
Antioxidant Properties of Curcuma longa L. and Curcuma
xanthorriza Rhizomes
Dian Ratih Laksmitawati
1,* a
, Diah Kartika Pratami
1b
, Wahyu Widowati
2c
,
Hanna Sari Widya Kusuma
3d
, Cahyaning Riski Wijayanti
3
, Cintani Dewi Wahyuni
3
,
Ervi Afifah
3e
and Rizal Rizal
3,4 f
1
Faculty of Pharmacy,Pancasila University, Jl. Raya Lenteng Agung No.56-80, Srengseng Sawah, Jakarta 12640,
Indonesia
2
Faculty of Medicine, Maranatha Christian University, Jl. Surya Sumantri No. 65, Bandung 40164, West Java, Indonesia
3
Biomolecular and Biomedical Research Center, Aretha Medika Utama, Jl. Babakan Jeruk 2 No. 9, Bandung 40163, West
Java, Indonesia
4
Biomedical Engineering, Department of Electrical Engineering, Faculty of Engineering, Universitas Indonesia, Depok
16426, West Java, Indonesia
cahyaningwidodo@gmail.com, cintannidewi@amubbrc.co.id, ervi.afifah@gmail.com, rizal_biotek@yahoo.com
Keywords: Antioxidant, Curcuma Longa L. , Curcuma Xanthorriza, Free Radical, Oxidative Stress.
Abstract: Oxidative stress can lead to tissue damage and result in disease or aggravate existing disease. Antioxidants
are required to protect cells from free radical damage. Temulawak (Curcuma xanthorriza L.) and turmeric
(Curcuma longa L.) are natural ingredients with polyphenol compound. Polyphenols has antioxidants that
can neutralize free radicals by donating an electron or hydrogen atom. This study was aimed to determine the
antioxidant properties of temulawak extract (TLE) and turmeric extract (TE). The antioxidant activity were
determined using total phenolic content (TPC), total flavonoid content (TFC), 2,2 diphenyl 1 picrylhydrazyl
(DPPH), 2,2′-Azinobis(3-Ethylbenzthiazoline-6-Sulfonate) (ABTS), hydrogen peroxide (H
2
O
2
), NO
(Nitrogen Oxide) scavenging and ferric reducing antioxidant power (FRAP). The result showed that the TPC
of value was 10.93 µg GAE/mg extract, and the TFC value was 5.67 µg QE/mg extract. Meanwhile, TPC
and TFC value of TLE were 4.83 and 2.68 µg GAE/mg, respectively. The IC
50
value of DPPH, ABTS, H
2
O
2
,
NO scavenging activity and FRAP activity of TE were 300.7; 39.19; 86.83; 88.03 µg/mL and 493.75 μm Fe
(ii)/μg respectively compared to TLE 197.5; 82.55; 205.94; 164.25 µg/mL and 451.00 μm Fe (ii)/μg
respectively. Turmeric has higher antioxidant properties than temulawak, both turmeric and temulawak are
potential natural antioxidants.
1 INTRODUCTION
Free radicals are a highly unstable substance. Free
radicals are generated in the body due to metabolic
processes or environmental factors like industrial
chemical exposure, X-ray exposure, smoking, ozone,
and air pollution (Lobo et al., 2010). If free radicals
are present in the human body, they can bind with
a
https://orcid.org/0000-0001-8484-0290
b
https://orcid.org/0000-0003-1052-5946
c
https://orcid.org/0000-0002-5401-7794
d
https://orcid.org/0000-0002-7422-0036
e
https://orcid.org/0000-0003-4205-2434
f
https://orcid.org/0000-0003-2783-0672
other molecules to become stable, allowing these
molecules to become free radicals (Phaniendra et al.,
2015). As a result of this chain reaction, cells, tissues,
and organs are damaged. Antioxidants can donate
electrons to free radicals, causing oxidative stress
through free radical chain reactions. Lipid
peroxidation is caused by free radicals, which
destroys liver cells. Antioxidants can minimize cell
104
Laksmitawati, D., Pratami, D., Widowati, W., Kusuma, H., Wijayanti, C., Wahyuni, C., Afifah, E. and Rizal, R.
Antioxidant Properties of Curcuma longa L. and Curcuma xanthorriza Rhizomes.
DOI: 10.5220/0010745300003113
In Proceedings of the 1st International Conference on Emerging Issues in Technology, Engineering and Science (ICE-TES 2021), pages 104-111
ISBN: 978-989-758-601-9
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

damage caused by the oxidative process, making
them hepatoprotective (Lobo et al., 2010). However,
synthetic antioxidants such as Butylated
hydroxyanisole (BHA), butylated hydroxytoluene
(BHT), propyl gallate (PG), and tert-butyl
hydroquinone (TBHQ) can give some side effects
such as skin allergies, gastrointestinal tract, and even
cancer (Caleja et al., 2017; Lourenço et al., 2019;
Wang & Kannan, 2019).
