
Reverse Translational Research
How Clinical Trials on Fluorescence Imaging for Vocal Cord Cancer Fuels
Fundamental Research
Olivier Gaiffe
1
, Christian Pieralli
2
, Laurent Tavernier
3
, Lionel Pazart
1
and Bruno Wacogne
1,2
1
INSERM CIT 808, Besançon University Hospital, Place Saint Jacques, 25030 Besançon cedex, France
2
FEMTO-ST Institute, UMR 6174, 16 Route de Gray, 25030 Besançon cedex, France
3
Besançon University Hospital ENT Unit, Bd Flemming, 25030 Besançon cedex, France
Keywords: Translational Research, Vocal Cords, Fluorescence Imaging.
Abstract: Translational research consists in translating fundamental research results as closely as possible to patients.
Researchers sometimes underestimate these studies because it is thought that, although essential for setting
up new investigation tools, they do not deepen fundamental knowledge. However, users face specific
difficulties due to the variability of the biological systems under study. Variability is easily understood from
one patient to another, but there is also variability in a single patient whose metabolism evolves together
with therapeutic actions. Results obtained in translational research often depend on this variability, and new
questions and scientific obstacles arise when research is applied to the real world. In order to address these
new challenges, reverse translational research is required. Fundamental research is fuelled by the results of
translational research. In this position paper, we consider vocal cord fluorescence imaging as an example of
bi-directional translational research. First, we briefly recall the basics of fluorescence imaging, and we
explain why commercial fluorescence systems lead to variable estimations of their efficiency by end-users.
Second, we describe solutions intended to improve fluorescence techniques. This position paper will then
make conclusions.
1 INTRODUCTION
Translational research is a fairly new and rapidly
evolving concept. The general idea is to translate
fundamental research results as closely as possible to
patients via pre-clinical and clinical trials. In other
words, it consists in taking research from bench to
bedside. Sometimes researchers underestimate these
studies because it is thought that, although essential
for setting up new investigation tools, they do not
deepen fundamental knowledge. However, users
face specific difficulties due to the variability of the
biological systems under study. Variability is easily
understood from one patient to another, but there is
also variability in a single patient whose metabolism
evolves together with therapeutic actions. Results
obtained in translational research often depend on
this variability, and new questions and scientific
obstacles arise when research is applied to the real
world. In order to address these new challenges,
reverse translational research is required.
Fundamental research is then fuelled by the results
of translational research.
Consequently, the concept of bidirectional
translational research is emerging: forward from
researchers to end-users and then back to research to
answer new questions or improve current results. In
this position paper, we illustrate this idea through
examples concerning fluorescence optical diagnosis,
and more precisely tools that could be used to
diagnose vocal fold disease.
In part two of this paper, we briefly recall the
basics of fluorescence and we highlight the fact that
both the excitation wavelength and observation
wavelength window must be carefully chosen to
efficiently assess the composition of the tissue under
examination. This section illustrates what is called
translational research.
In part three, we explain why end-user teams
often disagree on the performances of current
commercial fluorescence systems. Here, we will see
that multimodality may offer versatile systems
intended for most end-users. This section illustrates
how translational research can put to evidence new
282
Gaiffe O., Pieralli C., Tavernier L., Pazart L. and Wacogne B..
Reverse Translational Research - How Clinical Trials on Fluorescence Imaging for Vocal Cord Cancer Fuels Fundamental Research.
DOI: 10.5220/0004323802820287
In Proceedings of the International Conference on Biomedical Electronics and Devices (BIODEVICES-2013), pages 282-287
ISBN: 978-989-8565-34-1
Copyright
c
2013 SCITEPRESS (Science and Technology Publications, Lda.)
scientific obstacles.
Part four is devoted to the description of
hyperspectral fluorescence techniques that may be
used to improve diagnosis efficiency. We also
propose architectures based on multimodality. Early
experimental results will be presented for these
techniques. This section illustrates how translational
research fuels fundamental research by means of
what we call reverse translational research.
This paper will then make conclusions.
