
Governance and Privacy in a Provincial Data Repository
A Cross-sectional Analysis of Longitudinal Birth Cohort Parent Participants’
Perspectives on Sharing Adult Vs. Child Research Data
Shawn X. Dodd
1
, Kiran Pohar Manhas
2
, Stacey Page
2
, Nicole Letourneau
3
, Xinjie Cui
4
and Suzanne Tough
5
1
Medical Sciences Graduate Program, University of Calgary, 3820-24
th
Avenue NW, Calgary, Canada
2
Department of Community Health Sciences, University of Calgary, Calgary, Canada
3
Faculty of Nursing, University of Calgary, Calgary, Canada
4
PolicyWise for Children & Families, Edmonton, Canada
5
Department of Pediatrics, University of Calgary, Calgary, Canada
Keywords: Governance, Privacy, Stakeholder Engagement, Child Data, Controlled Access, Vulnerable Populations.
Abstract: Research data abound and are increasingly shared through a variety of platforms, such as biobanks for
precision health and data repositories for reuse of research and administrative data. Data sharing presents
great opportunities as well as significant ethical and legal concerns, such as privacy, consent, governance,
access, and communication. Respectful data governance calls for stakeholder engagement during platform
development. This stakeholder-engagement study used a web-based survey to capture the views of research
participants about governance strategies for secondary data use. Survey response rate was 60.8% (n = 346).
Parents’ primary concern was ensuring appropriate data re-use of data, even over privacy. Appropriate re-use
included project-specific access and limiting access to researchers with more-trusted affiliations like
academia. Other affiliations (e.g. industry, government and not-for-profit) were less palatable. Parents
considered pediatric data more sensitive than adult data and expressed more reluctance towards sharing child
identifiers compared to their own (p-value<0.001). This study stresses the importance of repository
governance strategies to sustain long-term access to valuable data assets via large-scale repository.
1 INTRODUCTION
Originating in the UK and USA, obligatory data
sharing expanding globally (CIHR, 2011; MRC,
2011; NIH, 2003; OECD, 2007). In Canada, national
research funding agencies, including the Social
Sciences and Humanities Research Council
(SSHRC), Canadian Institutes of Health Research
(CIHR), and Natural Sciences and Engineering
Research Council of Canada (NSERC), have issued
policies that highly recommend a range of data
sharing practices (CIHR, NSERC & SSHRC, 2016).
SSHRC funding requires Canadian researchers to
preserve their data and make it available within a
reasonable period of time to other researchers upon
project completion. Data informing peer-reviewed
publications that arose from SSHRC, CIHR or
NSERC funding must be made freely accessible
within 12 months of publication. This publication-
focused policy is expected to extend further to
promote greater accessibility of research outputs and
other research data (CIHR, 2011; CIHR, 2013).
To facilitate these requirements, data-sharing
platforms, such as biobanks and data repositories, are
proliferating. These platforms promote research
transparency and accountability by enabling further
analyses, replications, verifications and refinements
of results (El Emam et al., 2011; MRC, 2011; OECD,
2007). The frequency, diversity, novelty and
complexity of research opportunities increase due to
the expanding wealth of data available. Data sharing
introduces cost savings realized through economies
of scale, benefiting the public, funders, researchers
and trainees (McGuire et al., 2008; MRC, 2011;
OECD, 2007). The contributions of research
participants are maximized, while future research and
respondent burdens are lessened.
The emergence of data-sharing platforms,
highlighted the ethical tensions between individual
208
Dodd, S., Manhas, K., Page, S., Letourneau, N., Cui, X. and Tough, S.
Governance and Privacy in a Provincial Data Repository - A Cross-sectional Analysis of Longitudinal Birth Cohort Parent Participants’ Perspectives on Sharing Adult Vs. Child Research
Data.
DOI: 10.5220/0006430802080215
In Proceedings of the 6th International Conference on Data Science, Technology and Applications (DATA 2017), pages 208-215
ISBN: 978-989-758-255-4
Copyright © 2017 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

autonomy and the broader, societal good. While
individual consent is the norm for primary research
undertakings, it may or may not be sought when these
data are used for a secondary research purpose.
Currently, Canadian research ethics policy (TCPS2)
permits secondary use of research data to proceed
without consent, if data are de-identified (Article
5.5B, TCPS2) (CIHR, NSERC & SSHRC, 2010). The
implicit trade-off is that the risks of harms to
individuals through the use of their de-identified data
are lesser than the benefits arising to society through
data use and knowledge advancement. Where data are
identifiable, consent considerations direct secondary
use. Waiver of consent is possible, if several, specific
criteria are met (Article 5.5A, TCPS2) (CIHR,
NSERC & SSHRC, 2010). On balance, the
importance of the research question and knowledge
generated must outweigh possible harms to the
welfare of the person to whom the information relates
(CIHR, NSERC & SSHRC, 2010). The TCPS2
advocates engagement with relevant populations to
seek input on ethical issues and appropriate privacy
protection (CIHR, NSERC & SSHRC, 2010).
