
Morphologic Character of Haematopinus Sp. Nymph and Mature
Stadium of Fries Holland Cow from Jember with SEM
(Scanning Electron Microscope)
Aan Awaludin
1*
, Yudhi Ratna Nugraheni
2
, Kurniasih
3
, Hariadi Subagja
1
1
Department of Animal Sciences, Politeknik Negeri Jember
2
Department of parasitology, Faculty of Veterinary Medicine, Gadjah Mada University
3
Department of pathology, Faculty of Veterinary Medicine, Gadjah Mada University
Keywords: Ectoparasites, Haematopinus, Insect, Phthiriasis, SEM.
Abstract: Haematopinus is the biggest parasite of insect family of domestic animal having all lifecycles in the female,
and can only live some hours out of the female body. This research aims at seeing the ultra-structural
difference of nymph stadium with Haematopinus sp. of mature insect facing Fries Holland (FH) from Jember
district based on morphologic identification key of Meleney and Kim. Samples of nymph stadium and mature
insect of Haematopinus sp. was taken from tail end area, perineum vulvae, area, and around eyes of 5 Fries
Holland (FH) cows of each infected cow from Jember district. The samples were identified based on
morphologic identification key of Meleney and Kim and did Scanning Electron Microscope (SEM) of part of
caput and abdomen. Results of morphologic identification and ultra-structure were analyzed descriptively.
There are ultra-structural differences of nymph stadium from mature insect, especially ultra-structure of
abdomen area. Nymph stadium has not been found in gonopod development; while, in mature insect,
gonopod has developed and can be identified. SEM method can be used to change the morphologic
identification, especially in a mature insect. Nymph and mature insect of Haematopinus sp. in Fries Holland
(FH) from Jember district are Haematopinus quadripertusus species.
1 INTRODUCTION
Ectoparasites of Haematopinidae frequently parasites
in the cow. Haematopinus euryternus was reported
parasitizing on cows in areas with cold climate, while
Haematopinus quadripertusus was reported
parasitizing on cows in tropical and sub-tropical areas
(Scifield et. al., 2012).
In some cases, insect infestation was reported
causing a reduction in productivity, and some cases
were reported as a disease vector, such as babesiosis,
theileriosis, anaplasmosis and others (Norval et. al.
1984). The incidence of insect infestation in a great
number of cows causes itching, reduction in body
weight, irritating, uncomfortable feeling, reduction in
dairy water production and reduction in initial harvest
product quality (Lasisi et. al., 2010). Hadi and
Saviana (2000) suggested that incidence of insect
infestation is called as pediculosis and phthiriasis.
Haematopinus sp. has characteristics of 0.5 cm,
yellow or grey-brown with a black line of each
margin, having no eye, having three pairs of wide and
flat legs (Urquhart et. al., 1987). Haematopinus sp.
has spiracle of dorsal periphery of mesothorax (Noble
and Glenn, 1989). Size of the head is elongated with
the wider back part than front part and protrusion in
the back antenna, the antenna has 5 internodes, and
part of the thorax is wide and has sterna plate in the
lower part (Lapage, 1956).
Mouth area of Haematopinus sp. has fine and
small proboscis called as haustellum, internal part of
haustellum is completed with small teeth directing to
exit functioning to implant in female skin, having 3
prick organs whose shapes are like needles called as
stilet which may expel to function to absorb blood and
inject saliva gland into female body (Hado and
Soviana, 2000).
Meleney and Kim (1974) explained key of
identification for species of Haematopinus sp.,
namely, rounding or compact para tergite in
peripheral area of abdomen with 2 posterior setae,
posterior area of abdomen has a non-pointing
gonopod, sterna plate under thorax elongates,
Awaluddin, A., Nugraheni, Y., Kurniasih, . and Subagja, H.
Morphologic Character of Haematopinus Sp. Nymph and Mature Stadium of Fries Holland Cow from Jember with SEM (Scanning Electron Microscope).
