
Carryover Effect after Functional Electrical Stimulation Treatment
Pilot Study for a Quantitative Approach
Marta Gandolla
1
, Alessandra Pedrocchi
1
, Simona Ferrante
1
, Eleonora Guanziroli
2
,
Nick S. Ward
3
and Franco Molteni
2
1
Politecnico di Milano, NearLab – Dept. of Electronics, Information and Bioengineering,
Via G. Colombo 40, 20133 Milano, Italy
2
Valduce Hospital, Villa Beretta, Rehabilitation Center, Via N.Sauro 17, 23845 Costamasnaga, LC, Italy
3
Sobell Department of Movement Neuroscience, UCL Institute of Neurology, 33 Queen Square, London WC1N 3BG, U.K.
Keywords: Stroke, Foot Drop - FD, Carryover Definition, Functional Electrical Stimulation – FES.
Abstract: Functional Electrical Stimulation (FES) has been reported to be an effective treatment for neurological
patients, e.g. post-stroke patients. Besides beneficial effects at muscles themselves, a re-learning process
named carryover effect has been observed in some patients. This work aims at defining a quantitative
method to assess the carryover effect in a group of patients, starting from a set of outcome measures that are
specific to the considered treatment. Fifteen post-stroke chronic subjects have been recruited for 20 half an
hour sessions of FES-based treatment for Foot Drop correction during ambulation. Gait velocity, a spatial
asymmetry index, a temporal asymmetry index, endurance velocity and tibialis anterior activation index
during gait have been selected as outcome measures. After the analysis performed with the proposed
method based on principal component analysis, 50% of patients presented the carryover effect. The
proposed approach is a quantitative method that can be applied to any set of outcome measures of interest.
The results could inform further studies aimed at identifying the carryover effect mechanism of action.
1 INTRODUCTION
The aging of society and the continuously improved
ability to face acute clinical interventions are
enhancing the social impact of the neuro-motor
disabilities, and consequently, the relevance of
rehabilitation. Foot Drop (FD) is one of the common
gait impairments associated with hemiplegia; an
estimated 20% of all stroke survivors suffer from FD
(Heart Disease and Stroke Statistics—2007 Update).
FD is caused by total or partial paresis of ankle
dorsiflexor muscles (Kottink et al., 2004); it makes
ground clearance difficult during swing, and can
lead to inefficient gait compensations such as
circumduction and hip hiking (Olney and Richards,
1996; Richards et al., 1999). Residual gait deficits
such as FD contribute to increased energy
expenditure during gait, decreased endurance, and
an increased incidence of falls (Kesar et al., 2010).
The conventional approach to address FD is the
prescription of an ankle-foot orthosis, but this has
significant drawbacks as discussed by Ring and
colleagues (Ring et al., 2009). An alternative FD
treatment was introduced by Liberson and
colleagues (Liberson et al., 1961) and consisted in
externally induced ankle dorsiflexion through
peripheral neuromuscular Functional Electrical
Stimulation (FES) during the swing phase of gait.
Nowadays, FES rehabilitation treatment is a well-
known procedure in clinic rehabilitation (Sabut et
al., 2010; Pomeroy et al., 2006). FES has several
specific advantages as recently pointed out by Kesar
and colleagues (Kesar et al., 2010). Indeed, FES
promotes active muscle contractions, can help to
improve muscle strength, prevents disuse and
atrophy, reduces spasticity and spasms, produces a
more energy efficient use of proximal limb muscles,
and aids in motor relearning. FES has also been
shown to reduce the energy cost of walking post-
stroke.
Besides the peripheral effect on muscles
themselves, possible mechanisms about central
therapeutic benefits of FES have been hypothesized
(Rushton, 2003; Sheffler and Chae, 2007; Everaert
561
Gandolla M., Pedrocchi A., Ferrante S., Guanziroli E., Ward N. and Molteni F..
Carryover Effect after Functional Electrical Stimulation Treatment - Pilot Study for a Quantitative Approach.