Recognizing the need for stakeholder input,
researchers have sought adult and adolescent
perspectives on data sharing, primarily in biobanking
contexts. These findings reveal that adults generally
recognize the need to balance research utility (i.e., the
common good) and participant privacy in sharing
genetic data (McGuire et al., 2008; Trinidad et al.,
2012; Trinidad et al., 2010). Privacy risks were
recognized, but permission, notification and
communication issues between biobanks and
participants were their more pressing concerns
(Trinidad et al., 2010; Beskow and Dean, 2008;
Ludman et al., 2010). Research participants generally
express trust in researchers and institutions, but, many
still wish to be asked for permission for the re-use of
their data (Beskow et al., 2008; Ludman et al., 2010).
Parent concerns impacting biobank enrollment for
themselves and their children include lack of
information, risks of stigma, privacy, consent,
researcher credibility questions, and the inability to
be re-contacted for results (Brothers and Clayton,
2012; Neidich et al., 2008; Joseph et al., 2008).
Ethnicity appears to impact biobank participation in
the US, with minorities more reticent than Caucasians
(Halverson and Ross, 2012; Joseph et al., 2008;
Jenkins et al., 2009).
The perspectives of research participants, parents
and children on secondary use of data have
predominantly focused on biological and genetic
data. Treating biobank and epidemiological data
similarly is recognized as inappropriate
(Laurie,
2011; Brakewood and Poldrack, 2013). These data
diverge in their nature, collection, storage, research
potential and implications. Application of standards
developed for the protection of biological data to non-
biological data might not be appropriate. Such
standards may be overly restrictive, wholly
inappropriate, or may disregard unique concerns.
This has relevance for all sectors even beyond health
research as many commercial and research initiatives
deal with personal non-biological information rather
than the limited, unique circumstances of biologics
and genomics. The voices of research participants on
secondary data use are absent. The purpose of this
study is to describe the governance and privacy
preferences of Albertan parent participants from two
longitudinal birth cohorts, when sharing their and
their child’s non-biological research data with a
research data repository.
2 METHODS
A cross-sectional, web-based survey (Dillman et al.,
2009)
sought parent cohort participant views about
governance strategies for secondary use of their and
their child’s data. This survey is part of a broader
mixed-methods study in this population; the
qualitative findings that preceded and informed
survey development are published elsewhere
(Manhas et al., 2015; Manhas et al., 2016).
2.1 Study Population
The target population was all parent participants of
two longitudinal provincial pregnancy cohorts: All
Our Babies (AOB)
(McDonald et al., 2013) and
Alberta Pregnancy Outcomes and Nutrition (APrON)
(Kaplan et al., 2014). These cohorts recruited
pregnant women beginning at 26 weeks gestation and
continuing with nine collection time points over the
subsequent five years. Together, cohort participants
(approximately 6400 people) provided information
on their demographics, lifestyle, mental, psychosocial
and physical health, pregnancy history, health service
utilization, quality of life, and breastfeeding. Detailed
information on these cohorts is described elsewhere
(Kaplan et al., 2014; McDonald et al., 2013).
Email invitations were sent to 569 eligible
participants the AOB cohort and 348 from the APrON
cohort) as we aimed to capture 10% of the cohort
population within the constraints of which
participants consented to contact for survey
invitation). The survey link remained available for
14-days. Reminder emails were sent to participants
Governance and Privacy in a Provincial Data Repository - A Cross-sectional Analysis of Longitudinal Birth Cohort Parent Participants’
Perspectives on Sharing Adult Vs. Child Research Data
209
on days 3 and 11, if the survey remained incomplete.
The AOB cohort participants received a follow-up
call on day 7 to ensure that they received the
invitation and to answer any questions. APrON
cohort preferences did not permit our team to provide
these participants with a follow-up call. Following
completion of the online survey, participants could
submit their e-mail address for entry in a draw for an
iPod Touch, which was kept separate from any data
they provided during survey completion.
Stratified random sampling directed recruitment
toward the following groups to enhance diversity of
responses: (a) father participants; (b) maternal age ≥
30 years at birth of cohort child; (c) maternal age < 30
years at birth of cohort child; and (d) mothers who
self-identify as a minority and/or as new to Canada in
the last five years. Inclusion criteria required that the
parent permitted re-contact for future research on
their original cohort consent form. Exclusion criteria
was limited to previous participation in other aspects
of the
broader mixed-methods study
(Manhas et al.,
2015; Manhas et al., 2016).
2.2 Online Survey Development
Survey content and design based on our previous
qualitative findings, a literature review of stakeholder
engagement in data sharing especially involving
parent perspectives or pediatric data, and pre-testing
using cognitive interviewing with 9 participants (CI)
(Adair et al., 2011).