DOI: 10.5220/0010360905690575
In Proceedings of the 3rd International Conference of Computer, Environment, Agriculture, Social Science, Health Science, Engineering and Technology (ICEST 2018), pages 569-575
ISBN: 978-989-758-496-1
Copyright
c
2021 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
569

forming an elongating head and having parasite in
cows, so that the species directs to Haematopinus
euryternus species or maybe Haematopinus
quadripertusus. Female Haematopinus euryternus
species has body with 2.23 – 3.18 mm in long and
male Haematopinus euryternus species has body with
1.99 – 2.7 mm in long, having short and round
processus anterolatera; sterna plate, thin abdominal
trachea, subgenital plate median forming 9
subtrapezoid, tergite in abdomen with elongating and
more protruding processus anteromedial, short
forehead, and having subgenital plate in male insect,
completed with 6 anterior setae. Female
Haematopinus quadripertusus species has 3.42 –
4.75-mm body and male Haematopinus
quadripertusus species has 3.04 – 3.52-mm body,
having elongate processus anterolateral sterna plate,
long forehead, elongate processus anterolateral in
sterna plate thorax in female insect, thick abdominal
trachea trunks, long and narrow gonopod, wide
subgenital plate median subrectangular, 9
th
abdominal tergite has short and blunt processus
anteromedial, and male insect has subgenital plate
completed with 4 anterior setae.
Lapage (1956) explained lifecycle of
Haematopinus sp., 1-mm Haematopinus egg,
elongating shape, white in color, and sometimes
brown, eggshell is not hard, female insect lays one or
four eggs per day and female insect can expel 24 eggs,
eggs will hatch to be nymph (young insect) after 9 –
19 days at 27.5
o
C, nymph will come out from egg
and grow and molt perfectly and grow into adult.
2 METHODOLOGY
2.1 Location of Research
Samples of nymph and mature stadium of
Haematopinus sp. Were taken from Fries Holland
(FH) cows found suffering from pediculosis
(ptiriasis_ from Jember district.
The research was conducted in some locations.
Morphologic identification of insect was conducted
in the Parasitological laboratory, Faculty of
Veterinary Medicine, Gadjah Mada University,
Yogyakarta. Length scale and picture of insect were
measured using Lucida camera in Plant Infest
laboratory (Nematological Laboratory), faculty of
farming, Gadjah Mada University, Yogyakarta. ultra-
structure was identified by Scanning Electron
Microscope (SEM) in Central Research of Zoology,
Institute of Sciences of Indonesia (LIPI), Cibinong.
2.2 Tools and Materials
Tools to take samples included rubber glove, 2-ml
microtube, pinset, and labeling paper. Tools to
identify insect morphology included petri dish, slide
glass, cover glass, measurer (0.05-mm accuracy),
Lucida camera, pencil, HVS paper and tracing paper,
tools to examine body surface ultra-structure included
a holder, stibe, vacuum evaporator, Ion Coates, and
JEOL JSM-5310LV Scanning Electron Microscope
(SEM).
Required materials were absolute ethanol, 50%
ethanol, 70% ethanol, 85% ethanol, 95% ethanol,
2.5% glutaraldehyde, coccodylate buffer, 2% tannin
acid, 1% osmium tetraoxide, tertiary butanol, and
aquades.
2.3 Methods
2.3.1 Collection of Samples
Samples of nymph and mature stadium of
Haematopinus sp. In Fries Holland (FH) found
suffering from pediculosis (phthiriasis) were taken
from the tail area, especially fiber tail end, around
perineum vulvae, ear, and surrounding eyes of the
cow with 5 mature and nymph insects, using pinset,
then inserted into 2-ml microtube containing absolute
ethanol and labeled. Samples of mature insects and
nymph were collected in a separate microtube.
2.3.2 Morphologic Identification
Morphologic identification of mature insects and
nymph samples included macroscopic and
microscopic observations.
Macroscopic observation consisted of
identification of body, color, presence or absence of
wings, total extremities, and long measures body
using measurer.