DOI: 10.5220/0004659205610567
In Proceedings of the 5th International Joint Conference on Computational Intelligence (SSCN-2013), pages 561-567
ISBN: 978-989-8565-77-8
Copyright
c
2013 SCITEPRESS (Science and Technology Publications, Lda.)

et al., 2010). Liberson and colleagues reported the
following: “On several occasions, after training with
the brace, patients acquired the ability of
dorsiflexing the foot by themselves, although the
periods of spontaneous activity reported were only
transitory” (Liberson et al., 1961). This
phenomenon, introduced in literature under the name
of carryover effect, was further observed in
subsequent studies (Waters et al., 1985, Burridge et
al., 1997, Merletti et al., 1979, Ambrosini et al.,
2011). If the aim of a rehabilitation treatment is to
restore a lost motor function, the carryover effect
could be seen as a marker of the therapeutic
efficacy. However, a comprehensive quantitative
definition of carryover is yet not clear in literature in
distinguishing patients in those who report a
“carryover effect” after the treatment and those who
do not, based on selected outcome measures. This
work proposes a quantitative method to combine
different outcome measures to define an overall
outcome score. The overall outcome score could be
useful to inform about the carryover effect. Indeed,
it could inform further studies that directly measure
brain activity and plasticity (e.g., fMRI or TMS
studies) in order to directly address the reason why
an FES-based treatment is effective only for a pool
of patients.
2 METHODS
2.1 Participants
Patients were recruited from the outpatient and
inpatient services at the Villa Beretta Rehabilitation
Centre (Costamasnaga, LC, Italy). All patients had
suffered from first-ever stroke > 6 months
previously, resulting in weakness of at least the
tibialis anterior muscle (to <4+ on the Medical
Research Council (MRC) scale). Exclusion criteria
consisted of (i) responsiveness of less than 10° in
FES-induced ankle dorsiflexion; (ii) language or
cognitive deficits sufficient to impair cooperation in
the study; (iii) inability to walk even if assisted; (iv)
high spasticity at ankle joint plantar flexor as
measured by the modified Ashworth scale index,
MAS > 2 (Ashworth 1964).
Experiments were conducted with approval from
the Villa Beretta Rehabilitation Centre ethics
committee and all subjects gave informed written
consent.
Fifteen patients were recruited for the study and
10 completed the 20 sessions of training. The
carryover effect index was therefore possible to be
calculated only for the 10 participants that had all
measures.
2.2 Training
All patients were recruited for a specific FES-based
treatment for FD correction. Along with post-stroke
rehabilitation therapy appropriate to their clinical
needs, the patients were trained 5 times per week for
4 weeks, receiving a total of 20 sessions lasting 30
minutes of walking supported by a commercial
electrical stimulator. Two commercial devices were
available at the Villa Beretta Rehabilitation Centre:
Bioness L300 (Bioness Inc.) and WalkAide
(Innovative Neurotronics). The more suitable
commercial device was selected for each patients
depending on his/her best responsiveness to
stimulation and best wearability. Current threshold
was selected for each participant at the beginning of
each session so as to be able to elicit ankle
dorsiflexion during gait, but at the same time to
remain within the tolerance level. Two stimulating
electrodes were placed superficially along the
peroneal nerve to elicit tibialis anterior muscle
contraction during the swing phase of gait.
2.3 Clinical and Instrumental Measures
Patients impairment at the time of recruitment for
this study (t
1
) and after the intervention (t
2
) was
evaluated using a battery of clinical and instrumental
tests. In particular, they were evaluated through (i) a
gait analysis test performed following the standard
Davis evaluation protocol (Davis et al., 1991) in the
“Gait Lab” at Villa Beretta Rehabilitation Centre
along with (ii) the correspondent dynamic
electromyography test; and (iii) the 6-minute
walking test. Moreover they were scored by the
clinician on the (iv) MRC scale index at tibialis
anterior muscle.
