
A Microscope Image Auto-Focus Method Based on Colorful-Gradient
Po-Yi Li
1
, Chia-Jen Liu
2
, Cheng-Kuan Lin
3,∗
and Yu-Chee Tseng
3
1
Department of Biological Science and Technology, National Yang Ming Chiao Tung University, Taiwan
2
Institute of Emergency and Critical Care Medicine, School of Medicine, National Yang Ming Chiao Tung University,
Taiwan
3
Department of Computer Science, National Yang Ming Chiao Tung University, Taiwan
Keywords:
Auto-Focus Measurement, Gradient-Based Operators, Image Processing.
Abstract:
Blood testing has always been an important indicator for judging patients’ states and various types of lesions.
The typical way to observe the blood sample is by having medical personnel operate the conventional micro-
scope and classify the white blood cells in a patient’s blood sample. However, such a long period of observation
may cause visual fatigue. As a result, we built an automated microscope system to ensure the efficiency of the
observation. In addition, focus measurement has always been a huge topic in the auto-focus method, which
we implemented in the system. We proposed an automatic focus algorithm based on the gradient operator
and colorfulness value to fit with the automated microscope system. The color-gradient operator is compared
to conventional operators such as Laplacian-based, Wavelet-based, and DCT energy-based. By taking advan-
tage of the three components in the color-gradient operator, the standard of determining a microscope image
consists of both sharpness and colorfulness. The experimental results showed that the proposed microscope
automatic focus algorithm is significantly stable in all real-life blood cell microscope image dataset scenarios.
Such performance is discussed in specific situations that happened only in microscope auto-focus measure-
ments.
1 INTRODUCTION
Clinically, blood testing has always been one of the
critical indicators for judging the state of patients and
various types of lesions. For example, by count-
ing and classifying white blood cells in a patient’s
blood sample, you can know whether the patient has
leukemia and the type of leukemia. In addition, var-
ious indexes of white blood cells are also closely re-
lated to Covid-19. In 198 COVID-19 patients, the re-
search of Tong et al. shows that the survivors’ lym-
phocytes, basophils, and eosinophils levels were sig-
nificantly higher than those of non-survivors (Tong
et al., 2021). Blixt et al. observed that the most con-
valescent COVID-19 patients have robust and durable
B and T cell immunity (Blixt et al., 2022). There-
fore, improving the detection efficiency and accuracy
of white blood cells is very important, which will help
to judge the patient’s status accurately.
In the field of leukemia-related disease, acute
myeloid leukemia (AML) accounted for a large pro-
portion. Practically, medical personnel spends hours
∗
Corresponding author.
of microscope observation on patients’ blood sample
to identify their health. However, looking straight
into the microscope for hundreds or even thousands
of samples often cause visual fatigue or lower overall
efficiency. Therefore, many manufacturers of micro-
scope accessories have launched series for automatic
photography to solve the fatigue of repetitive oper-
ation behavior. Taking the Lionheart LX/FX series
of automated microscopes launched by BioTek as an
example, it costs about 40, 000 to 70, 000 US dollars
per device. Nonetheless, the whole product line of
the digital microscope DM B series launched by Le-
ica also costs about 5, 000 to 10, 000 US dollars. As
we can see, although the fatigue problem of medical
personnel has been solved at first glance, such an au-
tomated photographic microscope is too expensive for
many medical institutions with relatively insufficient
funds.
To solve the problems which people met in real
life, we came up with the idea of building an au-
tomated photography microscope system having the
following features: (1) modulized structure, (2) lower
price, (3) ability to analyze the image. The main idea
is to build a retrofit modular system that can easily in-
Li, P., Liu, C., Lin, C. and Tseng, Y.
A Microscope Image Auto-Focus Method Based on Colorful-Gradient.
DOI: 10.5220/0012017800003612
In Proceedings of the 3rd International Symposium on Automation, Information and Computing (ISAIC 2022), pages 693-698
ISBN: 978-989-758-622-4; ISSN: 2975-9463
Copyright
c
2023 by SCITEPRESS – Science and Technology Publications, Lda. Under CC license (CC BY-NC-ND 4.0)
693

stall on a conventional microscope which most med-
ical institutions own. At the same time, we have to
make sure that the price of this module is affordable,
which makes us abandon the method of installing an
auto-focus digital camera on top of the microscope.
We, however, chose the Raspberry Pi High-Quality
Camera as our image collector.