Microscopic observation consisted of
morphological observation of caput, thorax,
abdomen, extremity, and picture and measure scale
were made using a binocular microscope (zooming 4
x 10) completed with Lucida camera drawing the
shadow of sample objects using pencil on HVS paper
which was then moved to tracing paper.
2.3.3 Identification of Ultra-structure
Identification of ultra-structure with Scanning
Electron Microscope (SEM) included caput and
abdomen. Observation of ultra-structure in caput part
included an anterior end to see ultra-structure of
mouth type. Observation of ultra-structure in
ICEST 2018 - 3rd International Conference of Computer, Environment, Agriculture, Social Science, Health Science, Engineering and
Technology
570
abdomen part included pleural disk part to see ultra-
structure of para tergite and posterior setae and in the
posterior end, part to observe ultra-structure of
gonopod and subgenital plate median.
The process of Scanning Electron Microscope
(SEM) was conducted in Central Research of
Zoology, Institute of Sciences of Indonesia (LIPI),
Cibinong. Before the samples were delivered, the
samples were fixed in absolute ethanol.
The process of Scanning Electron Microscope
(SEM) included process of specimen preparation
consisting of 5 stages: first, the samples were washed
by soaking in coccodylate buffer (2 hours), then the
samples were agitated in Ultrasonic cleaner (5
minutes); second, the samples were prefixed by
inserting the samples in 2.5% glutaraldehyde (2-3
hours); third, the samples were fixed by using 2%
tannin acid (6 hours), then washed by coccodylate
buffer (5 minutes), the process of washing was
conducted repeatedly until 4 times, then processed by
second washing by 1% osmium tetraoxide (one hour),
third washing by aquades (15 minutes); fourth,
dehydration process was conducted by soaking the
samples in stratified ethanol of 5% ethanol ( 5
minutes), this process was continued by using 70%
ethanol (20 minutes), 85% (20 minutes), and 95% (20
minutes) and absolute ethanol (10 minutes)
conducted two times at room temperature; fifth,
drying process by soaking the samples in tertiary
butanol (10 minutes), this process was conducted
until 2 times, then frozen by Freezed Drier until dry.
The process of Scanning Electron Microscope
(SEM), the samples which had been covered by
copper with Ion Coates (15 minutes) and observed by
Scanning Electron Microscope (SEM) of JEOL JSM-
5310LV.
3 RESULTS AND DISCUSSION
Fries Holland (FH) from Jember found suffering
from pediculosis (phthiriasis) had nymph stadium and
mature insect of Haematopinidae family. The
ectoparasites infestation area covered areas of the
hairy tail end, around the ear, and around perineum
vulvae, especially in the lower part. In the area, insect
eggs binding to hair were also found.
3.1 Morphologic Identification
Nymph stadium of Haematopinus sp. In Fries
Holland (FH) from Jember was found black, body
divided into 3 parts (caput, thorax, and abdomen),
dorso-ventral flat body shape, having no wings,
having 3 pairs of legs (proleg, mesoleg, and metaleg)
where each leg is divided into 4 internodes (coxa,
femur, tibia, tarsus) with 1 claw in each leg (Figure
1). The mature insect of Haematopinus sp. In Fries
Holland (FH) from Jember was black-grey in color, a
body is divided into 3 parts (caput, thorax, and
abdomen), dorso-ventral flat body shape. Having no
wings, having 3 pairs of legs
(proleg, mesoleg, and
metaleg) where each leg is divided into 4 internodes
(coxa, femur, tibia, tarsus) with 1 claw in each leg
(Figure 2). Body lengths of nymph samples of
Haematopinus sp. In Fries Holland (FH) from
Jember are 3.10 mm, 2.60 mm, 2.90 mm, 2.75 mm,
and 2.95 mm (Table 1), or averagely body length of
nymph samples is 2.86 mm. Body lengths of mature
insect samples of Haematopinus sp. In Fries Holland
(FH) from Jember are 4.70 mm, 4.60 mm, 4.20 mm,
4.15 mm, and 4.20 mm (Table 2), or averagely body
length of mature insect is 4.37 mm.