A set of outcome measures (N=5) was designed
to assess different aspects of patients’ functional
current condition. All patients were therefore scored
on the following outcome measures within 5 days
before the beginning of the intervention (t
1
) and
within 5 days after the end of the treatment (t
2
): (i)
gait velocity (Vonschroeder et al., 1995; Perry et al.,
1995); (ii) a spatial asymmetry index – SA defined
as the absolute value of 1 minus the ratio between
paretic leg step length and non-paretic leg step
length (Lin et al., 2006); and (iii) a temporal
asymmetry index – TA defined as the absolute value
of 1 minus the ratio between paretic single support
time and non-paretic single support time (Lin et al.,
IJCCI2013-InternationalJointConferenceonComputationalIntelligence
562

2006), as measured during the gait analysis test; (iv)
endurance velocity, as calculated during the 6-
minute walking test; (v) the tibialis anterior
activation index during gait - TAAI index defined as
the ratio between the activity of the tibialis anterior
muscle between toe off and toe strike and during the
whole gait cycle (Burridge et al., 2001).
2.4 Carryover Effect Definition
In order to define the carryover effect, we proposed
to obtain one representative vector of improvement
(overall outcome score) that included all outcome
measures assessing different aspects of recovery,
with respect to a reference population of control
subjects. So as to perform a correct analysis in
comparing these scores, all considered outcome
measures were converted such that increasing score
reflected minor residual disability. Therefore, TA
and SA indices were converted such that an
increasing score reflected improvement. In particular
they have been computed as following:
11