The final survey consisted of 35 fixed choice
items assessing 5 areas: (a) parents’ motivations and
reservations surrounding participation in research,
data and data repositories; (b) preferences for
protective and organizational approaches for data
repositories; (c) consent preferences; (d) perceptions
of the sensitivity of pediatric data and secondary
research using this data; and (e) communication
preferences with data repositories. Ethics approval
was received from the Conjoint Health Research
Ethics Board, University of Calgary.
2.3 Data Analysis
Descriptive statistics were used to summarize study
variables. Chi-square and Fisher’s exact tests were
used to describe the association between categorical
variables. As the online survey did not require
participants to answer each question, missing
responses varied by question. The reported
percentages are according to participants who
answered each question, where missing data was not
included in the denominator. When comparing
parents’ willingness to share their or their child’s
identifiers, the four-point willingness scale was
truncated and presented as “willing” and “unwilling”.
The response options “do not care”, “somewhat
willing” and “willing” were collapsed into one
category called “willing”. Analyses were conducted
using STATA for Mac version 14.1 and a significance
level of p<0.05 was used for all tests.
3 RESULTS
3.1 Sample Characteristics
A response rate of 60.8% was achieved (N=346). 105
participants originated from the AOB cohort, 188
originated from the APrON cohort and 50 participants
were members of both cohorts. Amongst these
participants, 96.2% had attended some form of post-
secondary education, 98.0% were female, 69.1%
were over 35 years of age and 86.7% were born in
Canada. Table 1 provides a summary of the
respondent characteristics.
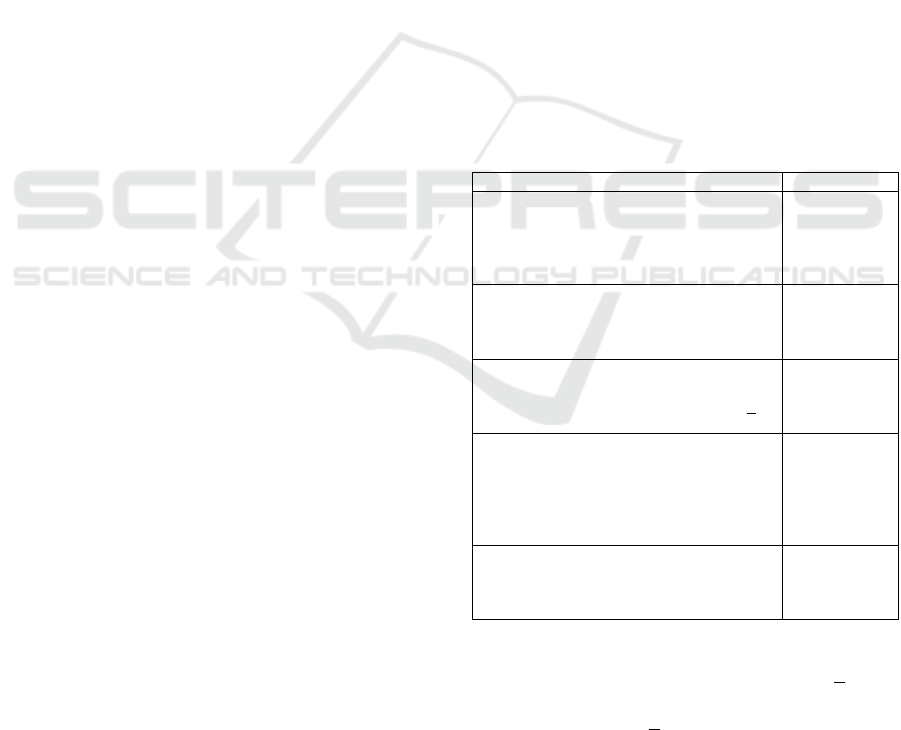
Table 1: Characteristics of Participants.
Characteristic N (%)
Longitudinal Birth Cohort
AOB Cohort
APrON Cohort
Both Cohorts
Missing
105 (30.3)
188 (54.3)
50 (14.5)
3 (0.8)
Sex
Male
Female
Missing
5 (1.4)
339 (98.0)
2 (0.5)
Age (years of age)
<35
>35
Missing
138 (39.9)
180 (52.0)
28 (8.1)
Education (highest level, in/complete)
High School
Business, Trade, Technical School
Bachelor’s Degree
Graduate School
Missing
9 (2.6)
51 (14.7)
190 (54.9)
92 (26.6)
4 (1.2)
Country of Origin
Canada
Other
Missing
300 (86.7%)
34 (9.8)
12 (3.4)
Study recruitment involved stratified random
sampling towards 4 strata, including mothers >30 and
<30 years of age, our sample included a greater
proportion of women >35 years of age than the AOB
and APrON cohorts (Table 2). The remaining
demographic characteristics of our sample were
reflective of both cohorts.