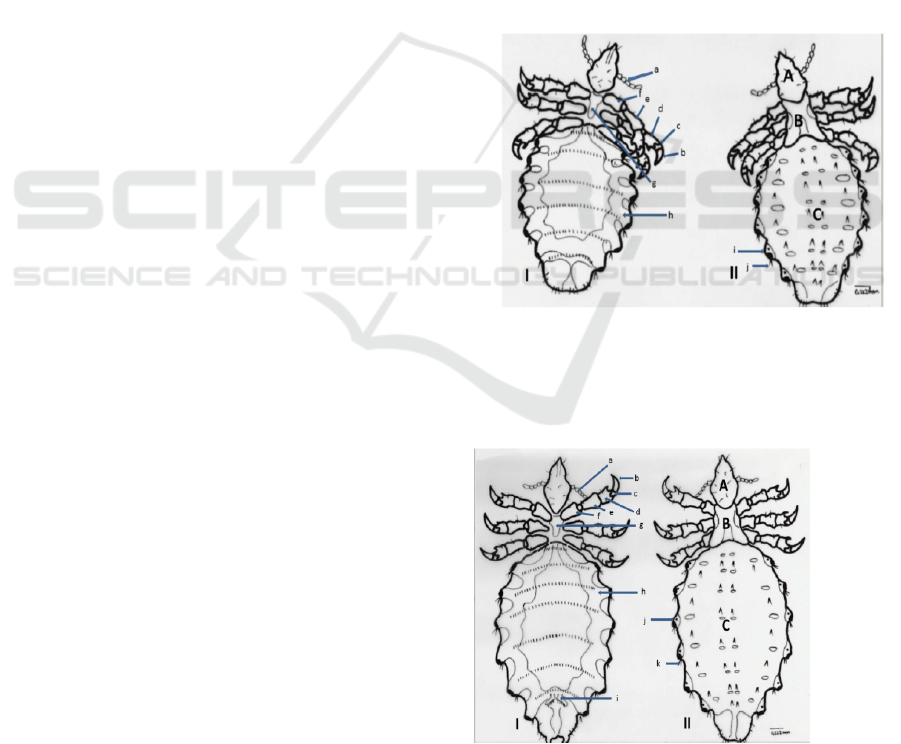
Figure 1. Nymph of Haematopinus sp. Of Fries Holland
(FH) from Jember: I. Ventral ( a. antenna, b. claw, c. tarsus,
d. tibia, e. femur, f. coxa, g. sterna plate, h. abdominal
tracheae trunks, i. gonopod); II. Dorsal (A. caput, B. thorax,
C. abdomen, i. para tergite, j. setae). Scale bar: 0.222 mm.
Figure 2. The mature insect of Haematopinus sp. Of Fries
Holland (FH) from JemberL I. Ventral (a. antenna, b. claw,
c. tarsus, d. tibia, e. femur, f. coxa, g. sterna plate, h.
Morphologic Character of Haematopinus Sp. Nymph and Mature Stadium of Fries Holland Cow from Jember with SEM (Scanning Electron
Microscope)
571

abdominal tracheae trunks, i. gonopod); II. Dorsal (A.
Caput, B, Thorax, C. Abdomen, j. para tergite, k. setae).
Scale Bar: 0.222.
Table 1. Samples of the nymph of Fries Holland (FH) from
Jember.
