
Screen-printed Biochemical Sensors for Detection of Ammonia Levels
in Sweat – Towards Integration with Vital Parameter Monitoring
Sports Gear
Susanne Oertel
1
, Michael P. M. Jank
1
, Lothar Frey
1
, Christian Hofmann
2
,
Nadine
Lang
2
and Matthias Struck
2
1
Fraunhofer Institute for Integrated Systems and Device Technology IISB, Schottkystr. 10, 91058 Erlangen, Germany
2
Fraunhofer Institute for Integrated Circuits IIS, Am Wolfsmantel 33, 91058 Erlangen, Germany
Keywords: Screen-printed, Biosensor, Biomedical Sensor, Sweat, Ammonia, Exercise, Ion-selective Electrode,
Wearable Device.
Abstract: The fabrication of fully screen-printed biochemical sensors employing planar integrated solid state
electrodes is described. The sensors are developed to fit wearable devices and target the monitoring of
ammonia respectively ammonium levels in sweat. Increased ammonium levels in sweat correlate to physical
overstrain of muscles, indicated by the breakdown of proteins in muscle cells. The sensor on flexible foil
uses an ion-selective working electrode and a reference electrode for potentiometric measurements of the
electromotoric force, EMF. For the ammonium ion-selective electrode a cocktail of nonactin was deposited.
The printed sensors were calibrated with ammonium standard solutions at a working range between 10
-5
M
to 0.1 M which corresponds to the range of physiological levels of ammonium in sweat before and during
physical strain. The potentiometric characterization of the ion-selective sensor shows a linear behaviour of
the EMF versus pC values with a Nernstian slope of 59.3 mV ± 11.2 mV. The combination of low-cost
printed sensors, potentiometric sensing, and the integration with textiles represents a very attractive
approach for non-invasive monitoring of individual sports performance to prevent overload during physical
training.
1 INTRODUCTION
The monitoring of fitness status for identification of
the ideal workout conditions or the prevention of
muscular overstress is usually performed by logging
vital data during physical exercise and sequential
externally analysis by medical labs.
Direct analysis of body metabolism during
physical strain can be performed in sports medicine
laboratories using body fluids, e.g. blood and, more
and more common, also sweat. Generally, blood is
used to analyze the workout condition in
combination with the registration of vital parameters
like heart rate and respiratory gases. Analysis of
blood withdrawals during physical strain are
invasive, very much time-consuming, and costly and
can only be achieved in cooperation with a sports
medicine laboratory
Mobile fitness and health related data acquisition
becomes more and more common and is the first
success story for a range of wearable technologies.
Motion trackers that count the steps and estimate the
energy/calories consumption of the user have been
in the market for years. Vital parameters such as
respiration effort as well as heart rate and heart rate
variabilities derived from the electrocardiogram
(ECG), deliver more reliable information sources for
assessing training success and cardiovascular health.
Thus those systems are under research for
integration in future wearable systems (Tantinger et
al., 2012; Tobola et al., 2015). An even more
detailed picture of health and fitness status can be
derived with simultaneous monitoring of metabolic
parameters. Non-invasive approaches, e.g. detection
of relevant electrolyte concentrations are preferred
against blood analysis. The appropriate sensing units
along with challenging textile sensor adaptation and
biosignal processing methods that can cope with
motion artefacts have to be developed.
Generally, the body fluid sweat is only used for
medical analysis for diseases or for analysis for drug
160
Oertel, S., Jank, M., Frey, L., Hofmann, C., Lang, N. and Struck, M.
Screen-printed Biochemical Sensors for Detection of Ammonia Levels in Sweat – Towards Integration with Vital Parameter Monitoring Sports Gear.
DOI: 10.5220/0005691501600165
In Proceedings of the 9th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2016) - Volume 1: BIODEVICES, pages 160-165
ISBN: 978-989-758-170-0
Copyright
c
2016 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

levels inside the body (Heikenfeld, 2014).