2 BASICS OF FLUORESCENCE:
TRANSLATION
One way to discriminate unhealthy tissues from
healthy ones is by detecting an abnormal
concentration of particular proteins by means of
fluorescence measurements. Fluorescence can be
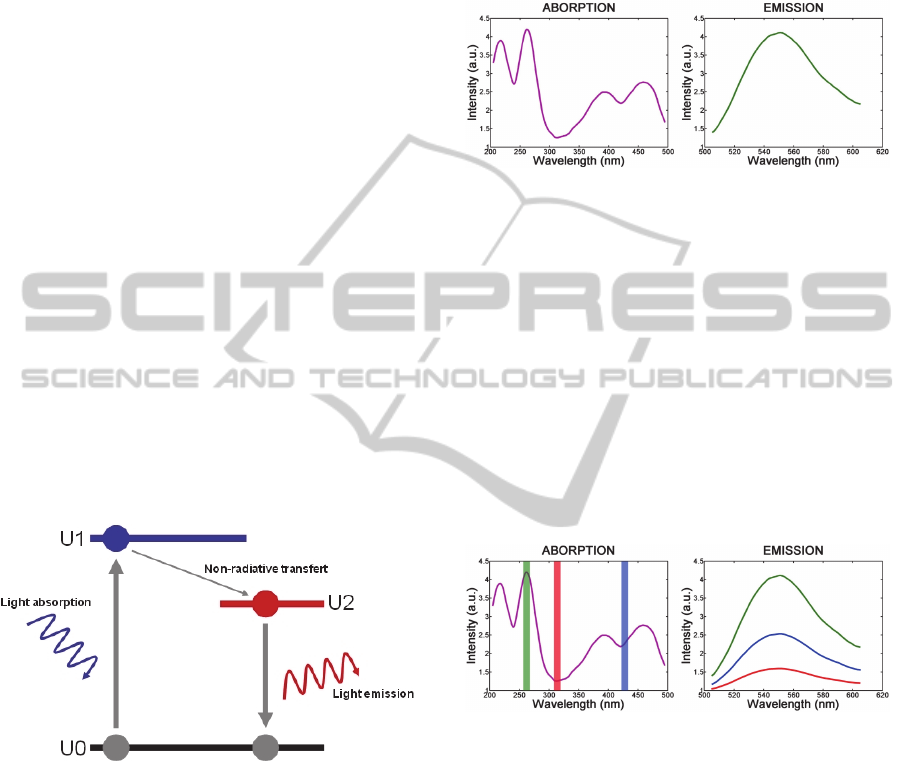
explained with the help of the Jablonski diagram
(figure 1). By illuminating the molecules, electrons
absorb light and “go upward” from energy state U0
to energy state U1. The molecules then relax, first by
a non-radiative loss of energy (from U1 to U2) and
finally down to energy state U0. This last step is a
radiative process and the light emitted at this stage is
called fluorescence.
Figure 1: Jablonski diagram.
Two different methods can be used to detect
proteins. The first deals with exogen fluorescence
which consists in applying a mixture of mono- or
polyclonal antibodies functionalized with
fluorophores. This method requires foreign
substances to be injected into the body, however.
The other way of detecting the target proteins is
to use endogen fluorescence, also termed
autofluorescence. It consists in studying the natural
fluorescence of the target proteins. In this case, once
the target proteins are determined, an illumination
source suited to their absorption spectrum must be
chosen. The first step of the study is therefore to
determine the target proteins adequately. For
instance, for vocal folds, we can name collagen,
elastin, NADH, flavins or porphyrins. Figure 2
shows the absorption and emission spectra of flavins
(Wagnieres 1998 – Richards-Kortum 1996).
Figure 2: Absorption and emission spectra of flavins.
2.1 Influence of the Excitation
Wavelength
Fluorescence intensity depends on different factors
such as the quantum properties of the fluorescent
protein, its concentration in the tissue and the
absorption of both excitation and emission
wavelengths by the tissue. These parameters are not
easily controllable. However, fluorescence intensity
also depends on the excitation wavelength used.
Figure 3 illustrates this with flavins. The shape of
the emission spectrum remains constant but its
amplitude depends on the excitation wavelength.
Figure 3: Effect of the excitation wavelength on
fluorescence intensity.
This illustrates the importance of the choice of
excitation wavelength. Note that the fluorescence
intensity also depends on the spectral width of the
excitation around the central excitation wavelength.