(1)
11
(2)
Where “a” means that measure refers to the
“affected leg” and “na” means the measure refers to
the “non-affected leg”.
The values of the outcome measures for the control
population were derived from literature. The
controls dataset was created as 50 points randomly
sampled from a normal distribution having mean and
standard deviation as reported in literature (Table 1).
The overall outcome score was calculated
following the hereby outlined steps.
Let cj be the outcome measures sampled from
the normal distribution for the control group. j
ranges from 1 to 5 (i.e. N) and indicates the outcome
measure considered. Moreover, let x
1j
be the
outcome measures acquired at t
1
and x
2j
the same
outcome measures acquired at t
2
for the patients
group.
Firstly, a transformed space defined on control
subjects data is defined:
1) normalisation – let μ
j
and σ
j
be the mean and
the standard deviation of c
j
outcome measures (i.e.,
j=1,2,…,5, number of outcome measures
considered) defined on control subjects population.
A novel set of standardised variables z_c
j
(i.e. zero
mean and unit standard deviation) can be defined as
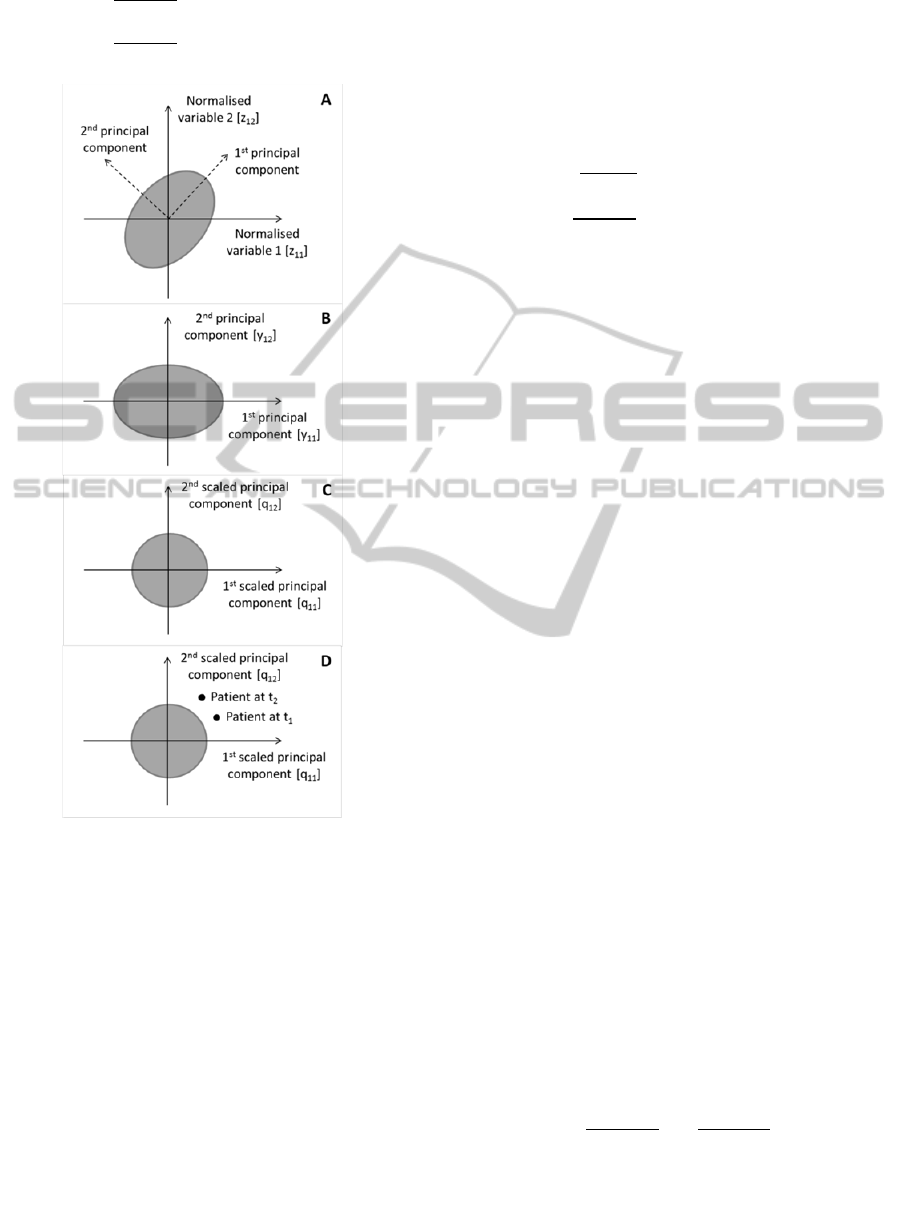
follows (Figure 1, panel A):
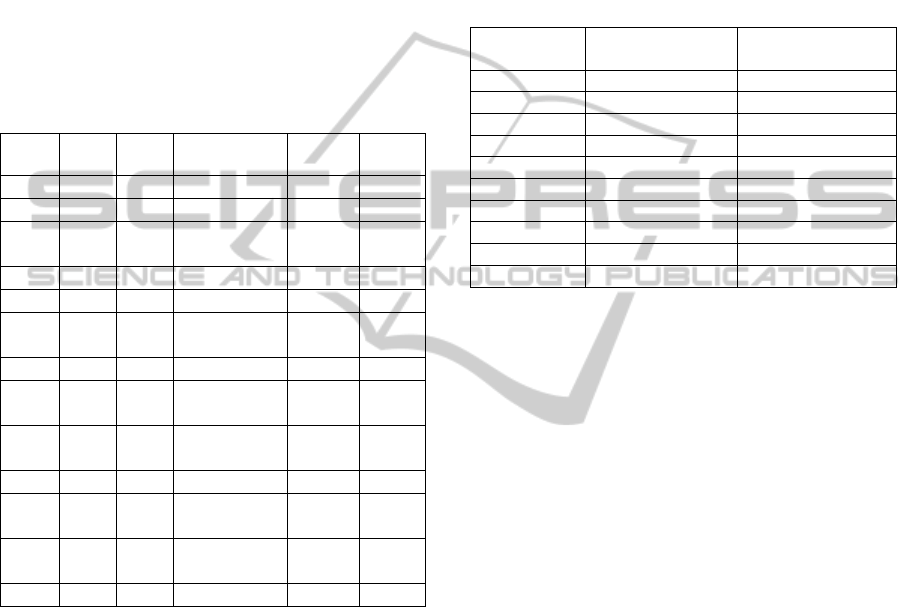
Table 1: Means and standard deviations of the selected
outcome measures for the control population as derived
from literature.
#
Outcome
measure
Mean
Standard
deviation
Reference
i
Gait
velocity
1.07
[m/s]
0.17 [m/s]
Vonschroeder
et al., 1995
ii
TA 1 10%*1 --
iii
SA
1 10%*1 --
iv
Enduranc
e velocity
1.4
[m/s]
0.2 [m/s]
Ilgin et al.,
2011
v
TAAI 0.70 0.12
Burridge et
al., 2001
_
; 1,2,…,5
(3)
The standardisation is useful so that the different
outcome measures units do not skew the results
(Shutte et al., 2000).