DATA 2017 - 6th International Conference on Data Science, Technology and Applications
210
3.2 Motivations and Reservations
When asked what motivated them to participate in
research generally, 77.4% of parents answered that
they felt research benefits society and wanted to help
advance science. Conversely, 6.1% participants felt
that there were potential benefits for their child, their
family or themselves. Some parents felt that past
experiences with research motivated them to continue
to participate in research (16.5%).
The most important reported benefit of research
data sharing centred on scientific advancement.
Parents indicated that the most important benefit of
data sharing (42.9%) was that new research questions
could be addressed from existing data sets. Another
11.9% of parents believed that data sharing would
benefit science by allowing the primary researchers’
work to be checked. The efficiencies of data sharing
were also viewed from a scientific, rather than
participant, lens. More parents prioritized the cost and
time savings for researchers and funders resulting
from data sharing (40.3%) over the reduced time and
effort burden on research participants (2.9%).
Parents were asked to prioritize their reservations
regarding research data sharing. Ensuring the
appropriate re-use of data was the primary concern
for 61.5% of parents. Protecting participant privacy
was of utmost importance for 36.3% of parents.
Finally, a small minority of parents highlighted
potential logistical concerns with the long-term costs
of supporting a data repository (1.5%).
3.3 Governing Data Access
The survey explored parent participant’s willingness
to make their data available to different types of
secondary users, based on their affiliation. Almost all
parents were quite willing to share their non-
biological research dataset with academic researchers
(e.g. universities: 97.4% willing). However, other
research affiliations were met with greater reluctance:
far fewer parents were willing to share their datasets
with industry (15.9% willing), government (41.6%
willing) and not-for-profit agencies (34.1% willing).
Parents also indicated that their support for data
sharing was dependant on researchers’ motives.
Support was largely received for initiatives that aimed
to uncover new knowledge about children, families
and society (91.6% willing); or to improve public
programs and policies (84.1% willing), and clinical
practices (83.5% willing). When motives were
commercial, parental reservations were noted. Only
38.2% of parents were willing to share datasets when
researcher motives aimed to improve products (i.e.
drug, baby food, etc.), while motives to use the shared
data to increase sales of a product were acceptable for
only 5.2% of parent participants.
Parental input was sought on how to control
secondary researcher’s access once approved. Nearly
three-quarter of participants felt that access should be
limited to the single project described on their
approved data access request (71.5%). Other parents
felt that access should be broader. Sixteen percent of
parents believed that once a researcher gained access,
the dataset could be used for multiple projects;
whereas 12.5% of parents felt that the researchers’
entire research team could access the data to complete
research projects.
The survey solicited parents on how repositories
could best monitor data reuse. There was diversity in
parents’ opinions. Some parents felt that the datasets
should never leave the repository facilities and
analyses should be performed in closed computing
areas (33.3%).
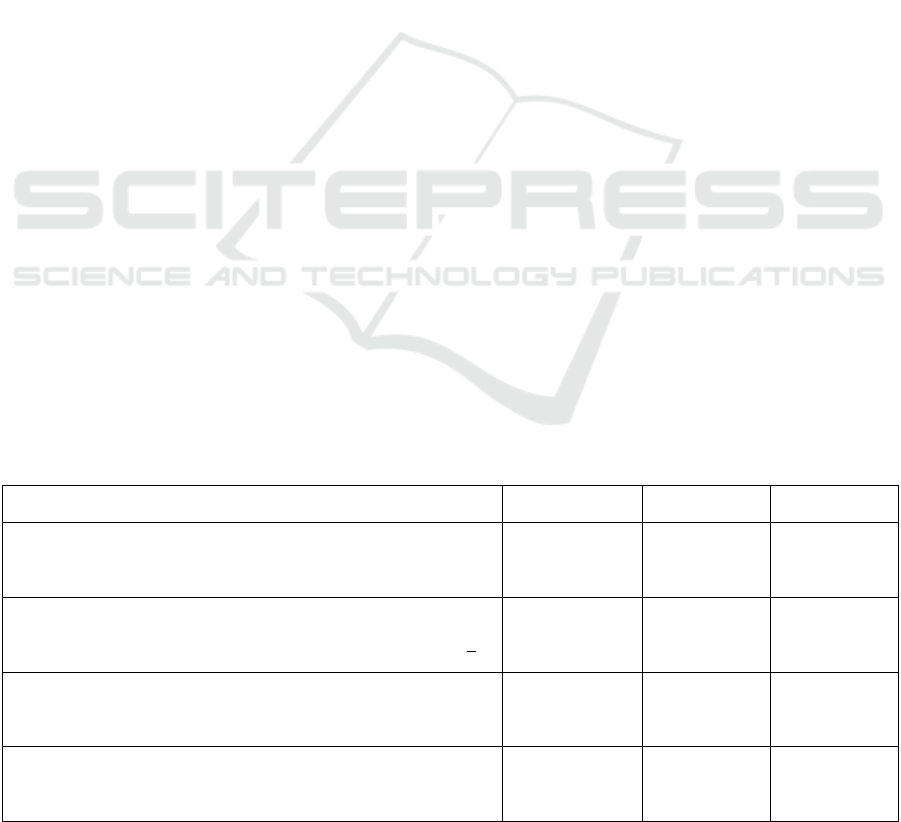
Table 2: Characteristics of Study Sample Compared to AOB and APrON Participant Characteristics.