2.2 Influence of the Observation
Wavelength Window
Several fluorescent proteins are usually present in
tissue. A single excitation wavelength often induces
fluorescence in different proteins even if the
excitation wavelength is carefully chosen. This does
not necessarily mean that observation provides
average information on each individual protein
ReverseTranslationalResearch-HowClinicalTrialsonFluorescenceImagingforVocalCordCancerFuelsFundamental
Research
283

contribution. Since different proteins emit
fluorescent light according to their own emission
spectra, the choice of the observation wavelength
window may help differentiate between the
information from each protein. This is illustrated in
figure 4 in the case where both flavins and
porphyrins are considered (Wagnieres 1998 –
Richards-Kortum 1996).
Figure 4: Influence of the observation window.
As can be seen, when higher observation
wavelengths are considered, the greatest
contribution comes from porphyrins. Conversely,
when shorter wavelengths are considered, the
greatest contribution comes from flavins.
3 TRANSLATIONAL RESEARCH:
CONFRONTATION WITH
REAL SITUATIONS
In real life, the situation is much more complicated
due to the large number of fluorescent proteins
present in the tissues. The figure below shows the
excitation and emission spectra of several proteins
(Wagnieres, 1998); (Richards-Kortum, 1996).
It can be seen that neither the choice of the
specific excitation wavelength nor the choice of the
observation window can help to dissociate the
fluorescence contribution of each individual protein.
Concerning translational research, a large
number of studies have been conducted in order to
assess the efficiency of experimental or commercial
devices. Highly interesting reviews have been
published (Piazza, 2011); (Kraft, 2010); (Shin,
2010); (Mehrotra, 2010); (Rethman, 2010).
Figure 5: Absorption and emission spectra of some
proteins.
Commercial apparatuses relying on this principle
are readily available but the clinical trials performed
to date do not present sufficient evidence of their
ability to provide a reliable diagnosis (Piazza, 2011);
(Rethman, 2010). These systems suffer from the fact
that the excitation is made over a large wavelength
band (Arens, 2007); (Mehrotra, 2010). In other
words, many proteins are excited: the useful signal is
buried in the various fluorescence signals emanating
from non-relevant proteins, thus preventing the
target protein from being detected. The same is true
of their detection principle, as the observation
wavelength range is also quite wide. Therefore,
superimposition of information from a large number
of proteins is observed and dilutes useful signal into
what can be considered as noise. Figure 6 shows the
excitation and observation wavelength windows
commonly used in commercial systems.
Although autofluorescence is considered as
highly effective in the early diagnosis of laryngeal
cancer and its precursor lesions (Kraft, 2010),
clinical trials lead to varying specificity and
sensitivity results even with the same commercial
system: 98% sensitivity and 100% specificity (Lane,
2006), 50% sensitivity and 38.9% specificity
(Mehrotra, 2010) and 97% sensitivity and 94%
specificity (Poh, 2006).
Figure 6: Wavelength windows commonly used in clinical
trials.
BIODEVICES2013-InternationalConferenceonBiomedicalElectronicsandDevices
284
In fact, in the case of upper aerodigestive tract
cancer evaluation, optical techniques such as tissue
staining, chemiluminescence, autofluorescence and
tissue reflectance analysis have given unconvincing
results. For the latter two techniques, the following
description was reported (Rethman, 2010):
- "There is insufficient evidence that commercial
devices based on autofluorescence enhance visual
detection of potentially malignant lesions beyond
that achieved through a conventional visual and
tactile examination (Patton, 2008).
- There is insufficient evidence that commercial
devices based on tissue reflectance enhance visual
detection of potentially malignant lesions beyond
that achieved through a conventional visual and
tactile examination (Patton, 2008)."
The conclusion to be made regarding these
translational research results is that none of the
techniques is entirely satisfactory. Because these
techniques can be greatly enhanced in terms of
sensitivity and specificity, they often require
subjective interpretation and depend on the visual
recognition skills of the examiner (Shin 2010). The
same authors also explain that "the combination of
wide-field and high-resolution fluorescence imaging
systems with automated image analysis should be
investigated to maximize overall diagnostic
performance".
At this stage, forward translational research was
conducted. Research on fluorescence led to the
testing of advanced systems in clinical trials. These
trials concluded that systems are largely improvable
in terms of specificity and/or sensitivity. We have
explained the reasons for information loss in the
present paper. Reverse translational research should
now be envisaged to explore the performance of
advanced fluorescence techniques, possibly coupled
with other optical investigations of tissue properties.