Identification Result
Color : Black
Body Shape : Dorsoventral flat
Body parts : 3 parts (caput,
thorax, abdomen)
Caput
Shape : Narrow and
pointing (smaller
than thorax part )
Mouth type : Pricking and
absorbing
Total antennas : 2 pairs
Antenna internodes : 5 internodes
Eye : Absent
Thorax
Shape : No segment
Total extremities : 3 pairs: Proleg,
Mesoleg, Metaleg
Extremity internodes : 4 ruas: Coxae,
Femur, Tibia,
Tarsus
Claw : Single
Wing : No wing
Abdomen
Shape : Segmental
Paratergite : Rounding, having
pleural disks with
2 pairs of setae in
angular part
Respiratory tract : Abdominal
tracheae tract is
seen clearly
Reproduction tool : Unobservable
Body length size
Nymph 1 : 3.10 mm
Nymph 2 : 2.60 mm
Nymph 3 : 2.90 mm
Nymph 4 : 2.75 mm
Nymph 5 : 2.95 mm
Table 2. Samples of mature insect of Fries Holland (FH)
from Jember
Identification Result
Color : Black-grey
Body Shape : Dorsoventral flat
Body parts : 3 parts (caput,
thorax, abdomen)
Caput
Shape : Narrow and
pointing (smaller
than thorax part)
Mouth type : Pricking and
absorbing
Total Antenna : 2 pairs
Internodes : 5 internodes
Eye : Absent
Thorax
Shape : No segment
Total extremities : 3 pairs: Proleg,
Mesoleg, Metaleg
Internodes : 4: Coxae, Femur,
Tibia, Tarsus
Claw : Single
Wing : No wing
Abdomen
Shape : Segmental
Paratergite : Rounding, having
5 setae hairs in
angular part
Respiratory tract : Abdominal
tracheae tract is
seen clearly
Reproduction tool : Long and narrow
Gonopod
Body length size
Insect 1 : 4.70 mm
Insect 2 : 4.60 mm
Insect 3 : 4.20 mm
Insect 4 : 4.15 mm
Insect 5 : 4.20 mm
Results of observation for nymph samples and
mature insect) Table 1 and Table 2) macroscopically
and microscopically indicate that there is no
morphologic difference of nymph from mature insect,
except nymph samples are seen black than the mature
ICEST 2018 - 3rd International Conference of Computer, Environment, Agriculture, Social Science, Health Science, Engineering and
Technology
572
insect. Key of morphologic identification of
Haematopinus sp., according to Meleney and Kim
(1974) is that abdomen has round and compact para
tergite with 2 posterior setae, and gonopod is not
pointing in posterior part. Haematopinus
quadripertusus has body lengths of 3.42 – 4.75 mm
(female) and 3.04 – 3.52 mm (male), having thick
abdominal tracheae trunks, long and narrow gonopod.
Haematopinus euryternus species has body length