Characteristic
Study
Sample (%)
AOB
33
(%)
APrON
38
(%)
Sex
Male
Female
Missing
1.4
98.0
0.5
0
100
-
1.4
98.1
0.5
Age (years of age)
<35
>35
Missing
39.9
52.0
8.1
75.8
24.1
-
76.6
23.4
-
Education
High School or less
Post-secondary education
Missing
2.6
96.2
1.2
11.0
89.0
-
9.7
90.3
-
Country of Origin
Canada
Other
Missing
86.7
9.8
3.4
78.1
21.9
-
81.3
18.7
-
Governance and Privacy in a Provincial Data Repository - A Cross-sectional Analysis of Longitudinal Birth Cohort Parent Participants’
Perspectives on Sharing Adult Vs. Child Research Data
211

Other parents felt that repositories should collaborate
with either the researchers’ home institutions (26.1%)
or the researchers’ scientific or professional societies
(7.0%) to monitor researchers. Other parents believed
that regular update reports submitted to the repository
(17.7%) or surprise audits of the secondary
researchers’ facilities and activities (14.8%) would
best safeguard the datasets.
Most parents preferred that the primary researcher
should be involved with the secondary data access
process. Most parents felt that either the primary
researcher should be a member of the decision-
making data access committee (40.8%) or the primary
researcher should at least be informed of all access
requests and given the opportunity to provide their
non-binding opinion on access (42.3%). Few
participants felt that primary researchers should
advise secondary researchers (11.1%) or actively
participate in the secondary research (4.1%).
3.4 Perceptions of Child vs. Adult Data
Parents were asked to consider whether there was a
difference between adult and pediatric data. Parents
generally agreed (69.8%) that there is a difference,
with 59.2% of parents believing that pediatric data
were more sensitive. The survey solicited parent
willingness to share specific identifiers about
themselves and their child with a qualified, secondary
researcher.
As illustrated in Table 3, of the 13 identifiers
described for adults and the 9 identifiers described for
children, parents were generally willing to share 11
and 7 identifiers, respectively. Parents exhibited little
concern sharing their full name (82.8% willing), their
complete address (72.5% willing) and their complete
date of birth (88.4% willing). Only a small percentage
of parents were unwilling to share their gender (0.3%)
or marital status (1.2%). Parents did, however,
express reluctance to share health care numbers
(40.5% of adults willing to share, 38.0% willing to
share their child’s) and social insurance numbers
(15.1% of adults willing to share, 12.9% willing to
share their child’s).
When comparing willingness of parents to share
their own identifiers compared to their child’s
identifiers, parents’ attitudes changed. Where
applicable, parents expressed more reluctance
towards sharing their child’s identifiers compared to
their own (p-value<0.001; Table 3). The sole
exception was gender (p-value= 0.924), where only
1.2% of parents were unwilling to share their child’s
gender.
The survey also asked parents when identifiers
should be removed from the dataset and by whom.
Parents generally felt that identifiers should be
removed by the primary researcher before submitting
the dataset to a data repository (71.6%). A minority
of parents indicated that removing identifiers was the
role of the data repository and should be done before
the dataset is released to the secondary researcher
(11.7%). Interestingly, 16.7% of parents had no
preference on when or by whom the identifiers were
removed.
Table 3: Willingness of Parent’s to share their, or their child’s identifiers.