In Asia, the Zingiberaceae family is the most
commonly grown crop. This plant is beneficial to
human health as a source of food, spices, dyes, food
colouring, and herbal medicine. Some of the
Zingiberaceae family are turmeric (Curcuma longa
L.) and temulawak (Curcuma xanthorriza L.).
Turmeric and temulawak are both available and can
be consumed as a beverage or used as a cooking spice.
Turmeric and temulawak have been shown in
previous studies to have various health benefits,
including anti-inflammatory, antibacterial,
antioxidant, and hepatoprotective properties
(Cavaleri, 2018; Lukitaningsih, 2020). Curcuminoid
compounds in turmeric and temulawak (curcumin,
demethoxycurcumin, bisdemethoxycurcumin) are the
main components that function as antioxidants.
This research has done as preliminary data to
prove turmeric extract (TE) dan temulawak extract
(TLE) as antioxidants potential and this research will
be continued to prove TE dan TLE with Good
Manufacturing Practice (GMP) as hepatoprotective
potential.
This study was aimed to determine the antioxidant
properties of TLE and TE using method of 2,2
diphenyl 1 picrylhydrazyl (DPPH), 2,2 ′ -
Azinobis(3-Ethylbenzthiazoline-6-Sulfonate)
(ABTS), hydrogen peroxide (H
2
O
2
), NO (Nitrogen
Oxide) scavenging activities and ferric reducing
antioxidant power (FRAP) potential.
2 METHODS (AND MATERIALS)
2.1 Samples
Temulawak and turmeric were extracted with 70%
ethanol solvent. The standardized extract powder of
turmeric and temulawak were produced based on
current Herbal Good Manufacturing Practices by
FAST Co. (Jakarta, Indonesia).
2.2 Total Phenolic Content
The total phenolic content (TPC) was determined
using method described by Prahastuti and Utami with
slight modification (Prahastuti et al., 2020; Utami et
al., 2018). A 0,015 mL standard gallic acid (Sigma
398225) solution in 6 concentration level (50.00 -
1.56 µg/mL) and sample of TE and TLE in
concentration of 2000; 1000; and 500 µg/mL were
added into well in 96-well plate, respectively. Then,
added 60 µl of Na
2
CO
3
7.5% (Merck A897992745)
and 75 µl Folin- Ciocalteu reagent 10% (Merck
1.090.010.500) into well. The mixed solution was
incubated at 50ºC for 10 minutes, then the absorbance
was measured in a wavelength of 760 nm using a
microplate reader (Multiskan Go Reader, Thermo
Fisher Scientific 1510). The phenolic content (TPC)
calculation was compared to the gallic acid linear
regression using equations 1.
y
= 0.0429x + 0.152 (1)
2.3 Total Flavonoid Content
The total flavonoid content (TFC) was performed
using an AlCl
3
colorimetric assay method described
by Prahastuti and Utami with slight modification
(Prahastuti et al., 2020; Utami et al., 2018). An
amount of 75 µL standard quercetin (Sigma Q4951)
solution in 7 concentration level (500.00 - 7.80
µg/mL) and TE and TLE in concentration of 2000 and
1000 µg/mL, were added into well respectively and
each well was mixed with 75 µl AlCl
3
2% (Merck
449598). Using microplate reader (Multiskan Go
Reader, Thermo Fisher Scientific 1510), the
absorbance was measured in 415 nm of wavelength.
The concentration of flavonoid content was
calculated from calibration linear regression equation
2.
y
= 0.0095x+0.037 (2)
2.4 DPPH Free Radical Scavenging
Assay
The antioxidant activity using DPPH free radical
scavenging assay was perfomed using method
described by Prahastuti and Widowati with slight
modification (Prahastuti et al., 2020; Widowati et al.,
2018). An aliquot of 0.05 mL of TE and TEE samples
solution was poured into well respectively, then 200
μL of DPPH solution (D9132, Sigma Aldrich,
Missouri, USA) was added to each well. The mixture
was incubated at the dark room temperature for 30
mins. The absorbance was measured at 517 nm by the
microplate reader (Multiskan GO Microplate
Spectrophotometer, Thermo Scientific,
Massachusetts, USA). The IC
50
of free radical
Antioxidant Properties of Curcuma longa L. and Curcuma xanthorriza Rhizomes
105

inhibition activity calculation was obtained from the
Equation 3 scavenging activity.