Sweat is an electrolyte solution that mostly
consists of water with sodium, potassium, and
bicarbonate. Additional inorganic compounds such
as urea, pyruvate, lactate, as well as proteins,
peptides, amines, amino acids, and ammonia are
contained in sweat. Furthermore, metal ions,
inhibitors, antigens, antibodies, and a variety of
xenobiotics such as drugs, cosmetics, and ethanol
have been found in sweat (Sato et al., 1989; Mena-
Bravo and Luque de Castro 2014).
Next to these physical species, ammonia content
in sweat is known for some decades to be a key
parameter for sports performance diagnostics
(Ament et al., 1997). The correlation of muscular
overstrain to production of ammonia is known since
last century. Ammonia and ammonium in blood are
mostly a result of metabolic degradation of proteins
(Schulz and Heck, 2001).
Czarnowski et al., (1992) published the
mechanism of the ammonia transport from muscle
cells into sweat. As ammonia generally is a
cytotoxin, the human body tends to minimize the
ammonia content (Czarnowski et al., 1992). The
difference between the pH value of sweat (4.0-6.8)
and blood (7.35-7.45) forms a pH gradient.
Ammonia diffuses from a higher to a lower pH
value, i.e. from blood to sweat. The size of ammonia
molecules is similar to water molecules. As such
they are permeable through cell membrane.
Some 20 years ago, investigations verified the
mechanism published by Czarnowski et al., (1992)
and therefore the correlation between physical strain
and sweat ammonia. Ament et al., (1997) published
the evaluation of ammonia in blood and sweat in
correlation to physical strain. This study was
performed utilizing an incremental cycle ergometer
exercise. During physical strain the content of
ammonia in sweat is in the range of millimol
whereas it is only in the µmol range in blood at the
same time.
Further publications from Alvear-Ordenes et al.,
(2005) and Meyer et al., (2007) show a direct
correlation between muscle overload of rugby
players during physical workout and also a gender
effect of ammonia content in sweat. All ammonia
diagnostics were done by analyzing sweat collected
with gauze pads (Alvear-Ordenes et al., 2005; Meyer
et al., 2007). One of the major challenges in
monitoring ion concentrations in sweat is the sample
recording. Extraction from human skin is time-
consuming, complex, and subject to errors, e.g. in
analyte concentration due to evaporation of solvent
or contamination of the sweat sample. This explains
also the low number of publications on sports related
sweat diagnostics.
For these reasons, direct monitoring of sweat
composition on the skin before and during physical
strain is a very attractive alternative. Bandodkar et
al. (2013), Wang et al., (2010), and Guinovart et al.,
(2013) developed skin applicable microfluidic
devices for sweat extraction and integrated pH
monitoring as well as flexible screen-printed
electrodes for application to skin-wearable devices.
Rose et al., (2013) published a sodium-selective
sensor in combination with a RFID chip.
Ion-selective electrodes are the most
fundamental features of sensors for analysis of
activity of ions in physiological fluids. In the last
century they mostly were electrochemical glass
electrodes an automated apparatus (Schulz and
Heck, 2001; Czarnowski et al., 1992; Ament et al.,
1997; Alvear-Ordenes et al., 2005; Meyer et al.,
2007).
Koncki et al., (1999) and Tymecki et al., (2006)
published screen-printed and planar electrodes
designed from printed silver, carbon and insulating
inks on flexible substrates. The potentiometric
measurements were taken against commercially
acquired reference electrodes.
In the last years, several potentiometric sweat
electrolyte measurements were published using
planar ion-selective sensors directly on skin (Yang et
al., 2010; Guinovart et al., 2013; Rose et al., 2014).
Koncki et al., (1999); Tymecki et al., (2006) and
Guinovart et al. (2013) developed ammonium-
selective electrodes by means of screen printing on
flexible foils.
Guinovart et al. (2013) developed a
potentiometric tattoo style ammonium sensor
including the reference electrode that can be directly
stuck to the skin.