Identifier
Willingness to share their
identifier
Willingness to share child’s
identifier
P-value
Willing Unwilling Willing Unwilling
Full Name 279 (82.8) 58 (16.8) 263 (76.0) 83 (24.0) <0.001
Complete Address 251 (72.5) 95 (27.5) 208 (60.1) 138 (39.9) <0.001
Full Postal Code
If no, first 3 Digits of Postal Code
202 (89.4)
87 (92.6)
24 (10.6)
7 (7.4)
189 (75.6)
99 (83.2)
61 (24.4)
20 (16.8)
<0.001
<0.001
Complete Birth Date
If no, month and Year of Birth
If no, year of Birth
306 (88.4)
134 (91.2)
67 (94.4)
40 (11.6)
13 (8.8)
4 (5.6)
280 (81.2)
137 (91.3)
54 (91.7)
65 (18.8)
13 (8.7)
5 (8.3)
<0.001
<0.001
0.001
Contact Information N/A N/A 253 (73.1) 93 (26.9) N/A
E-mail Address 297 (86.3) 47 (13.7) N/A N/A N/A
Phone Number 244 (71.1) 99 (28.9) N/A N/A N/A
Marital Status 340 (98.8) 4 (1.2) N/A N/A N/A
Job 329 (96.2) 13 (3.8) N/A N/A N/A
Gende
r
341 (99.7) 1 (0.3) 338 (98.8) 4 (1.2) 0.924
Ethnicity or Race 335 (99.4) 2 (0.6) 337 (97.7) 8 (2.3) <0.001
Level of Education 339 (99.1) 3 (0.9) N/A N/A N/A
Health Care Number 139 (40.5) 204 (59.5) 131 (38.0) 214 (62.0) <0.001
Social Insurance Number 52 (15.1) 292 (84.9) 44 (12.9) 298 (87.1) <0.001
DATA 2017 - 6th International Conference on Data Science, Technology and Applications
212

4 DISCUSSION & CONCLUSIONS
In general, parents supported the inclusion of their
non-biological research data with a research data
repository. Parents understood the impact that
research has on society and believe that data sharing
supports research initiatives while reducing the time
and cost to researchers and participants. This support
for data sharing came with some reservations.
Ensuring the appropriate re-use of data was the pri-
mary concern for parents. Governance was of greater
concern than privacy. Most empirical explorations of
stakeholder concerns have focused on privacy and
consent questions (Joseph et al., 2008; Kaufman et
al., 2008; Ludman et al., 2010; Malin et al., 2013;
McGuire et al., 2008; Trinidad et al., 2012), while the
more theoretical, ethico-legal analyses have
discussed governance issues (Laurie, 2011). Distinct
from the previous research studies, this study asked
participants to consider and rank between governance
and privacy concerns.
Appropriate re-use of data included sharing it with
researchers that participants trusted such as academic
researchers. Other research affiliations, such as
industry, government and not-for-profit, were met
with greater reticence. This information supports
previous findings that research participants place a
great deal of trust in academic researchers, the
governance and security afforded by their institutions
and their motives towards the greater good, and they
are wary of researchers outside of institutions
(O’Doherty et al., 2011). Even when profit is clearly
not the motive, as for not-for-profit organizations, a
lack trust exists, likely due to perceived institutional
unfamiliarity and possibly lack of security.
Regarding privacy, parents believed that
identifiers should be removed prior to the primary
researcher submitting the data to a repository.
Parents, however, expressed little reservation towards
sharing identifiers, with parents only noticeably less
willing to share their and their child’s health care
numbers and social insurance numbers. This
represents an inconsistency on two levels. Parents
were willing to share information that could combine
to proffer a great deal of information; information that
was available in a single number identifier that
parents were much less willing to share. It is unclear
if the question framing had each parent considering
the identifiers in isolation, or if they felt that their
names and birthdates were less crucial to their privacy
than their health care number and social insurance
number. The former seems unlikely because most
participants seemed to understand that datasets
include more than one variable. The other
inconsistency related to the fact that many parents
wanted the primary researcher to remove identifiers
prior to data sharing; but that parents were willing to
share the majority of identifiers about themselves
(and even their children).
Parental perspectives about governance and
control of data access is quite novel and informative.
Once a primary researcher has submitted a dataset,
parents felt that the primary researcher should either
be a member of a decision-making data access
committee or should be informed of all access
requests and given the opportunity to provide their
opinion on access. Once access was granted, parents
felt that access should be limited to the single project
described by the secondary researcher on the
approved data access request. This conservative
approach to governance and preference for project-
specific access provides complementary information
to the literature supporting broad consent models over
project-specific consent (Caulfield, 2007; Master et
al., 2012; Willison et al., 2008). While project-
specific consent from participants is unpalatable for
many stakeholders due to time and feasibility issues
(Master et al., 2012; McGuire et al., 2008; Trinidad et
al., 2012; Willison et al., 2008), project-level scrutiny
is desired by participants to ensure the security and
respect desired. This coincides with some
commentators in the literature that have recognized
the link between trust in institutions, responsible data
governance, and long-term practical sustainability,
interest and support in large-scale data repositories
including biobanks (Laurie, 2011; O’Doherty et al.,
2011)
There was some diversity amongst parents’
opinions regarding how to ensure that data are being
appropriately re-used. Some parents preferred that
data never leave the repositories and analyses should
be performed in a closed computing area, whereas
others felt that repositories should collaborate with
researcher’s home institutions (if applicable) or
societies to effectively monitor researchers. The key
finding from this paper is that there should be clear
governance at access and monitoring, with parent
participant stakeholders being more flexible on how
monitoring is realized.