sizes of 2.23 – 3.18 mm in female and 1.99 – 2.7 mm
in male, thin abdominal trachea, short and compact
gonopod.
Based on the key of identification, samples of
insect are from Haematopinus quadripertusus species.
3.2 Identification of Ultra-structure
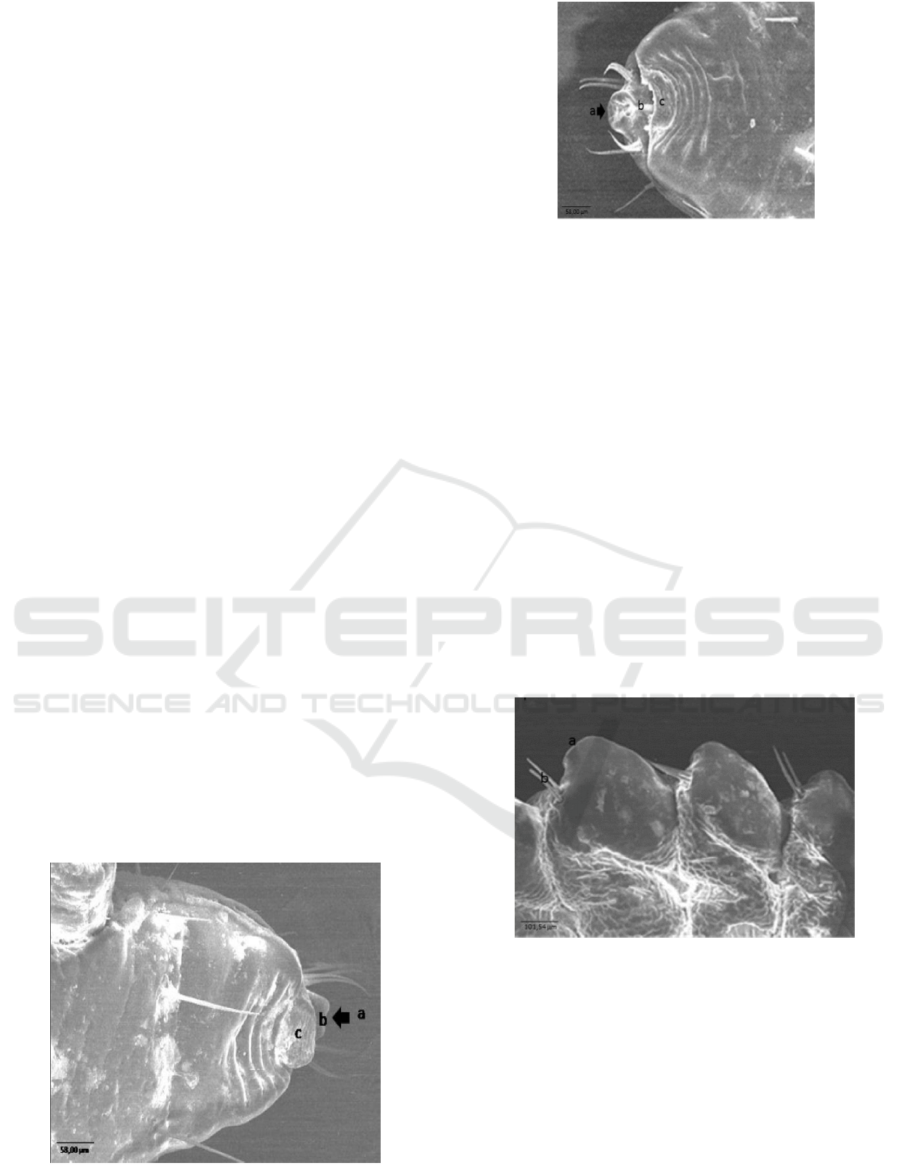
Ultra-structure of the anterior end of nymph samples
and mature insect (Figure 3 and Figure 4) is seen as
pointing shape of the suction tube in haustellum with
similar function to proboscis, labrum (lip), and 4 pairs
of setae. Organ of suction tube in haustellum belongs
to an insect with suction tube functioning to suck
blood from hospes as main food insect and flexible to
pull and expel according to need. Haustellum is an
organ with similar function to mouth of higher level
animals. Whitlock (1960) explained that
Haematopinus sp. Is different from other insects with
blood suction tubes because they have no long
proboscis pricked into the female body. Hadi and
Soviana (2000) indicated that Haematopinus sp. Has
mouth consisting of the fine and small proboscis
(haustellum). The internal part of haustellum is
completed with small teeth directing to exit to implant
into the female skin, having 3 organs of the suction
tube to suck blood and inject saliva gland into a female
body.
Figure 3. Ultra-structure of the ventral anterior end of
Haematopinus sp. Nymph of Fries Holland (FH) from
Jember: a. suction tube, b. Labrum, c. Setae. Scale bar:
58.00 m.
Figure 4. Ultra-structure of the ventral anterior end of
mature insect of Haematopinus sp. Of Fries Holland (FH)
from Jember: a. suction tube, b. Labrum, c. Setae. Scale
Bar: 58.00 m.
Ultra-structure of abdomen area of pleural disk
part of nymph samples and mature insect of
Haematopinus sp. (Figure 5 and Figure 6) indicates
that para tergite is rounded with spiraculum in end
part and has 2 setae hairs in posterior part in the lateral
area of the abdomen. In ventral part, mature female
insect samples have elongated and narrowing
gonopod shape and posterior part of gonopod has
subgenital plate median in subrectangular shape with
hollow in the central part (Figure 8), different from
the picture of ultra-structure in nymph samples
unseen for gonopod shapes (Figure 7). According to
Meleney and Kim (1974), Haematopinus
quadripertusus has long and narrow gonopod and
subgenital plate median of subrectangular shape.