% scavenging =
× 100
(3)
Ac : negative control absrobance
As : sample absorbance
2.5 FRAP Assay
The antioxidant activity using FRAP assay was
perfomed using method described by Prahastuti and
Widowati with slight modification (Prahastuti et al.,
2020; Widowati et al., 2018). The FRAP reagent
(mixture of 10:1:1 of 300mM sodium acetate buffer,
pH 3.6; 10mM 2,4,6-tris(2-pyridyl)-1,3,5-triazine in
40mM HCl; 20mM FeCl
3
.6H
2
O), 142.5μL and 7.5μL
of TE and TLE samples respectively were mixed into
96 well plate then incubated at 37°C for 30 min. The
absorbance of the mixture was measured at 760 nm
by microplate reader (Multiskan GO Microplate
Spectrophotometer, Thermo Scientific,
Massachusetts, USA). FRAP analysis was measured
by comparing a linear regression equation of
FeSO
4
.7H
2
O standard solution.
2.6 ABTS Reducing Activity Assay
The antioxidant activity using ABTS assay was
perfomed using method described by Ginting,
Prahastuti and Widowati with slight modification
(Ginting et al., 2020; Prahastuti et al., 2020;
Widowati et al., 2018). The solution of ABTS was
made by reacting 14 mM 2,2'-Azino-bis (3-
ethylbenzothiazoline-6- sulphonic acid)(ABTS•+)
[Sigma Aldrich A1888-2G, USA] with 4.9 mM
potassium persulfate [Merck EM105091, USA] in 1:1
volume ratio, for 16 h at the dark room temperature.
Then, the mixture was diluted with 5.5 mM PBS (pH
7.4) until the solution’s absorbance was was 0.70 ±
0.02 at 745 nm. The 2 µL of samples were added into
microplate of 96 well, followed by 198 µL of ABTS
solution. The mixture was then incubated at 30º C for
6 min and measured by microplate reader (Multiskan
GO Microplate Spectrophotometer, Thermo
Scientific, Massachusetts, USA) at 745 nm. ABTS-
reducing activity was then used to measure the
median inhibitory concentration (IC50). The equation
of ABTS reducing activity was calculated with
equation 4.
% reducing activity =
× 100
(4)
Ac : control absorbance
As : sample absorbance
2.7 H
2
O
2
Scavenging Activity Assay
H
2
O
2
scavenging activity was performed using
phenanthroline method by Mukhopadhyay et al.
(2016) with slight modification (Mukhopadhyay et
al., 2016). The samples (TE and TEE) 60 μL was
added into plate of 96 well, respectively and followed
12 μL of 1mM Ferrous ammonium sulfate (215406,
Sigma Aldrich. Then, mixed with 3 μL of 5mM H
2
O
2
(1.08597.1000, Merck). The mixture were incubated
at dark room temperature for 5 min. Then, 75μL of
1mM 1,10-phenanthroline (131377, Sigma Aldrich)
was added to the mixture and incubated for 10 min at
the dark room temperature. The mixture absorbance
was measured by microplate reader at 510nm
wavelenght. The IC
50
scavenging activity of H
2
O
2
was calcuated by Equation 3.
2.8 No Scavenging Activity Assay
The antioxidant activity using NO assay was
perfomed using method described by Utami with
slight modification (Utami et al., 2018). An amount
of 10 μL of samples (TE and TLE, respectively) in
various concentrations were mixed with 40 μL 10
mM sodium nitroprusside (106541, Merck,
Germany) in phosphate buffered saline (PBS)
(1740576, Gibco, Canada). Then the mixture was
incubated at room temperature for 2 hours followed
by the addition 100μL of Griess reagent (1:1 of 1%
sulfanilamide [Merck 111799, Germany] in 2%
H
3
PO
4
[Merck 100573, Germany] and 0.1% N-(1-
napththyl) ethylenediamine dihydrochloride) [Sigma
222488, USA]). The formation of chromophore
absorbance due to diazotization of nitrite with
sulfanilamide and coupling of
Naphthylethylenediamine dihydrochloride was
measured by a microplate reader (Thermo Scientific
Multiscan GO) at a wavelength of 546 nm. The
scavenging activity of NO was calculated by
Equation 3.
3 RESULTS AND DISCUSSION
3.1 Total Phenolic Content and Total
Flavonoid Content of TE and TLE
Curcuma longa and C. xanthorrizha are a rhizomes
having a yellow or an orange color due to the
present of curcuminoids (Rafi et al., 2015). This
pigment belongs to the family of flavonoid and
flavonoid is one group of polyphenols. The precence
ICE-TES 2021 - International Conference on Emerging Issues in Technology, Engineering, and Science
106
of secondary metabolites phenolic and flavonoid in
the Curcuma longa and C. xanthorrhiza were further
used for the standardization of the extract by using
determination of phenolic and flavonoid content
(Ab Halim et al., 2012).