4 REVERSE TRANSLATIONAL
RESEARCH: HOW TO
ANSWER NEW OBSTACLES
HIGHLIGHTED BY CLINICAL
TRIALS
We entirely agree with (Shin, 2010) concerning the
utility of combined techniques. Furthermore, we
believe that other modalities may be included in
advanced devices.
For example, we may consider a device that can
excite target molecules at several specific
wavelengths and also perform hyperspectral signal
detection. We can thus analyze one or more small
spectral bandwidths centered on the fluorescence
wavelengths of several proteins (Muller, 2003);
(Gillenwater, 1998).
4.1 Possibilities with Hyperspectral
Fluorescence
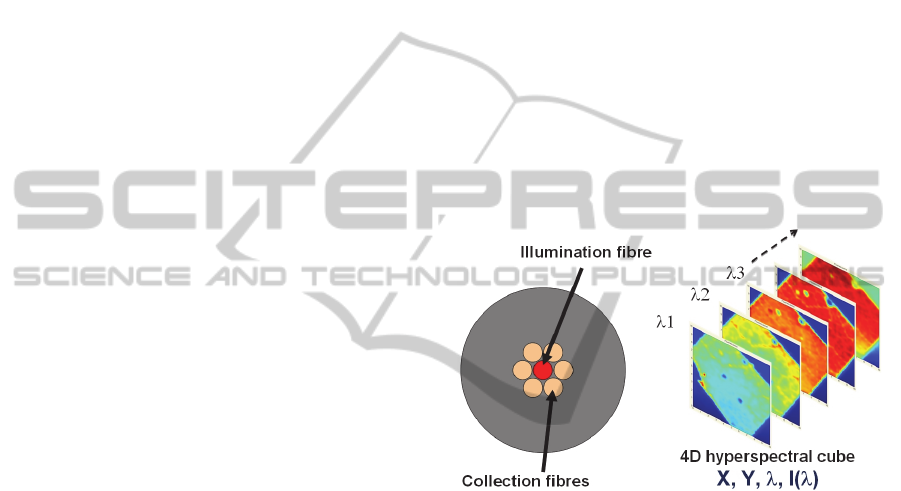
Our basic experimental set-up consists of a fiber
probe containing several optical fibers (figure 7:
left). The central one is used to illuminate the tissue
with a series of monochromatic wavelengths (via
optical switches). Collection fibers are used to
collect the emitted fluorescence. The light is then
launched into a spectrometer. For each pixel of the
image, the whole fluorescence spectrum is recorded
as depicted in figure 7 (right) in the case of a tree
leaf.
Figure 7: Optical fiber probe (left) and hyperspectral
fluorescence image (right).
In our preliminary experiments, the image is
formed by scanning the sample. In a more advanced
system, fiber bundles coupled with hyperspectral
CCD cameras will be employed.
The image obtained in this case consists of a 4D
hyperspectral cube. There are different ways of
investigating this hyperspectral cube. Figure 8
illustrates this. On the one hand we can select
different observation wavelength windows (top
right). It can be clearly seen that different pieces of
information appear depending on the observation
window. On the other hand, we can try to define
hypervolumes in the hyperspectral cube. These
hypervolumes can be specific to the possible
pathological nature of the tissue. This can be
achieved through the use of data processing
algorithms, such as the Kernel Principal Component
Analysis or the Support Vector Machine (Diaz-Ayil,
2007); (Adbat, 2012).