Our present work combines the previously
reported sensor designs of Koncki et al., (1999) and
Guinovart et al., (2013). A flexible screen-printed
sensor is prepared combining an ammonium-
selective electrode based on nonactin ionophore with
an integrated reference electrode. Screen-printing
technology enables the deposition of thick layers,
short fabrication time, and precise sensor patterns
with a resolution of few hundreds of microns leading
to the fabrication of cheap and disposable
electrochemical sensors. The ion-selective
biomedical sensor published in this paper shows a
promising approach. The fully-printed biomedical
sensor shows a sensitivity or Nernstian slope of over
2 orders of magnitude (0.001 M to 0.1 M).
Furthermore, this work presents an outlook towards
Screen-printed Biochemical Sensors for Detection of Ammonia Levels in Sweat – Towards Integration with Vital Parameter Monitoring
Sports Gear
161

the sensor integration in textiles to extent a
functional sport shirt with low-power appliance for
various electrolyte monitoring tasks (Tantinger et
al., 2012; Tobola et al., 2015).
2 EXPERIMENTAL
2.1 Materials and Reagents
Silver-based (125-13), silver-silver chloride with a
ratio of 65:35 (125-21) and carbon filled (120-24)
pastes for screen printing were acquired from
Creative Materials (Ayer, MA, USA).
The pastes were printed on flexible polyester
(Hewlett Packard ink jet foil, 125µm) and polyimide
(Kapton HN, 125µm, Müller GmbH) foils.
Analytical grade salts of ammonium chloride and
sodium chloride were purchased from Bernd Kraft
for standard calibration solutions.
Sodium chloride (NaCl, ≥ 99.5%, BioXtra),
methanol 99.8% anhydrous) and polyvinyl butyral
(PVB, Butvar® B-98) were purchased from Sigma
Aldrich and used for an insulating layer on top of the
reference electrode (Guinovart et al., 2013).
Ammonium ionophore (nonactin in Cocktail A,
Fluka) was obtained from Sigma Aldrich for
fabrication of the ion-selective electrode. Cocktail A
consisted of Nonactin (6.9 wt%), 2-Nitrophenyl
octyl ether (92.40 wt% NPOE) as plasticizer and
potassium tetrakis(4-chlorophenyl)borate (0.7 wt%
KTClPB).
For the insulating layer, a Barium titanate (PE-
BT 101) paste from Conductive Compounds was
used. All screen printing pastes offered annealing
temperatures below 200°C. This is essential for
sensor application on flexible foil substrates.
2.2 Equipment
An automated screen- and pattern printer from Ekra
(series X1) was utilized to produce the printed
electrodes. Polyester screen (110 µm mesh - 34 µm
wire thickness x 22.5° cover angle, 10 µm -15 µm
emulsion over mesh, EOM) was used for silver-
silver chloride paste and a stainless steel screen (VA
270-0.036x22.5°, 5-10µm EOM) for silver- and
carbon-filled paste.
The printed layers on foils were annealed at
150°C for different times (5 minutes up to
15 minutes) on a hot plate (PZ 28-2 EZ, Harry
Gestigkeit). For potentiometric measurements a
2636B Sourcemeter from Keithley instruments
(Cleveland, OH, USA) was used.
2.3 Screen-printing for Sensor
Fabrication
The sensor design was adopted from two sensor
layouts published by Koncki et al., (1999) and
Guinovart et al., (2013). The schematic and a picture
of the screen-printed sensor electrodes are shown in
Figure 1. The layout of the sensor involves several
steps and consists of a bielectrode system that
combines silver working electrodes with silver-silver
chloride electrodes as the reference electrode.
At first the conductive layer with silver-based
paste (84% silver) was printed and afterwards the
silver-silver chloride electrode. Koncki et al., (1999)
proposed an additional carbon layer as a chemically
inert layer between the ion-selective layer and the
silver electrode. Guided by the same considerations
we choose to realize the reference electrode both by
a carbon/silver-based system as well as a pure silver-
silver chloride electrode.
Finally, printing the insulating paste with contact
openings for electrical contacts and opening at the
active area with ion-selective membrane was done as
fourth and last screen printing step.