Total phenolic content and total flavonoid content
of the sample TE and TLC are as shown in Table 1.
Table 1: The Average of TPC and TFC of TE and TLE.
Sample
TPC
(µg/mg extract)
TFC
(µg/mg extract)
TE 3.90 ± 0.04 1.86 ± 0.36
TLE 5.92 ± 0.33 2.68 ± 0.46
Note: The data was given in mean+SD, n=3
TLE had a higher polyphenol content, namely
5.92 ± 0.33 µg / mg, compared to TE (3.90 ± 0.04 µg
/ mg). Synchronous results were found on flavonoid
levels. The level of flavonoids in TLE (2.68 ± 0.46 µg
/ mg) was higher than in TE (1.86 ± 0.36 µg / mg).
The aim of TPC was to bind phenolic compounds
to a blue complex formed by Folin Ciocalteu's
reagent. Using the gallic acid calibration curve, total
polyphenols were measured (Prahastuti et al., 2020) .
The analysis show that TPC of TLE is higher than TE.
The theory behind total flavonoid content is that
AlCl
3
forms acid-stable complexes with flavonoid’s
keto groups and either hydroxyl groups, while it binds
to curcuminoids through the β-diketon group (Indira
Priyadarsini, 2013). The analysis show that theTFC
of TE is less than TLE.
The quantity of polyphenol content of TE and
TLE depend on sources, method of extraction,
phytogeographic region, and time of the collection of
rhizome (Adaramola & Onigbinde, 2017; Akinola et
al., 2014).
3.2 The Antioxidant Capacity of TE
and TLE using DPPH Assay
The results of antioxidant capacity of using DPPH
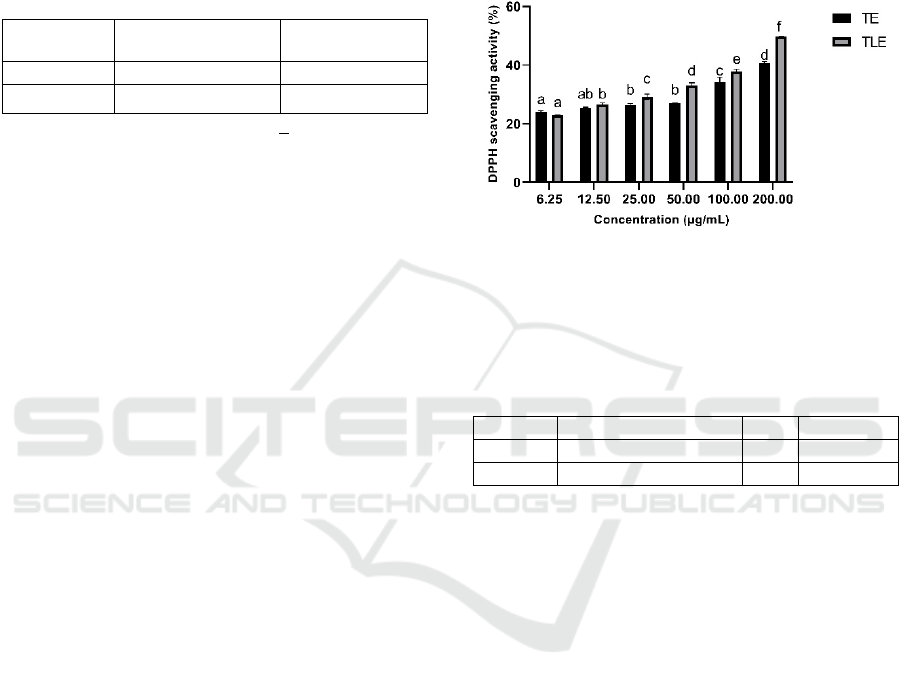
Assay are obtained as shown in Figure 1 and Table 2.
The color change purple to yellow that occurs when
free radicals interact with antioxidant compounds in
the extract, which is then measured using a
spectrophotometer, is the basic concept of this
process (Kedare & Singh, 2011). The reactive group
in DPPH (1,1-diphenyl-2-picrylhydrazyl) is a
nitrogen atom that forms a stable DPPH radical with
the antioxidant's hydrogen atom (1,1-diphenyl-2-
pikrihildrazil). The analysis that have been done show
that TE has less DPPH scavenging activity than TLE,
according to research findings. In the previous
research (Widowati et al., 2011), the lowest DPPH
scavenging activity was TE than TLE with the
scavenging capacity 8.33 µg/mL and 39.58 µg/mL
respectively. The turmeric extract was strongest
antioxidant than temulawak (Widowati et al., 2011).