ReverseTranslationalResearch-HowClinicalTrialsonFluorescenceImagingforVocalCordCancerFuelsFundamental
Research
285
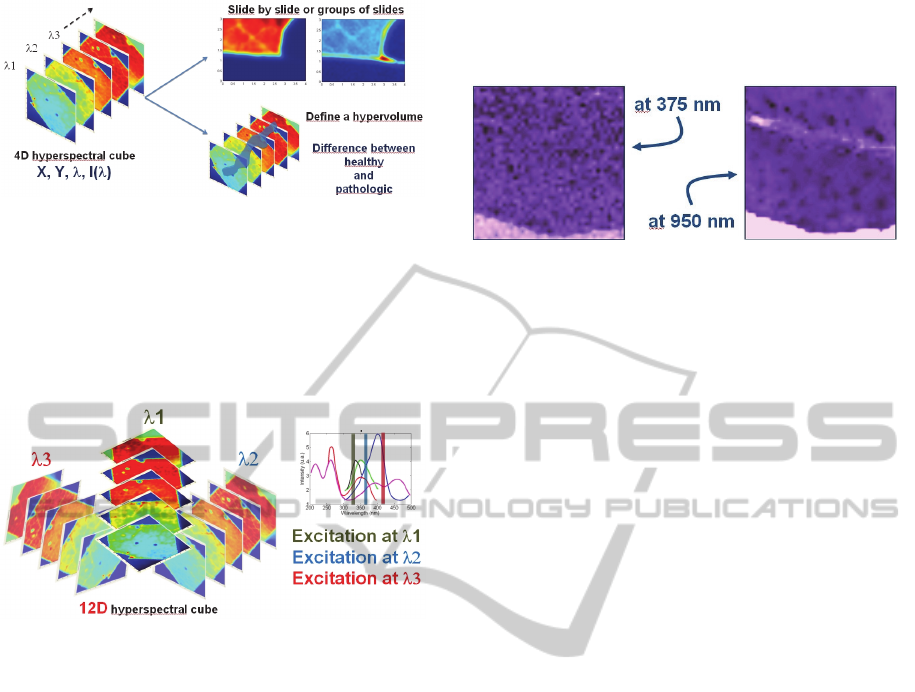
Figure 8: Different ways of exploiting hyperspectral
fluorescence.
This hyperspectral technique can be further
developed using images obtained with different
excitation wavelengths. For each supplementary
excitation wavelength, four dimensions are added to
the hyperspectral cube as depicted in figure 9.
Figure 9: Illustration of multiple excitations and
hyperspectral fluorescence imaging.
Note that fluorescence lifetime imaging could
also be included in this type of measurement.
4.2 Other Possibilities with Diffusion
This autofluorescence measurement can also be
coupled with an analysis of the diffuse reflectance of
the tissues, i.e. on the photon elastic scattering
process occurring in the biological sample. The
bimodality of the system obtained can then
significantly improve the sensitivity and specificity
of the diagnosis (Diaz-Ayil, 2007). Beyond sheer
data acquisition and direct analysis, further
information can be obtained from the clinical trials
by coupling and crossing the fluorescence and
scattering measurements, allowing us to retrieve the
intrinsic fluorescence characteristics of the sample
(Wu, 1993); (Muller, 2001).
Figure 10 illustrates the possibilities offered by
spectroscopic reflectance measurements. We can see
that different details can be observed depending on
the spectral observation window used to investigate
diffusion properties of tissues. Note that these
images were obtained with the same fiber probe as
the one used in the previous sub-section, thus
demonstrating that multimodality can be obtained
relatively easily.
Figure 10: Example of spectral analysis of tissue diffusion.
Now let us go back to the discussion of
translational research. In the previous section, we
have seen that translational research highlights
obstacles and questions that would not have arisen if
they had not been applied to real clinical situations.
In this section, we see that it is the role of reverse
translational research to go back to more
fundamental studies and to work at innovative
solutions in order to address these new questions.
5 CONCLUSIONS
In this position paper we look at optical fluorescence
imaging of the vocal folds as an example of bi-
directional translational research. As mentioned in
the introduction, translational research is sometimes
underestimated because it is thought that it does not
deepen fundamental knowledge.
However, users face specific difficulties due to
the variability of the biological systems under study.
Results obtained in translational research often
depend on this variability and new questions and
scientific obstacles arise when research is applied to
the real world. In order to address these new
challenges, reverse translational research is required.
Fundamental research is then fuelled by the results
of translational research and the latter should be
considered essential to fully understand the
biological system under study.
We focus herein on advanced fluorescence
techniques in order to illustrate bidirectional
translational research. The optical methods that are
currently used are in need of improvement. We thus
propose to develop a standalone device able to
assess the possible pathological nature of vocal
folds. Pre-clinical and clinical trials will then be
conducted in order to transform the expected
research results into new optical diagnosis tools.
To conclude, we believe that translational
research should not be underestimated because it
BIODEVICES2013-InternationalConferenceonBiomedicalElectronicsandDevices
286

fuels fundamental studies when new questions or
scientific obstacles have been highlighted by pre-
clinical or clinical trials. Through the example of
vocal cord fluorescence imaging, this position paper
illustrates that the concept of bi-directional
translational research should be applied to all work
aiming to develop new medical devices.