2) definition of principal components – define a
set of N independent/uncorrelated outcome measures
(i.e. y_z_c
j
) called principal components, that are
linear combination of the original N discrete
variables (Figure 1, panel B). Note that performing
principal component decomposition over normalised
variables corresponds to perform principal
component analysis on correlation matrix (Abdi and
Williams, 2010).
3) scaling of principal components – define a
new set of scaled principal components such that
each q
j
variable has equal variance over the control
group (Shutte et al., 2000). This is accomplished
through division by the standard deviation of each
principal component. Let s
j
be the standard deviation
of y_z_c
j
principal components (i.e., j=1,2,…,5,
number of principal components), the scaled
principal components are defined as follows (Figure
1, panel C):
_
_
; 1,2,…,5
(4)
At this stage we defined the transformed space on
scaled independent/uncorrelated variables (i.e.
scaled principal components) as defined on control
subjects outcome measures.
Secondly, all outcome measures as measured at
t
1
and t
2
for patients population are projected in the
transformed space defined at steps 1-3.
4) normalisation of t
1
and t
2
outcome measures –
consider now the same outcome measures as
acquired at t
1
and t
2
(i.e. x
1j
, x
2j
) and standardise
them as follows:
CarryoverEffectafterFunctionalElectricalStimulationTreatment-PilotStudyforaQuantitativeApproach
563
_
; 1,2,…,5
(5)
_
; 1,2,…,5
Figure 1: Graphical display of the proposed method with
two hypothetical variables represented by the grey shaded
ellipse. Only two hypothetical variables are represented
for the sake of graphical representation clarity. A)
graphical representation of the standardised two
hypothetical variables – each control subject is represented
by a combination of the two variables, i.e. he/she is a point
in the grey ellipse. Since the variables have been
standardised the mean of each outcome measure is 0. The
principal components axes can be seen as a rotated
coordinates system; B) projection of the original data on
the principal components axes; C) scaling of the principal
components, i.e. the ellipse representing the data becomes
a circle; D) an hypothetical patient represented as two
points in the transformed space.
Where μ
j
and σ
j
are the mean and the standard
deviation as calculated on outcome measures of
control subjects (see step 1).
5) projection of patients’ normalised outcome
measures in the principal component plane – i.e.,
project the t
1
and t
2
standardized outcome measures
in the principal component space defined at step 2
(i.e. define y_z_x
1j
and y_z_x
2j
).
6) scaling of patients’ principal components –
scale the t
1
and t
2
principal component as follows:
_
_
; 1,2,…,5
(6)
_
_
;
1,2,…,5
Where s
j
are the standard deviation of y_z_c
j
principal components defined at step 3.
At this stage all patients are represented by two
points in the transformed space (Figure 1, panel D)
where the origin of the reference system represents
the controls mean.
7) patients’ distance from control group – In
accord with clinicians, in order to define a threshold
for significant improvement, a threshold point was
added to the dataset defined as the Minimum
Detectable Change (MDC) for each outcome
measure. The MDC for each outcome measure was
again derived from literature and in particular it was
considered equal to 0.3 m/s for gait velocity (Fulk
and Echternach, 2008), 0.1 m/s for endurance speed
(Eng et al., 2004), 0.12 for TAAI index (i.e., two
times the interquartile interval for a group of health
subjects - Burridge et al., 2001), 0.032 and 2% for
the SA index and TA index respectively (Kesar et
al., 2011). This threshold point (i.e. x
MDCj
) was
projected and scaled on the transformed plane
defined by controls following steps 4 to 6 as for
patients outcome measures (i.e., q
MDCj
) in order to
get a minimum significant threshold for all the
scaled uncorrelated variables. Moreover, it has to be
taken into account that for some outcome measures
it might be possible that the patients value passes the
control mean, but this has not to be considered as an
impairment. For example gait velocity might be
higher than the controls mean, but this would have
to be considered as improvement and not as
impairment. Therefore, in this study gait and
endurance velocity are set to the respective controls
mean if the value passes the controls mean itself.