The squeegee speeds during screen-printing of
pastes were between 50 mm/s and 80 mm/s. After
each printing step the flexible foil substrates were
annealed at 150°C on a hotplate with annealing
times between 5 minutes and 15 minutes. At the end
of the printing process, the sensors were cut for
further individual use.
For preparation of the measurements the
reference electrode was covered with a mixture of
PVB, methanol, and NaCl as was published by
Guinovart et al., (2013). This process was done by
dispensing the liquid by hand and letting the layer
dry for a minimum of 12 hours. The final
preparation step was the insertion of the ion-
selective membrane. The cocktail of ammonium
ionophore was drop-cast onto the inner circle
(insulator opening, layer 4 in Figure 1a) with
amounts of 20 µl and also dried overnight. The
finished ammonium-selective biomedical sensor is
shown in Figure 1b.
The additional silver electrodes shown left and
right next to the sensor in Figure 1a can be used for
conductivity measurements. The line gap was 100
µm (right) and 200 µm (left). The first generation of
screen-printed ammonium-selective sensors had a
dimension of 20 mm in width and 40 mm in length.
The resolution pattern of the ink allows for shrinking
the sensor size in future layouts.
BIODEVICES 2016 - 9th International Conference on Biomedical Electronics and Devices
162

(a)
(b)
Figure 1: (a) Schematic and (b) photograph of screen-
printed ammonium selective potentiometric sensor (1)
silver-silver chloride reference electrode, (2) silver
electrodes, (3) carbon-filled electrode, (4) insulating layer
and (5) ion-selective area with ammonium ionophore.
2.4 Measurements
Calibration curves were obtained by standard
solutions with different activities of the analyst ion.
Potentiometric measurements were recorded using
the high-impedance voltage measurement mode of
the 2636B sourcemeter that delivers 10
14
ohms of
internal resistance, i.e. the measurement is current-
less minimizing the feedback on the built-up
potential difference at the electrodes. Data points
were acquired with time intervals of 200
milliseconds (ms) or 500 ms respectively in a
continuous data collection cycles of up to 1 hour in
duration. The measurement schematic is depicted in
Figure 2. The connection between flexible sensor
and measurement equipment was realized via a
flexible flat cable (FFC) connector with a 0.1 in.
pitch.
Figure 2: Schematic of cabling of potentiometric ion-
sensitive sensor for measurement ammonium levels.
The ammonium ionophore, Nonactin, extracts its
preferred cation, NH
4
+
, out of the solution into the
membrane. The potential difference measured at the
device inputs is the sum of the potentials built-up at
all interfaces: solid-solid, solid-liquid and liquid-
liquid. In ideal case, all potentials except the
potential at the ionophore membrane can be
considered to be constant. The electromotive force,
EMF is forming across the membrane when both
reference and working electrodes are in contact with
the solution. This potential over the ammonium-
selective membrane depends on the NH
4
+
ion
activity (Spichiger, 1998).
Potassium, K
+
, is a critical interfered ion due to
the same ionic diameter as of ammonium. The
sensor selectivity will be tested in further work.
3 RESULTS AND DISCUSSION
3.1 Characterization of the Sensor
The potentiometric measurements of the
ammonium-selective sensor were conducted by
recording the voltage response upon modification of
activity of the ammonium by exchanging standard
solutions of concentrations from 0.1 mol/l (pC=1) up
to 0.0001 mol/l (pC=4). The negative of the
logarithm of base 10 of the ion activity (a
NH4+
) is pC.
A calibration of the sensor was realized by recording
the potential deviation (electromotive force, EMF)
versus the time and changing the activity of
ammonium ions by adding drops of solutions on top
of the sensor electrodes. After a delay time of
5 minutes, the first standard solution with pC=4 was
added and measured for further 5 minutes.
Afterwards the sensor was purged with deionized
water and dried using nitrogen flow. This procedure
was repeated for all standard solutions from pC=4
up to pC=1.