Figure 5. Ultra-structure of pleural (ventral) disk of
Haematopinus sp. Nymph of Fries Holland (FH) from
Jember: a. spiraculum, b. Setae. Scale bar: 101.54 m.
Morphologic Character of Haematopinus Sp. Nymph and Mature Stadium of Fries Holland Cow from Jember with SEM (Scanning Electron
Microscope)
573
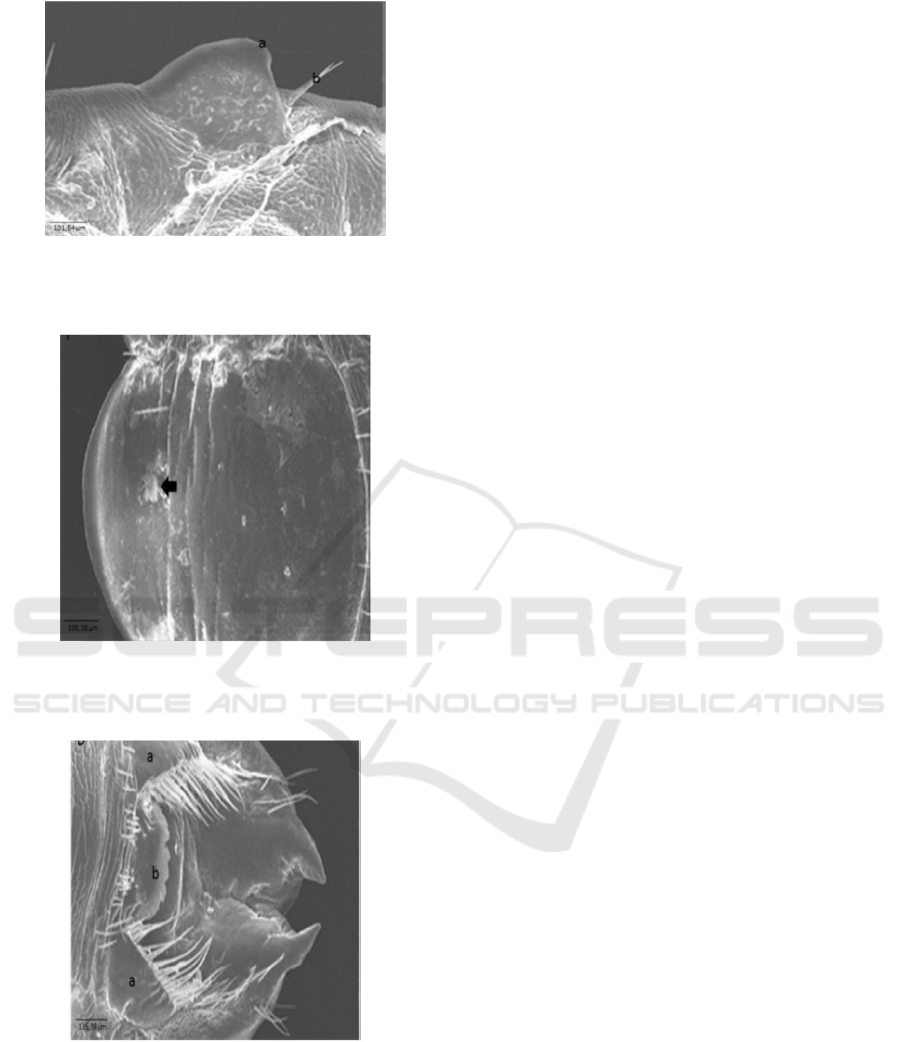
Figure 6. Ultra-structure of pleural (ventral) disk of
Haematopinus sp. A mature insect of Fries Holland (FH)
from Jember: a. spiraculum, b. Setae. Scale bar: 101.54 m.
Figure 7. Ultra-structure of ventro posterior nymph of
Haematopinus sp. Of Fries Holland (FH) from Jember
Gonopod shape is not seen (arrow). Scale bar: 135.38 m.