For each patient the overall outcome score (oos)
was defined as follows:
;
(7)
IJCCI2013-InternationalJointConferenceonComputationalIntelligence
564
8) definition of carryover effect – If oos > 0, the
patient overall worsened, whereas if oos < 0 the
patient overall improved beyond the predefined
threshold. The carryover effect would therefore be
achieved by those patients whose overall outcome
score is negative, i.e. they present an overall
functional improvement based on selected outcome
measures.
Table 2: Participant characteristics. Part = participant;
age = age of the participant at the time of t
1
acquisition in
years; M = male; F = female; R = right; L = left; MCA =
middle cerebral artery; ACA = anterior cerebral artery;
parac = paracentral; type = type of stroke;
H = haemorrhagic; I = ischemic; time = time since stroke
at the time of t
1
acquisition in months.
Part Age Sex
Site of
lesion
Type Time
PP
37 F R ACA H 10
AF
23 M R MCA TCE 23
SF
38 F
R globus
pallidus
I 23
EM
64 F L MCA H+ I 13
MT
19 M L MCA H 44
RM
47 F
L Globus
pallidus
H 44
MF
25 F R MCA I 30
SB
46 M
R globus
pallidus
I 13
DB
33
M
L parac.
lobule
I 6
LF
61
F
R MCA
H 158
LL
57
M
L Caudate
nucleus
I 6
GR
53
M
L globus
pallidus
H 37
PR
49
M
R MCA I 89
3 RESULTS
3.1 Participants
Table 2 outlines participants characteristics.
3.2 Carryover Effect Definition
The means (±standard deviations) reported for each
outcome measure at t
1
and t
2
acquisitions are the
following: (i) gait velocity - t
1
: 0.45 (±0.17) [m/s];
t
2
: 0.55 (±0.17) [m/s]; (ii) 1-SA - t
1
: 0.81 (±0.14); t
2
:
0.81 (±0.12); (iii) 1 – TA - t
1
: 0.71 (±0.20); t
2
: 0.72
(±0.13); (iv) endurance velocity - t
1
: 0.72 (±0.29)
[m/s]; t
2
: 0.82 (±0.35) [m/s]; (v) TAAI index - t
1
:
0.64 (±0.16); t
2
: 0.57 (±0.21).
The overall outcome score and the relative
achieved/non achieved carryover effect for each
participant is outlined in Table 3. Five patients out
of ten (i.e., 50%) reported a carryover effect as
defined by the outlined procedure.
Table 3: Overall outcome score calculated for each
participant, along with his/her definition of carryover
effect.
Participant
Overall outcome
score
Carryover effect
[yes/no]
PP
-4.86 Yes
AF
-2.12 Yes
SF
0.98 No
EM
0.89 No
MT
-1.01 Yes
RM
0.07 No
MF
0.14 No
SB
-0.81 Yes
DB
-0.65 Yes
LF
0.52 No
4 DISCUSSION
This work proposes a quantitative method to
distinguish patients undergoing a specific FES
treatment in those who report a carryover effect and
those who do not. This is an useful approach, since it
is common in literature to perform statistical
analysis between pre and post treatment sessions
looking at the patients as a group that statistically
improves or not, possibly with respect to a reference
group that does not get the treatment (e.g., Burridge
et al., 1997). However the clinical use of FES
demonstrated that patients differ in the
responsiveness to a FES based treatment (e.g.,
Merletti et al., 1979). Merletti and colleagues
approached the same issue, and demonstrated in 50
post-stroke patients that 34% reported a carryover
effect. However they based their results principally
on clinical considerations, whereas a rigorous
method could be of help when quantitative
evaluation is needed. In our study, 50% of patients
reported a carryover effect, even if assessed for a
smaller group of patients (i.e., 10 subjects). There
are contradictory conclusions in literature about the
validity of an FES treatments for FD (i.e., Schuhfrie
et al., 2012). The proposed step forward of this work
is about putting forward that the treatment could be
differentially effective for different patients, even
with the same functional baseline. This could have
CarryoverEffectafterFunctionalElectricalStimulationTreatment-PilotStudyforaQuantitativeApproach
565

its bases in a central effect of FES that responds to
differences in the lesion and consequent recovery at
the central nervous system level. Further studies are
required to investigate the relationship between the
carryover effect and what is happening in terms of
plasticity and/or connectivity between the involved
areas at central nervous system level.
