusion artefacts in areas where no diffusion was needed. Application of
our PDE was given here for illustration in the domain of plant science with crossing
seedlings during their elongation stage. In our case the segmentation of the crossing
seedlings was obtainable from a simple threshold and the partial differential equation
was only applied in the crossings area to separate the seedlings. However, for some
species with very thin roots, the contrast between background and seedlings may not
be so favorable and it could be interesting to apply the oriented anisotropic diffusion
locally in the whole image to separate seedling around crossings and also to restore the
edges along the curvated roots in non crossing areas. Plants are highly anisotropic struc-
tures organized along branching structures. Plants growth or plant pathogens spreading
along these structures therefore constitute a natural field of applications for anisotropic
diffusion with PDE to analyze or modelize these spatio-temporal phenomenon.
Acknowledgements
The work received support from the ANR in the framework of the AKER Investissements
d’Avenir project. Landry BENOIT thanks Angers Loire M
´
etropole and SNES-GEVES
for financial support of his PhD Grant.
References
1. Belin, E., Rousseau, D., Rojas-Varela, J., Demilly, D., Wagner, M., Cathala, M., and Durr, C.
(2011). Thermography as non invasive functional imaging for monitoring seedling growth.
Computers and Electronics in Agriculture, 79:236–240.
2. B
¨
odvarsson, B., Klim, S., Morkebjerg, M., Yoon, S. M. C., Chen, J., Maclaren, J., Luther, P.,
Squire, J., Bones, P., and Millane, R. (2008). A morphological image processing method for
locating myosin filaments in muscle electron micrographs. Image and Vision Computing,
26:1073–1080.
3. Chainais, P. and Lebental, B. (2011). Caract
´
erisation statistique d’une assembl
´
ee de nan-
otubes en imagerie microscopique. Actes du 23
`
eme Colloque GRETSI sur le Traitement du
Signal et des Images, Bordeaux, France, 8-11 sept. 2011.
4. Frangi, A., Niessen, W., Vincken, K., and Viergever, M. (1998). Multiscale vessel enhance-
ment filtering. Lecture Notes in Computer Science, Springer, Berlin, pages 130–137.
5. Histace, A., M
´
enard, M., and Cavaro-M
´
enard, C. (2009). Selective diffusion for oriented
pattern extraction: Application to tagged cardiac MRI enhancement. Pattern Recognition
Letters, 30:1356–1365.
8

6. Histace, A. and Rousseau, D. (2010). Noise-enhanced nonlinear PDE for edge restoration
in scalar images. 2nd IEEE Conference on Soft Computing and Pattern Recognition, Cergy,
France, 7-11 dec. 2010.
7. Kimura, K. and Yamasaki, S. (2003). Accurate root length and diameter measurement using
nih image: use of pythagorean distance for diameter estimation. Plant and Soil, 254:305–315.
8. Kirbas, C. and Quek, F. (2003). Vessel extraction techniques and algorithms : a survey.
3rd IEEE Symposium on Bioinformatics and Bioengineering, Bethesda, USA, 10-12 march
2003, pages 238–245.
9. Laptev, I., Mayer, H., Lindeberg1, T., Eckstein, W., Steger, C., and Baumgartner, A. (2000).
Automatic extraction of roads from aerial images based on scale space and snakes. Machine
Vision and Applications, 12:23–31.
10. Naeem, A., French, A., Wells, D., and Pridmore, T. (2011). High-throughput feature counting
and measurement of roots. Bioinformatics, 27:1337–1338.
11. Nagy, F. and Schafer, E. (2002). Phytochromes control photomorphogenesis by differentially
regulated, interaction signaling pathways in higher plants. Annual Review of Plant Biology,
53:329–355.
12. Perona, P. and Malik, J. (1990). Scale-space and edge detection using anistropic diffusion.
IEEE Transactions on Pattern Analysis and Machine Intelligence, 12:629–639.
13. Wang, L., Assadi, A. H., and Spalding, E. P. (2009). Tracing branched curvilinear structures
with a novel adaptive local PCA algorithm. Plant Physiology, 53:1632–1637.
14. Weickert, J. (1998). Anisotropic Diffusion in Image Processing. Teubner-Verlag, Stuttgart.
15. Yazdanbakhsh, N. and Fisahn, J. (2010). Analysis of arabidopsis thaliana root growth kinet-
ics with high temporal and spatial resolution. Annals of Botany, 105:783–791.
9