Figure 8. Ultra-structure of ventro posterior mature insect
of Haematopinus sp. Of Fries Holland (FH) from Jember
Gonopod shape is not seen (arrow). Scale bar: 135.38 m.
Based on identification key of Meleny and Kim
(1974), specially strengthened by the picture of ultra-
structure of mature insect sample consisting of a
suction tube, para tergite rounding with 2 setae hairs
in posterior part, long and narrow gonopod,
subrectangular subgenital plate median, then the
sample was identified as Haematopinus
quadripertusus species. Identification of insect using
Scanning Electron Microscope (SEM) can be used to
strengthen morphologic identification of insect,
especially in the mature stadium. In nymph,
especially in the posterior end, it cannot be a
reference of identification because gonopod did not
have developed, but the shape of pleural disk part
could be observed.
4 CONCLUSIONS AND
RECOMMENDATIONS
Scanning Electron Microscope (SEM) method can be
used to strengthen morphologic identification of
insect of Haematopinus sp. In mature stadium.
Samples of nymph and mature insect of Fries
Holland (FH) from Jember is Haematopinus
quadripertusus species.
Recommendations
Research of identification of species needs to add
molecular methods to see the phylogenetic tree of
researched species.
ACKNOWLEDGEMENT
Researcher team thanks, Prof. drh. Kurniasih,
M.VSc., Ph.D. for guidance and fund have given for
individual research so that this research could be
conducted. The team also thanks, Dr. drh. R. Wisnu
Nurcahyo for all guidance forms and
recommendations, parasitological department of
Veterinary Medicine Faculty and Laboratory of Plant
Infest (Nematology Laboratory), Faculty of Farming,
Gadjah Mada University, Yogyakarta, who had
provided place and tools for these research activities.
REFERENCES
Hadi, U.K. and Saviana S. 2000. Ektoparasit: Pengenalan,
Diagnosis dan Pengendaliannya. Faculty of Veterinary
Medicine, Farming Institute of Bogor. Bogor. pp.Hal.:
9-14.
Lapage, G. 1956. Veterinary Parasitology. Oliver and Boyd
Ltd. London. p: 377.
Lasisi, O. T., Eyarefe dan Adejinmi, J. O. 2010. Anaemia
and Mortality in Calves Caused by the Short-Nosed
ICEST 2018 - 3rd International Conference of Computer, Environment, Agriculture, Social Science, Health Science, Engineering and
Technology
574

Sucking Louse (Haematopinus eurysternus) (Nitzsch)
in Ibadan. Nigerian Veterinary Journal. 31 (4) 295-299.
Meleney, W.P. and Kim K. C. 1974. A Comparative study
of cattle-infesting Haematopinus with redescription of
H. quadripertusus Fahrenholz,1916 (Anoplura:
Haematopinidae)*. The journal of parasitology. 60 (3)
: 507-522.
Noble, E.R. and Glenn, A. 1989. Parasitologi Biologi
Parasit Hewan Edisi kelima. Gadjah Mada University
Press. Yogyakarta. Hal : 386,717.
Norval, R. A. I, Fivaz, B. H, Lawrence, J.A . and Brown,
A.F. 1984. Epidemiology of tick-borne diseases of
cattle in Zimbabwe. Tropical Animal Health and
Production. 16:63-70.
Scofield, A, Campos, K. F, Melo da Silva A, M, Oliveira C,
H, S, Barbosa, J. D, and Goes-Cavalcante G. 2012.
Infestation by Haematopinus quadripertusus on cattle
in São Domingos do Capim, state of Pará, Brazil. Rev.
Bras. Parasitol. Vet., Jaboticabal, v. 21, n. 3, p. 315-318.
Urquhart, G. M., J. Armour., J. L. Duncan, A. M. Dunn, F.
W. Jennings. 1987. Veterinary Parasitology.
Departement of Veterinary Parasitology, Faculty of
Veterinary Medicine. University of Glasgow. pp: 164-
69.
Morphologic Character of Haematopinus Sp. Nymph and Mature Stadium of Fries Holland Cow from Jember with SEM (Scanning Electron
Microscope)
575