The range of the calibration concentrations from
0.00001 mol/l up to 0.1 mol/l covers the typical
ammonium level in sweat with and without physical
strain (Czarnowski et al., 1992; Guinovart et al.,
2013). The curve progression of lower
concentrations (pC values of 5 to 3 in Figure 3) is
nonlinear. Between pC values of 3 and 1 the sensor
shows a linear calibration function, that correlates
with published calibration graphs of other groups
(Koncki et al., 1999). The Nernstian slope in the
linear range from pC values of 3 (0.001 M) to 1
(0.1 M) is 59.3 mV/pC ± 11.2 mV/pC.
Screen-printed Biochemical Sensors for Detection of Ammonia Levels in Sweat – Towards Integration with Vital Parameter Monitoring
Sports Gear
163
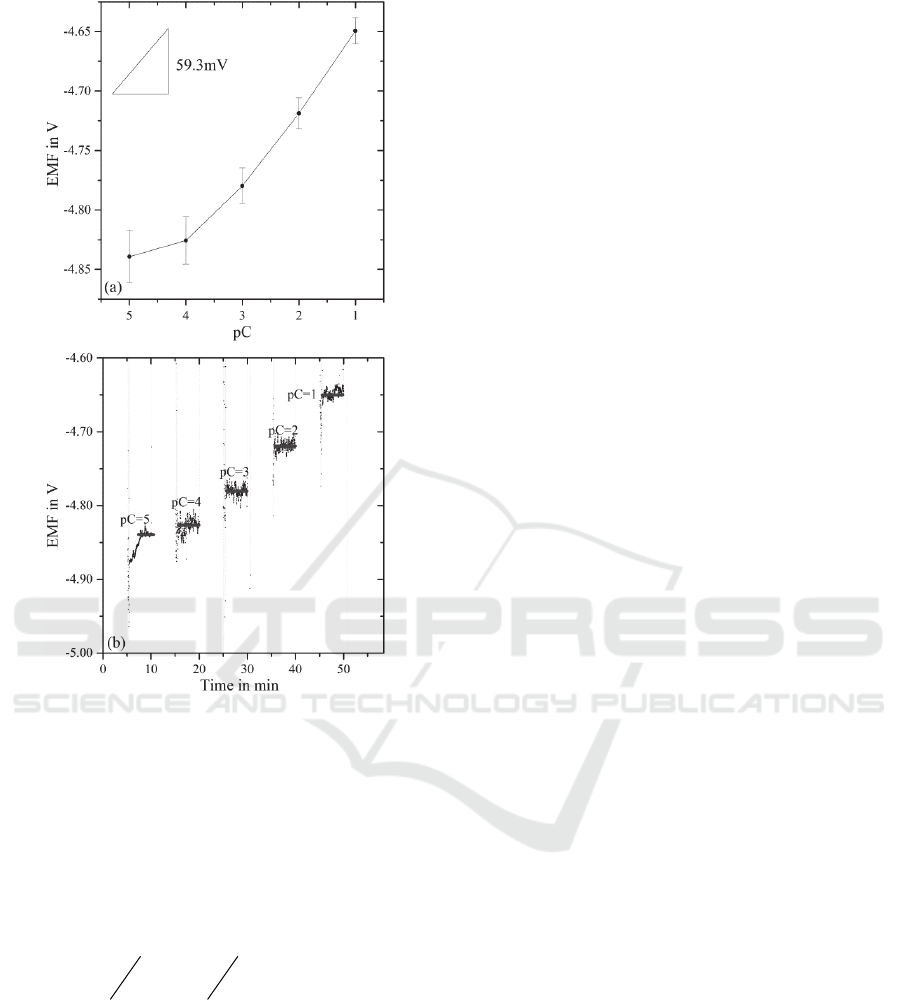
Figure 3: (a) Calibration graph and (b) potentiometric
measurement (potential deviation, EMF in mV vs. changes
of ion activity, -log(a
NH4+)
) of screen-printed ammonium-
selective biosensor with integrated screen-printed
reference electrode.