This work is preliminary and in particular two
further issues would be of interest. Firstly the
treatment only lasts 20 sessions, and it has been
proposed that the longer the treatment the better the
results (Schuhfried et al., 2012). It could therefore be
interesting to follow up the evolutions of the
carryover effect along a longitudinal study.
Moreover, a validation of the carryover effect
quantitative definition by a group of clinicians that
separately assess the presence/absence of carryover
would be an interesting further development.
It is interesting to note that this quantitative
method could be applied to any other group of
outcome measures in order to define the carryover
effect on any other particular district (e.g. upper
limbs)
5 CONCLUSIONS
The proposed method allows to quantitatively
distinguish patients that report a carryover effect
following an FES-based treatment for FD. The two
groups are easily identified thanks to clear
mathematical steps based on principal component
analysis that starts from a battery of outcome
measures. In our group of post-stroke chronic
patients, 50% reported a carryover effect after 20
sessions of FES-based treatment. This could inform
further studies aimed at identifying the carryover
effect mechanism of action.
ACKNOWLEDGEMENTS
This work was made possible thanks to the patients
that volunteered to participate to the project, thanks
to Mauro Casarin and Stefano Tagliaferri that gave
their availability to help with scanning.
REFERENCES
Abdi, H., Williams, L.J., 2010. Principal component
analysis. Wiley Interdisciplinary Reviews:
Computational Statistics 2: 433–459.
Ambrosini, E., Ferrante, S., Pedrocchi, A., Ferrigno, G.,
Molteni, F., 2011. Cycling Induced by Electrical
Stimulation Improves Motor Recovery in Postacute
Hemiparetic Patients: a Randomized Controlled Trial.
Stroke 42:1068–1073.
Ashworth, B., 1964. Preliminary trial of carisoprodol in
multiple sclerosis. The Practitioner 192: 540–542.
Burridge, J. H., Taylor, P. N., Hagan, S. A., Swain, I. D.,
1997. The effects of common peroneal stimulation on
the effort and speed of walking: a randomized
controlled trial with chronic hemiplegic patients. Clin
Rehabil. 11:201–210.
Burridge, J. H., Wood, D. E., Taylor, P. N., McLellan D.
L., 2001. Indices to describe different muscle
activation patterns, identified during treadmill
walking, in people with spastic drop-foot. Medical
Engineering and Physics 23: 427–434.
Davis, R. B., Ounpuu, S., Tyburski, D. J., Gage J. R.,
1991. A gait analysis data collection and reduction
technique. Hum. Mov. Sci. 10: 575-587.
Eng, J. J., Dawson, A. S., Chu, K. S., 2004. Submaximal
exercise in persons with stroke: test-retest reliability
and concurrent validity with maximal oxygen
consumption. Arch Phys Med Rehabil 85: 113-118.
Everaert, D. G., Thompson, A. K., Chong, S. L., Stein, R.
B., 2010. Does functional electrical stimulation for
foot drop strengthen corticospinal connections?
Neurorehabil Neural Repair. 24: 168-77.
Fulk, G. D, Echternach, J. L., 2008. Test-retest reliability
and minimal detectable change of gait speed in
individuals undergoing rehabilitation after stroke. J
Neurol Phys Ther 32: 8-13.
Ilgin, D., Ozalevli, S., Kilinc, O., Sevinc, C., Cimrin, A.
H., Ucan, E.S., 2011. Gait speed as a functional
capacity indicator in patients with chronic obstructive
pulmonary disease. Annals of Thoracic Medicine 6:
141-146.
Kesar, T. M., Perumal, R., Jancosko, A., Reisman, D. S.,
Rudolph, K. S., Higginson, J. S., Binder-Macleod, S.
A., 2010. Novel Patterns of Functional Electrical
Stimulation Have an Immediate Effect on Dorsiflexor
Muscle Function During Gait for People Poststroke.
Physical Therapy 90: 55-66.