ACKNOWLEDGEMENTS
The studies presented in this paper are conducted in
the frame of the STREP project "µRALP" funded by
the European Commission's 7th Framework
Program. It addresses the FP7-ICT-2011-7 call for
cognitive and robotic systems.
REFERENCES
Adbat, F., Amouroux, M., et al., 2012, Hybrid feature
selection and SVM-based classification for mouse skin
precancerous stages diagnosis from bimodal
spectroscopy, Opt. Express, Vol. 20, pp. 228-244.
Arens, C., Reussner, D., et al., 2007, Indirect fluorescence
laryngoscopy in the diagnosis of precancerous and
cancerous laryngeal lesions, Eur. Arch. Oto-Rhino-L.,
Vol. 264, pp. 621–626.
Diaz-Ayil, G., Amouroux, M., et al., 2007, Bimodal
spectroscopic evaluation of ultra violet-irradiated
mouse skin inflammatory and precancerous stages:
instrumentation, spectral feature extraction/selection
and classification (k-NN, LDA and SVM), Eur. Phys.
J-Appl. Phys. Vol. 47, pp. 12707.
Gillenwater, A., Jacob, R., et al., 1998, Noninvasive
Diagnosis of Oral Neoplasia Based on Fluorescence
Spectroscopy and Native Tissue Autofluorescence,
Arch. Otolaryngol., Vol.124, pp. 1251-1258.
Kraft, M., Betz, C. S., et al., 2010, Value of fluorescence
endoscopy for the early diagnosis of laryngeal cancer
and its precursor lesions, Head Neck, Vol. 33
pp. 941-948.
Lane, P.M., Gilhuly, T., et al., 2006, Simple device for the
direct visualization of oral-cavity tissue fluorescence,
J. Biomed. Opt., Vol 11, pp. 024006-1 – 024006-7.
Mehrotra, R., Singh, M., et al., 2010, A cross-sectional
study evaluating chemiluminescence and
autofluorescence in the detection of clinically
innocuous precancerous and cancerous oral lesions, J.
Am. Dent. Assoc., Vol. 141, pp. 151–156.
Muller, M. G., Georgakoudi, I., et al., 2001, Intrinsic
fluorescence spectroscopy in turbid media
disentangling effect of scattering and absorption, Appl.
Optics, Vol. 40, pp. 4633-4646.
Patton, L. L., Epstein, J. B., et al., 2008, Techniques for
oral cancer examination and lesion diagnosis: a
systematic review of the literature, J. of American
Dental Association JADA, Vol. 139, pp. 896-905.
Piazza, C., Bon, F. D., et al., 2011, Biologic endoscopy’:
optimization of upper aerodigestive tract cancer
evaluation, Otolaryng. Head Neck, Vol. 19, pp. 67–76.
Poh, C. F., Zhang, L. et al., 2006, Fluorescence
visualization detection of field alterations in tumor
margins of oral cancer patients, Clin. Cancer Res.,
Vol. 12, pp.6716-6722.
Rethman, M. P., Carpenter, W., et al., 2010, Evidence-
Based Clinical Recommendations Regarding
Screening for Oral Squamous Cell Carcinomas, J. of
American Dental Association JADA, Vol. 141, pp.
509-520.
Richards-Kortum, R; Sevick-Muraca, E., 1996,
Quantitative optical spectroscopy for tissue diagnosis,
Annu. Rev. Phys. Chem. Vol. 47, pp. 555–606.
Shin, D., Vigneswaren, N. et al., 2010, Advances in
fluorescence imaging techniques to detect oral cancer
and its precursors, Future Oncol., Vol. 6, pp. 1143–
1154.
Wagnieres, G. A., Star, W. M., et al., 1998, In vivo
fluorescence spectroscopy and imaging for
oncological applications, Photochem. Photobiol.,
Vol.68, pp.603–632.
Wu, J., Feld, M. S., et al., 1993, Analytical model for
extracting intrinsic fluorescence in turbid media, Appl.
Optics, Vol. 32, pp. 3585-3595.
ReverseTranslationalResearch-HowClinicalTrialsonFluorescenceImagingforVocalCordCancerFuelsFundamental
Research
287