The slope of an ideal electrode for the
selectively-measured ion is given by the Nernstian
equation:
(1)
(2)
with E
0
being the reference potential, s being the
Nernstian slope, a
i
the activity of the ion i, R the
universal gas constant, T the temperature, F the
Faraday constant, and z
i
the valency of the ion i.
The performance of the sensor was achieved
after considerable efford towards optimization of the
integrity of the ion-selective membrane on the
working electrode. Frequent defects include
delamination, cracks or insufficient drying behavior
and lead to dramatically changed sensing
characteristics. Guinovart et al., (2013) fabricated an
ammonium-selective sensor in a style of a skin
tattoo with integrated PVB-based reference
electrode. The range of the calibration
concentrations from 0.00001 mol/l up to 0.1 mol/l is
also used and the calculated Nernstian slope was
59.2 mV. Our approach of integrating the nonactin
ionophor in a polymeric matrix lowers dramatically
the consumption of the ionophor which is by far the
most expensive ingredient of the sensor system.
However we could show that the performance is
kept at the same level.
In comparison to this work and the sensors
published by Guinovart et al., (2013), Koncki et al.
(1999) fabricated a screen-printed sensor which is
measured against an external calomel reference
electrode. The Nernstian slope of 53mV/pC was
calculated in a range of the calibration
concentrations from 0.00001 mol/l up to 0.01 mol/l.
However the non-planar approach is less flexible
with respect to sensor processing and system
integration.
Our fastly and cheaply fabricated ammonium-
selective screen-printed sensors show a high
potential for analysis of physiological electrolytes as
sweat in sports applications. In further work, the
layout of the sensor will be shrinked. The resolution
of the screen-printed silver pastes was better than
200 µm. The size minimization will on the one hand
increase the amount of sensors yielded by one
fabrication cycle as well as the spatial options for
integration with functional sport textiles and will
also reduce the materials and fabrication costs per
sensor.
3.2 Integration with LokVitalTag
The printed sensor circuit will be attached to a
multifunctional electronics box. The so called
LokVitalTag combines various electronic modules
that acquire data from the physiological sensor front
ends (ECG-electrodes, respiration-band, ammonia-
sensitive sensor front-end) and also tracks
movement information from an inertial sensor. The
gathered data will be processed and stored locally.
Further on, the calculated values can be transmitted
wirelessly. In addition the LokVitalTag carries
electronics for the real-time-localization technology
RedFIR 2.0, so localization information and vital
parameters can be analysed in combination.
Under real conditions, in sweat, also interfering
ions interact with the ionophore membrane. The
effect on the overall potential can be related to the
concentration of the analyte by applying the
modified Nernstian equation, the so-called Nicolsky
i
asEE log
0
C25atmV
Fz
59.16
Fz
RT
2.303s
ii
BIODEVICES 2016 - 9th International Conference on Biomedical Electronics and Devices
164

equation. In further work, the selectivity factor will
be calculated by this evaluation. The experimental
selectivity coefficients in sweat are dependent on the
method of determination, e.g. separate solution
(SSM) or fixed interference method (FIM).
4 CONCLUSIONS
A facile route to the fabrication of planar, solid-
state, ion-selective sensors for ammonium ions using
screen-printing technology is presented. The
performance of the fully-printed ion-selective sensor
allows for the detection of ammonium ion
concentration in the physiological levels of human
sweat.
The results shown in this paper give the base for
further research into especially the potentiometric
analysis, the optimization of the sensor layout and
area consumption, and the integration with textiles
for wearable functional sport clothing.
ACKNOWLEDGEMENTS
This contribution was supported by the Bavarian
Ministry of Economic Affairs and Media, Energy
and Technology as a part of the Bavarian project
”Leistungszentrum Elektroniksysteme (LZE)”.
REFERENCES
Heikenfeld, J. 2014. Your sweat may bring medical
diagnostics to Fitbits and Fuelbands. IEEE Spectrum,
Nov. issue, 47-63.
Sato, K., Kang, W. H., Saga, K., Sato, K. T. 1989. Biology
of sweat glands and their disorders.I. Normal sweat
gland function, J. Am. Acad. Dermatol., 20(4), 537–
563.