Kesar, T. M., Binder-Macleod, S. A., Hicks, G. E.,
Reisman, D. S., 2011. Minimal detectable change for
gait variables collected during treadmill walking in
individuals post-stroke. Gait Posture 33: 314-317.
Kottink, A. I., Oostendorp, L. J., Buurke, J. H., Nene,
A.V., Hermens, H.J., IJzerman, M.J., 2004. The
orthotic effect of functional electrical stimulation on
the improvement of walking in stroke patients with a
dropped foot: a systematic review. Artif Organs
28:577–586.
Liberson, W. T., Holmquest, H. J., Scot, D., Dow, M.,
1961. Functional electrotherapy: stimulation of the
peroneal nerve synchronized with the swing phase of
the gait of hemiplegic patients. Arch Phys Med
Rehabil. 42:101–105.
Lin, P., Yang, Y., Cheng, S., Wang, R., 2006. The
Relation Between Ankle Impairments and Gait
IJCCI2013-InternationalJointConferenceonComputationalIntelligence
566

Velocity and Symmetry in People With Stroke. Arch
Phys Med Rehabil 87: 562-568.
Medical Research Council. Aids to the examination of the
peripheral nervous system, Memorandum no. 45. Her
Majesty's Stationery Office, London, 1981.
Merletti, R., Andina, A., Galante, M., Furlan, I., 1979.
Clinical experience of electronic peroneal stimulators
in 50 hemiparetic patients. Scand J Rehabil Med.
11:111–121.
Olney, S. J., Richards, C.,1996. Hemiparetic gait
following stroke, part I: characteristics. Gait Posture
4:136 –148.
Perry, J., Garrett, M., Gronley, J.K., Mulroy, S.J., 1995.
Classification of Walking Handicap in the Stroke
Population. Stroke 26: 982–989.
Pomeroy, V. M., King, L., Pollock, A., Baily-Hallam, A.,
Langhorne, P., 2006. Electrostimulation for promoting
recovery of movement or functional ability after
stroke. Cochrane Database Syst Rev. CD003241.
Richards, C. L., Malouin, F., Dean, C., 1999. Gait in
stroke: assessment and rehabilitation. Clin Geriatr
Med. 15: 833–855.
Ring, H., Treger, I., Gruendlinger, L., Hausdorff, J.M.,
2009. Neuroprosthesis for Footdrop Compared with an
Ankle-Foot Orthosis: Effects on Postural Control
during Walking. Journal of Stroke and
Cerebrovascular Diseases 18: 41-47.
Rushton, D. N., 2003. Functional Electrical Stimulation
and rehabilitation – an hypothesis. Med Eng Phys 25:
75-78.
Sabut, S. K., Sikdar, C., Mondal, R., Kumar, R.,
Mahadevappa, M., 2010. Restoration of gait and motor
recovery by functional electrical stimulation therapy in
persons with stroke. Disabil Rehabil. 32: 1594-603.
Sheffler, L.R., Chae, J., 2007. Neuromuscular electrical
stimulation in neurorehabilitation. Muscle Nerve
35:562-90.
Schuhfried, O., Crevenna, R., Fialka-Moser, V.,
Paternostro-Sluga, T., 2012. Non-invasive
neuromuscular electrical stimulation in patients with
central nervous system lesions: an educational review.
J Rehabil Med 44: 99-105.
Schutte, L. M., Narayanan, U., Stout, J. L., Selber, P.,
Gage, J. R., Schwartz, M. H. An index for quantifying
deviations from normal gait. Gait and Posture 11:25-
31.
Vonschroeder, H. P., Coutts R. D., Lyden, P. D., Billings,
E., Nickel, V.L., 1995. Gait Parameters Following
Stroke - a Practical Assessment. Journal of
Rehabilitation Research and Development 32 : 25–31.
Waters, R. L., McNeal, D. R., Faloon, W., Clifford, B.,
1985. Functional electrical stimulation of the peroneal
nerve for hemiplegia: long-term clinical follow up. J
Bone Joint Surg 67A:792–93.
CarryoverEffectafterFunctionalElectricalStimulationTreatment-PilotStudyforaQuantitativeApproach
567