When compared to local and provincial data
sources, the participants are generally representative
of the pregnant and parenting population in Calgary
and Alberta
(Leung et al. 2013). Therefore, this study
provides a good sample of Albertan parent
perspectives on data sharing, with certain limitations.
Given that participants were required to have
previously consented to participate in additional
research in their original cohort consent form, our
Governance and Privacy in a Provincial Data Repository - A Cross-sectional Analysis of Longitudinal Birth Cohort Parent Participants’
Perspectives on Sharing Adult Vs. Child Research Data
213

participants may be more supportive of research than
the general population. This may have resulted in an
overestimation of the support the Albertan pregnant
and parenting population may have towards data
sharing.
Another study limitation was a result of the
complexity of the topic. Given the novelty and many
nuances associated with data sharing, it was
recognized during the qualitative component of the
project that participants required additional
information to inform their decisions
(Manhas et al.
2015; Manhas et al. 2016). As such, detailed
background information was provided to participants
for each section of the survey. Though efforts were
made to avoid including information that may
influence participants’ responses, it is possible that
the included information may have altered
participants’ perspectives.
ACKNOWLEDGEMENTS
We would like to acknowledge and thank the
participants for this research project for their time and
insight. We are grateful for the AOB and APrON
research teams. We acknowledge Carol Adair, and
her significant contributions during study design and
proposal development; and Lawrence Richer and his
team’s significant contributions during data
collection in providing ePRO technical support. We
are grateful to PolicyWise for Children & Families,
which provided the external grant funding for the
study and salary support for a principal investigator
(KPM).
REFERENCES
Adair, CE, Holland, AC, Patterson, ML, et al. 2011
‘Cognitive interviewing methods for questionnaire pre-
testing in homeless persons with mental disorders.’
Journal of Urban Health: Bulletin of the New York
Academy of Medicine, vol. 89, no. 1, pp. 36-52.
Beskow LM, Dean E, 2008 ‘Informed consent for
biorepositories: Assessing prospective participants'
understanding and opinions.’ Cancer Epidemiology,
Biomarkers & Prevention, vol. 17, pp. 1440-1451.
Brakewood B, Poldrack RA, 2013 ‘The ethics of secondary
data analysis: Considering the application of Belmont
principles to the sharing of neuroimaging data.’
NeuroImage, vol 82, pp. 671-676.
Brothers KB, Clayton EW, 2012 ‘Parental perspectives on
a pediatric human non-subjects biobank.’ AJOB
Primary Research, vol 3, no. 3, pp. 21-29.
Canadian Institutes of Health Research (CIHR), 2013 CIHR
open access policy page, http://www.cihr-
irsc.gc.ca/e/46068.html. February 28, 2013.
Caulfield T, 2007 ‘Biobanks and blanket consent: The
proper place of the public good and public perception
rationales.’ Kings Law Journal, vol. 18, pp. 209-226.
CIHR, Natural Sciences and Engineering Research Council
of Canada (NSERC), Social Sciences and Humanities
Research Council of Canada (SSHRC), 2010. Tri-
council policy statement: Ethical conduct for research
involving humans. Government of Canada, Ottawa,
ON.
Collins D, 2003, ‘Pretesting survey instruments: An
overview of cognitive methods.’ Quality of Life
Research, vol. 12, pp. 229-238.
Dillman DA, Smyth JD, Christian LM 2009, Internet, mail
and mixed-mode surveys: The tailored design method.
3rd ed. John Wiley & Sons, Hoboken, NJ.
El Emam K, Buckeridge D, Tamblyn R, Neisa A, Jonker E,
Verma A 2011, ‘The re-identification risks of canadians
from longitudinal demographics.’ BMC Medical
Informations and Decision Making, vol. 11, pp. 46.
Freedom of information and protection of privacy act.
R.S.A. 2000, c. F-25.
Halverson CME, Ross LF, 2012, ‘Attitudes of african-
american parents about biobank participation and return
of results for themselves and their children.’ Journal of
Medical Ethics, vol. 38, no. 9, pp. 561-566.
Health information Act. R.S.A. 2000, c. H-5.
Jenkins MM, Reed-Gross E, Rasmussen SA, et al., 2009
‘Maternal attitudes toward DNA collection for gene-
environment studies: A qualitative research study.’
American Journal of Medical Genetics, Part A, vol.
149A, pp. 2378-2386.
Joseph JW, Neidich AB, Ober C, Ross LF, 2008, ‘Empirical
data about women's attitudes towards a biobank focused
on pregnancy outcomes.’ American Journal of Medical
Genetics, Part A, vol. 146A, pp. 305-311.
Kaplan BJ, Giesbrecht GF, Leung BM, et al. 2014, ‘The
Alberta Pregnancy Outcomes and Nutrition (APRON)
cohort study: Rationale and Methods’, vol. 10, no. 1,
pp. 44-60.