The different of quantity of scavenging capacity of
TE and TLE in our previous research in 2011 with
this research due to the different method of extraction
and the addition lactose in powder extract.
*Data are presented as means ± standard deviation, differences
letter (a,ab,b,c,d) for TE and differences letter (a,b,c,d,e,f) for TLE
show significant differences among concentrations at P <0.05
(Tukey HSD post hoc test)
Figure 1: DPPH scavenging activity of TE and TLE.
Table 2: The IC
50
value of DPPH scavenging activity of TE
and TLE.
Sample Equation R
2
IC
50
(µM)
TE y = 0.0866x + 23.945 0.98 300.87
TLE y = 0.1274x + 24.838 0.97 197.50
A lower IC
50
correlate better with higher DPPH
radical scavenging activity, which represents the
concentration of the extract to decrease 50% of the
DPPH solution initial absorbance. Antioxidant
potency is usually associated with the content of
phenolic compounds due to their extensive
conjugated π-electron systems that facilitate the
donation of electrons from the hydroxyl moieties to
oxidizing radical species (Pratami et al., 2018).
3.3 The Antioxidant Capacity of TE
and TLE using FRAP Assay
The FRAP assay determines the test sample's
reducing potential by using antioxidants in the sample
as reductants in a redox reaction. Antioxidants break
the chain reaction of radicals by donating electrons or
hydrogen atoms to the ferric complex, which then
converts to the ferrous complex (Fe
3+
to Fe
2+
- TPTZ
complex) (Bolanos De La Torre et al., 2015). The
absorbance calculation can be linked to antioxidant
activity and shows the amoun of Fe
2+
that have been
decreased (Al-Salahi et al., 2018). An increase in
Antioxidant Properties of Curcuma longa L. and Curcuma xanthorriza Rhizomes
107

absorbance indicates a high reducing power. Based
on the research that have been done, the FRAP
activity of TE, TLE respectively 493.75; 451.00 μm
fe (II)/μg at the highest concentration (50 µg/mL)
(Figure 2).
*Data are presented as means ± standard deviation, differences
letter (a,b,c,d,e,f) for TE and differences letter (a,b,c,d,e,) for TLE
show significant differences among concentrations at P <0.05
(Tukey HSD post hoc test)
Figure 2: FRAP activity of TE and TLE.
The reducing power obtained for the TE is greter
than TLE. The reducing power capacity of the
samples is probably due to the phytochemical
compounds present in TE and TLE.
3.4 The Antioxidant Capacity of TE
and TLE using ABTS Assay
The research show that TE has better antioxidant
capacity with higher ABTS scavenging activity and
lower IC
50
compare to TLE (Figure 3 and Table 3).
*Data are presented as means ± standard deviation, differences
letter (a,ab,b,c,d,e,) for TE and differences letter (a,ab,b,c) for
TLE show significant differences among concentrations at P <0.05
(Tukey HSD post hoc test)
Figure 3: ABTS scavenging activity of TE and TLE.
Table 3: The IC
50
value of ABTS scavenging activity of TE
and TLE.
Sample Equation R
2
IC
50
(µM)
TE y = 1.2269x + 1.9177 0.99 39.19
TLE y = 0.4243x + 14.975 0.98 82.55
The ABTS assay determine the antioxidant
capacity of samples by donating hydrogen to cation
radical, then the solution become colorless (Pisoschi
& Negulescu, 2011).
3.5 The Antioxidant Capacity of TE
and TLE using H
2
O
2
Assay
The result of antioxidant capacity of TE and TLE
using H
2
O
2
assay was shown in Figure 4 and Table 4.
In H
2
O
2
assay, the reaction between ferrous
ammonium and phenantroline was inhibited by the
presence of H
2
O
2
. Thus, it can determine the
antioxidant capacity of the sample against H
2
O
2.
(Pisoschi & Negulescu, 2011). Turmeric has lower
IC
50
and higher ABTS scavenging activity. It means
that TE has higher antioxidant capacity than TLE.
*Data are presented as means ± standard deviation, differences
letter (a,b) for TE and differences letter (a,b) for TLE show
significant differences among concentrations at P <0.05 (Tukey
HSD post hoc test)
Figure 4: H
2
O
2
scavenging activity of TE and TLE.
Table 4: The IC
50
value of H
2
O
2
scavenging activity of TE
and TLE.
Sample Equation R
2
IC
50
(µM)
TE y = 0.3135x + 22.779 0.99 86.83
TLE y = 0.0806x + 33.401 0.99 205.94
In our previous research the H
2
O
2
scavenging
activity of TE was greater than and TLE extract, with
the value C. longa 55.82% than C. xanthorrhiza
49.04% (Widowati et al., 2011). The turmeric extract
was strongest antioxidant than temulawak by using
H
2
O
2
scavenging assay.