Mena-Bravo, A., Luque de Castro, M. D. 2014. Sweat: A
sample with limited present applications and
promising future in metabolomics, J. Pharm. Biomed.
Anal. 90, 139– 147.
Schulz, H. & Heck, H. 2001. Ammoniak in der
Leistungsdiagnostik. Deutsche Zeitschrift für
Sportmedizin, 52(3), 107-108.
Czarnowski, D., Gorski, J., Jozwiuk, J. and Boron-
Kaczmarska, A. 1992. Plasma ammonia is the
principal source of ammonia in sweat. Eur. J. Appl.
Physiol., 65, 135-137.
Ament, W., Huizengau, J. R., Mook, G. A., Gips, C. H.,
Verkerke, G. J. 1997. Lactate and ammonia
concentration in blood and sweat during incremental
cycle ergometer exercise. Int. J. Sports Med., 18, 35-
39.
Alvear-Ordenes, I., Garcia-Lopez, D., De Paz, J.,
Gonzalez-Gallego, J. 2005. Sweat lactate, ammonia,
and urea in rugby players. Int. J. Sports Med., 26, 632-
637.
Meyer, F., Laitano, O., Bar-Or, O., Mc Dougall, D.,
Heigenhauser, G. J. F. 2007. Effect of age and gender
on sweat lactate and ammonia concentrations during
exercise in the heat. Braz. J. Med. Biol. Res., 40(1),
135-143.
Bandodkar,A. J., Hung, V. W. S., Jia, W., Valdés-
Ramírez, G., Windmiller, J. R., Martinez, A. G.,
Ramírez, J., Chan, G., Kerman, K. and Wang, J. 2013.
Tattoo-based potentiometric ion-selective sensors for
epidermal pH monitoring. Analyst, 138, 123-128 .
Yang, Y.-L., Chuang, M.-C., Lou, S.-L., Wang, J. (2010).
Thick-film textile-based amperometric sensors and
biosensors. Analyst, 135, 1230-1234 .
Guinovart, T., Bandokar, A. J., Windmiller, J. R.,
Andrade, F. J., Wang, J. 2013. A potentiometric tattoo
sensor for monitoring ammonium in sweat. Analyst,
138, 7031-7038.
Rose, D.P., Rattermann, M., Griffin, D. K., Hou., L.,
Kelley-Loughnane, N., Naik, R. R., Hagen, J. A.,
Papausky, I., Heikenfeld, J. 2014. Adhesive RFID
Sensor Patch for Monitoring of sweat electrolytes.
IEEE Transc. Biomed. Eng., 0018-9294, 1-9.
Koncki, R., Glab, S., Dziwulska, J., Palchetti, I., Mascini,
M. 1999. Disposable strip potentiometric electrodes
with solvent-polymeric ion-selective membranes
fabricated using screen-printing technology. Anal.
Chim. Acta, 385, 451-549.
Tymecki, L., Glab, S., Koncki, R. 2006. Miniaturized,
planar ion-selective electrodes fabricated by means of
thick-film technology. Sensors, 6, 390-396.
Tantinger D, Feilner S, Schmitz D, Weigand C, Hofmann
C, Struck M. 2012. Evaluation of QRS detection
algorithm implemented for mobile applications based
on ECG data acquired from sensorized garments.
Biomed Eng Biomed Tech ,57(1), 635-368.
Tobola, A., Espig, C., Streit, F. J., Korpok, O., Schmitz,
B., Hofmann, C. 2015. Scalable ECG hardware and
algorithms for extended runtime of wearable sensors.
Medical Measurements and Applications (MeMeA),
IEEE International Symposium. 255–60. 209.
Spichiger-Keller, U. E., 1998. Chemical Sensors and
Biosensors for Medical and Biological Applications.
Wiley-VCH, Weinheim.
Screen-printed Biochemical Sensors for Detection of Ammonia Levels in Sweat – Towards Integration with Vital Parameter Monitoring
Sports Gear
165