Kaufman DJ, Murphy-Bollinger J, Scott J, Hudson K, 2009
‘Public opinion about the importance of privacy in
biobank research.’ The American Journal of Human
Genetics, vol. 85, pp. 643-654.
Laurie G 2011, ‘Reflexive governance in biobanking: On
the value of policy led approaches and the need to
recognize the limits of law.’ Human Genetics, vol. 130,
pp. 347-356.
Leung BM, McDonald SW, Kaplan BJ, Giesbrecht GF,
Tough SC, 2013 ‘Comparison of sample characteristics
in two pregnancy cohorts: community-based versus
population-based recruitment methods.’ BMC Med Res
Methodol, vol. 13, pp. 149.
Ludman EJ, Fullerston SM, Spangler L, et al. 2010, ‘Glad
you asked: Participants' opinions of re-consent for
dbGaP data submission.’ Journal of Empirical
Research on Human Research Ethics, vol. 5, no. 3, pp.
9-16.
DATA 2017 - 6th International Conference on Data Science, Technology and Applications
214

Malin BA, El Emam K, O'Keefe CM, 2013, ‘Biomedical
data privacy: Problems, perspectives, and recent
advances.’ Journal of the American Medical
Informatics Association, vol. 20, no. 1, pp. 2-6.
Manhas KP, Page S, Dodd SX, Letourneau N, Ambrose A,
Cui X, Tough SC, 2015 ‘Parental perspectives on
privacy and governance for a pediatric repository of
non-biological data.’ Journal of Empirical Research on
Human Research Ethics, vol. 10, no. 1, pp. 88-99.
Manhas KP, Page S, Dodd SX, Letourneau N, Ambrose A,
Cui X, Tough SC, 2016 ‘Parental perspectives on
consent for participation in large-scale, non-biological
data repositories.’ Life Sciences, Society & Policy, vol.
12, pp. 1.
Master Z, Nelsn E, Murdoch B, Caulfield T, 2012
‘Biobanks, consent and claims of consensus.’ Nature
Methods, vol. 9, no. 9, pp. 885-888.
McDonald SW, Lyon AW, Benzies KM, McNeil DA, Lye
SJ, Dolan SM, Pennell CE, Bocking AD, Tough SC,
2013, ‘The All Our Babies cohort: Design, Methods,
and Participant Characteristics.’ BMC Pregnancy &
Childbirth, vol. 13, no. suppl 1, pp. S2.
McGuire AL, Hamilton JA, Lunstroth R, McCullough LB,
Goldman A 2008. ‘DNA data sharing: Research
participants' perspectives.’ Genetics in Medicine, vol.
10, no. 1, pp. 46-53.
Medical Research Council 2011, MRC policy and guidance
on sharing of research data from population and
patient studies. MRC, London.
National Institutes of Health 2003, NIH data sharing policy
and implementation guidance page.
http://grants.nih.gov/grants/policy/data_sharing/data_s
haring_guidance.htm. February 28, 2013.
Neidich AB, Joseph JW, Ober C, Ross LF, 2008 ‘Empirical
data about women's attitudes towards a hypothetical
pediatric biobank.’ American Journal of Medical
Genetics Part A, vol. 146A, pp. 297-304.
Organization for Economic Co-operation and Development
(OECD) 2007. OECD principles and guidelines for
access to research data from public funding. OECD,
Paris.
O'Doherty KC, Burgess MM, Edwards K, et al. 2011,
‘From consent to institutions: Designing adaptive
governance for genomic biobanks.’ Social Science &
Medicine, vol. 73, pp. 367-374.
Personal information protection and electronic documents
act. S.C. 2000, c. 5.
SSHRC 2012, Research data archiving policy page.
http://www.sshrc-crsh.gc.ca/about-au_sujet/policies-
politiques/statements-enonces/edata-
donnees_electroniques-eng.aspx. February 28, 2013.
Trinidad SB, Fullerton SM, Bares JM, Jarvik GP, Larson
EB, Burke W, 2010, ‘Genomic research and wide data
sharing: Views of prospective participants.’ Genetics in
Medicine, vol. 12, no. 8, pp. 486-495.
Trinidad SB, Fullerton SM, Bares JM, Jarvik GP, Larson
EB, Burke W, 2012, ‘Informed consent in genome-
scale research: What do prospective participants think?’
AJOB Primary Research, vol. 3, no. 3, pp. 3-11.
Willison DJ, Swinton M, Schwartz L, et al. 2008,
‘Alternatives to project-specific consent for access to
personal information for health research: Insights from
a public dialogue.’ BMC Medical Ethics, vol. 9, pp. 18.
Governance and Privacy in a Provincial Data Repository - A Cross-sectional Analysis of Longitudinal Birth Cohort Parent Participants’
Perspectives on Sharing Adult Vs. Child Research Data
215