ICE-TES 2021 - International Conference on Emerging Issues in Technology, Engineering, and Science
108
3.6 The Antioxidant Capacity of TE
and TLE using NO Scavenging
Assay
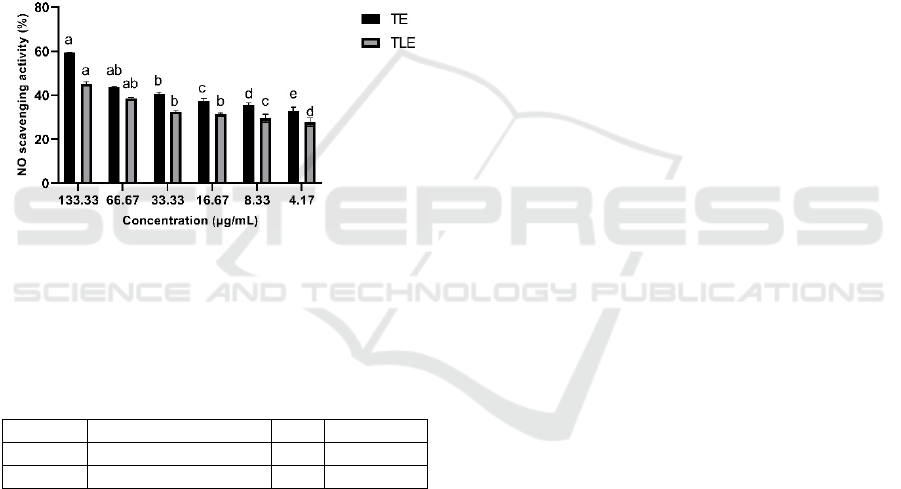
The result of antioxidant capacity of TE and TLE
using NO scavenging assay was shown in Figure 5
and Table 5. Determination of antioxidant by NO
scavenging assay have been done. Specific nitric
oxide synthases catalyze a biochemical reaction that
creates NO in biological tissues, which is the basis of
this assay. In buffered saline, sodium nitroprusside
reacts with oxygen to form nitrite ions, which can be
measured with Griess reagent (Alam et al., 2013).
The higher NO scavenging activity and lower IC
50
indicates that the sample has better antioxicant
activity. The result show that turmeric has higher
antioxidant activity than temulawak.
*Data are presented as means ± standard deviation, differences
letter (a,ab,b,c,d,e) for TE and differences letter (a,ab,b,c,d) for
TLE show significant differences among concentrations at P <0.05
(Tukey HSD post hoc test)
Figure 5: NO scavenging activity of TE and TLE.
Table 5: The IC
50
value of NO scavenging activity of TE
and TLE.
Samples Equation R2 IC
50
(µM)
TE y = 0.1912x + 33.169 0.98 88.03
TLE y = 0.1312x + 28.449 0.98 164.26
3.7 The Comparison of Antioxidant
Capacity of TE and TLE
Curcumin, from family Zingiberaceae, has an unique
conjugated structure that show a typical radical
trapping ability as a chain-breaking antioxidant,
including two methoxylated phenols and an enol form
of β-diketon (Nurrochmad, 2004). The antioxidant
activity of TE and TLE are due to curcuminoids
(Widowati et al., 2011). Curcuminoid is a phenolic
group, has benzene ring. Thus, it can function as free
radical scavengers. A phenolic antioxidant has a
distinct hydroxyl group (–OH) attached to its
composition's benzene loop (Asouri et al., 2013).
When reactive oxygen species (ROS) are present at a
specific concentration, they affect the function of
electron-releasing substituents contained as
substituents on the phenyl ring in phenolic
antioxidants. The O–H bond is broken as a result, and
the hydrogen ion is released. This hydrogen ion was
made accessible to nucleophilic free radicals,
quenching their reactive tendencies in the process
(Malik & Mukherjee, 2014). Previous studies have
shown that the phenolic hydroxyl and the methoxyl
groups on the phenyl ring and the 1,3-diketone system
are essential structural features for antioxidant
activity.
The reason that curcumin elicited the higher total
phenolic content maybe since it contains two phenolic
groups (Sepahpour et al., 2018). The number of
phenolic groups present in an antioxidant molecule
structure is not always the only factor to determine its
antioxidant activity. Positions of the phenolic groups,
presence of other functional groups in the molecules
such as double bonds, and conjugation to phenolic
and ketone groups also play essential roles in
antioxidant activities (Borra et al., 2013).
Based on 7 antioxidant methods namely: total
phenolic content (TPC), total flavonoid content
(TFC), 2,2 diphenyl 1 picrylhydrazyl (DPPH), 2,2′-
Azinobis (3-Ethylbenzthiazoline-6-Sulfonate)
(ABTS), hydrogen peroxide (H
2
O
2
), NO (Nitrogen
Oxide) scavenging and ferric reducing antioxidant
power (FRAP) that have been done, TE have better
antioxidant activity compared to TLE. This is due to
curcuminoids in TLE is less than turmeric. Previous
study have shown that curcumin in turmeric is
74.57%, while in TLE is 20.04 mg/g (Kusuma, 2012).
Curcumin is strong anti-oxidant and anti-
inflammatory effects and thus it possesses
hepatoprotective properties. The damage cells in the
liver is due to lipid peroxidation mechanism.
Antioxidant inhibit lipid peroxidation and enhance
antioxidant enzyme. Thus, it prevent the damage of
cells in the liver.
4 CONCLUSIONS
In conclusion, turmeric has higher antioxidant
properties than temulawak, but both turmeric and
temulawak are potential natural antioxidants.
Antioxidant Properties of Curcuma longa L. and Curcuma xanthorriza Rhizomes
109

ACKNOWLEDGEMENTS
The authors like to thank Seila Arumwardana, Aditya
Rinaldy, Muhamad Aldi Maulana from Biomolecular
and Biomedical Research Center, Bandung West
Java, Indonesia for their valuable assistance. The
author also acknowledges the financial support from
the Ministry of Research, Technology and Higher
Education of the Republic of Indonesia through
PTUPT Grant 2021.
REFERENCES
Ab Halim, M. R., Tan, M., Ismail, S., Mahmud, R. (2012).
'Standardization and phytochemical studies of Curcuma
xanthorrhiza Roxb'. Int. J. Pharm. Sci. 4(3), 606–610.
Adaramola, B., Onigbinde, A. (2017). 'Influence of
extraction technique on the mineral content and
antioxidant capacity of edible oil extracted from ginger
rhizome'. Chem. Int. 3(1), 1–7.
Akinola, A.A., Ahmad, S., Maziah, M. (2014). 'Total anti-
oxidant capacity, flavonoid, phenolic acid and
polyphenol content in ten selected species of
Zingiberaceae rhizomes'. Afr. J. Trad. Complement.
Altern. Med. 11(3), 7–13.
Al-Salahi, R., Anouar, E.-H., Marzouk, M., Taie, H. A.A.,
Abuelizz, H.A. (2018). 'Screening and evaluation of
antioxidant activity of some 1, 2, 4-triazolo [1, 5-a]
quinazoline derivatives'. Future Med. Chem. 10(4),
379–390.
Alam, M. N., Bristi, N. J., Rafiquzzaman, M. (2013).
'Review on in vivo and in vitro methods evaluation of
antioxidant activity'. Saudi Pharm. J. 21(2), 143–152.
Asouri, M., Ataee, R., Ahmadi, A.A., Amini, A., Moshaei,
M.R. (2013). 'Antioxidant and free radical scavenging
activities of curcumin'. Asian J. Chem. 25(13), 7593–
7595.
Bolanos De La Torre, A.A.S., Henderson, T., Nigam, P.S.,
Owusu-Apenten, R. K. (2015). 'A universally calibrated
microplate ferric reducing antioxidant power (FRAP)
assay for foods and applications to Manuka honey'.
Food Chem. 174, 119–123.
Borra, S.K., Gurumurthy, P., Mahendra, J. (2013).
'Antioxidant and free radical scavenging activity of
curcumin determined by using different in vitro and ex
vivo models'. J. Med. Plant Res. 7(36), 2680–2690.
Caleja, C., Barros, L., Antonio, A. L., Oliveira, M.B.P.P.,
Ferreira, I.C.F.R. (2017). 'A comparative study between
natural and synthetic antioxidants: Evaluation of their
performance after incorporation into biscuits'. Food
Chem. 216, 342–346.
Cavaleri, F. (2018). 'Presenting a new standard drug model
for turmeric and its prized extract, curcumin. Int. J.
Inflamm. 2018.
Ginting, C.N., Lister, I.N.E., Girsang, E., Riastawati, D.,
Kusuma, H.S.W., Widowati, W. (2020). 'Antioxidant
Activities of Ficus elastica leaves ethanol extract and
its compounds'. Mol. Cell. Biomed. Sci. 4(1), 27–33.
Indira Priyadarsini, K. (2013). 'Chemical and structural
features influencing the biological activity of
curcumin'. Curr. Pharm. Des. 19(11), 2093–2100.
Kedare, S.B., Singh, R.P. (2011). 'Genesis and development
of DPPH method of antioxidant assay'. J. Food Sci.
Technol. 48(4), 412–422.
Lobo, V., Patil, A., Phatak, A., Chandra, N. (2010). 'Free
radicals, antioxidants and functional foods: Impact on
human health'. Pharmacogn. Rev. 4(8), 118–126.
Lourenço, S.C., Moldão-Martins, M., Alves, V.D. (2019).
'Antioxidants of natural plant origins: From sources to
food industry applications'. Molecules, 24(22), 4132.
Lukitaningsih, E. (2020). 'In vivo antioxidant activities of
Curcuma longa and Curcuma xanthorrhiza'. Food
Research, 4(1), 13–19.
Malik, P., Mukherjee, T.K. (2014). 'Structure-function
elucidation of antioxidative and prooxidative activities
of the polyphenolic compound curcumin'. Chin. J. Biol.
2014.
Mukhopadhyay, D., Dasgupta, P., Roy, D. S.,
Palchoudhuri, S., Chatterjee, I., Ali, S., Dastidar, S. G.
(2016). 'A sensitive in vitro spectrophotometric
hydrogen peroxide scavenging assay using 1, 10-
phenanthroline'. Free Rad. Antioxid. 6(1), 124–132.
Nurrochmad, A. (2004). 'REVIEW: The new paradigm of
curcumin and its anticancer activity'. Rumphius J. Nat.
Prod. Biochem. 2(2), 75–80.
Phaniendra, A., Jestadi, D. B., Periyasamy, L. (2015). 'Free
radicals: properties, sources, targets, and their
implication in various diseases'. Indian J. Clin.
Biochem. 30(1), 11–26.
Pisoschi, A.M., Negulescu, G.P. (2011). 'Methods for total
antioxidant activity determination: a review'. Biochem.
Anal. Biochem. 1(1), 106.
Prahastuti, S., Hidayat, M., Hasiana, S.T., Widowati, W.,
Widodo, W.S., Handayani, R.A.S., Rizal, R., Kusuma,
H.S.W. (2020). 'The ethanol extract of the bastard cedar
(Guazuma ulmifolia L.) as antioxidants'. Pharmaciana.
10(1), 77–88.
Pratami, D.K., Mun, A., Sundowo, A., Sahlan, M., Pratami,
D.K., Mun, A. (2018). 'Phytochemical profile and
antioxidant activity of propolis ethanolic extract from
tetragonula bee'. Pharmacogn. J. 10(1), 73–80.
Rafi, M., Wulansari, L., Heryanto, R., Darusman, L.K.,
Lim, L.W., Takeuchi, T. (2015). 'Curcuminoid’s
content and fingerprint analysis for authentication and
discrimination of Curcuma xanthorrhiza from
Curcuma longa by high-performance liquid
chromatography-diode array detector'. Food Anal.
Methods. 8(9), 2185–2193.
Sepahpour, S., Selamat, J., Abdul Manap, M.Y., Khatib, A.,
Abdull Razis, A.F. (2018). 'Comparative analysis of
chemical composition, antioxidant activity and
quantitative characterization of some phenolic
compounds in selected herbs and spices in different
solvent extraction systems'. Molecules. 23(2), 402.
Utami, S., Sachrowardi, Q.R., Damayanti, N.A., Wardhana,
A., Syarif, I., Nafik, S., Arrahman, B.C., Kusuma, H.S.
ICE-TES 2021 - International Conference on Emerging Issues in Technology, Engineering, and Science
110

W., Widowati, W. (2018). 'Antioxidants,
anticollagenase and antielastase potentials of ethanolic
extract of ripe sesoot (Garcinia picrorrhiza Miq.) fruit
as antiaging'. J. Herbmed Pharmacol. 7(2), 88–93.
Wang, W., Kannan, K. (2019). 'Quantitative identification
of and exposure to synthetic phenolic antioxidants,
including butylated hydroxytoluene, in urine'. Environ.
Int. 128, 24–29.
Widowati, W., Janeva, W., Nadya, S., Amalia, A.,
Arumwardana, S., Kusuma, H. S. W., Arinta, Y. (2018).
'Antioxidant and antiaging activities of Jasminum
sambac extract, and its compounds'. J. Rep. Pharm. Sci.
7(3), 270–285.
Widowati, W., Sardjono, C.T., Wijaya, L., Laksmitawati,
D.R., Darsono, L. (2011). 'Free radicals scavenging
activities of spices and curcumin'. Proceedings of the
Second International Syposium on Temulawak and the
40th Meeting of National Working Group on
Indonesian Medicinal Plant.
Antioxidant Properties of Curcuma longa L. and Curcuma xanthorriza Rhizomes
111