Such a choice has to pay the price: There is no
auto-focus system in the pi camera; we have to control
the adjustment wheel on the microscope with motors
and take several photos while scrolling it. As a result,
we’ll get a set of images that involve different heights
from the microscope stage to the microscope objec-
tive, which means we have to figure out the way to
pick out the non-blurry image from the set. However,
there were quite a few differences between the daily
photos and cell images captured with a microscope.
And that’s the primary purpose of the algorithm we
designed and implemented in this paper.
In Section 2, we introduce related works for mi-
croscope auto-focus. Section 3 describes our micro-
scope image auto-focus method based on Colorful-
Gradient and propose our Colorful-gradient auto-
focus algorithm (CGAF). And we analyze the experi-
mental results in Section 4. Finally, we conclude this
paper in Section 5.
2 RELATED WORKS
In this section, we review several previous studies of
focus measurement. We search for how people solve
the blurry image filtering problem nowadays. A fo-
cus measure is a stated quantity used to calculate the
pixel sharpness locally. Sharpness is a general term
for every focus measurement score to be taken as a
sharpness value. To calculate it, several focus opera-
tors are proposed by different researchers. In the re-
search of Pertuz et al. (Pertuz et al., 2013), six main
groups of sharpness operators were used in the work.
We pick four of them as our competitors to evaluate
our performance. The four sharpness operator groups
are shown in the following lines.
1 Gradient-Based Operators: This group of focus
measures uses the first image derivative or gradi-
ent to measure the focus level. Considering the
images as a function f of the intensity value of
pixels(x, y), the gradient g can be computed by:
g =
s
∂ f
∂x
2
+
∂ f
∂y
2
(1)
This is also one of the most commonly used fo-
cus measurements. However, there is an assump-
tion that this operator is based on: focused images
present more sharp edges than blurred ones. This
also means that it is pretty sensitive to the sharp
edges in an image.
2 Laplacian-Based Operators: These operators
are similar to the Gradient-based operator group.
These focus measures are based on the second
derivative of an image. Considering the images as
a function f of the intensity value of pixels(x, y),
the Laplacian can be computed by the equation
below, which is usually defined as divergence of
the gradient of a function f :
∆ f (x, y) = div(grad( f )) (2)
Group members such as Energy of Laplacian and
Variance of Laplacian have been used as a fo-
cus measure for auto-focus in many pieces of re-
search. A focus measure based on an alternative
definition of the Laplacian, known as the modified
Laplacian, showed the ability to become a focus
measurement value (Nayar and Nakagawa, 1994).
To conclude, Laplacian has shown a remarkable
ability to deal with accurate shape recovery, mak-
ing us later pick the Laplacian-based operator as
our first experimental option.
3 Wavelet-Based Operators: These operators are
based on the discrete wavelet transform coef-
ficients. Thus, these coefficients are used to
compute the focus amount of an image. How-
ever, these kinds of operators have primarily been
based on the statistical properties of the discrete
wavelet transform (DWT) coefficients. It is a fo-
cus measurement for auto-focus computing from
the sub-bands of a higher-level discrete wavelet
transform which is basically the sum of wavelet
coefficients (Yang and Nelson, 2003).
4 DCT-Based Operators: This group of operators
utilizes the discrete cosine transform coefficients
to calculate the degree of focus. The discrete co-
sine transform is now part of many images and
video encoding algorithms. The sum of the AC
components of the DCT was proved to be equal
to the variance of the image intensity and can be
used as a focus measure (Baina and Dublet, 1995).
Shen and Chen proposed a ratio between DC and
AC components in the DCT as a new focus mea-
surement method (Shen and Chen, 2006). Lee et
al. applied the DCT to 8 × 8 image sub-blocks
in order to measure focus (Lee et al., 2009). To
improve the computation time, they picked only 5
out of 63 AC coefficients to compute the AC en-
ergy, which made the whole calculation faster and
was named DCT reduced energy ratio.
Based on this literature review section, we started
everything from the one with higher accuracy of
ISAIC 2022 - International Symposium on Automation, Information and Computing
694

shape recovery, the Laplacian-based operator. How-
ever, we faced problems due to these high-accuracy
features and the particular situation while processing
microscopy images. Nonetheless, six different focus
measurement operators are finally chosen as compo-
nents in the simulation section. We will compare our
method of this research to others while processing the
same set of images.
3 METHODS
Picking a clear image is vital in our project. After
placing one patient’s blood samples onto the micro-
scope stage, we control the stage’s height with motors
and get a cluster of images that contains photos with
the variable focus of the same sample. Then, this clus-
ter of images will transfer to our server to begin pro-
cessing the clear image selection calculation. Finally,
when we get a single most explicit image, we run our
AI model to classify cells in the images and even an-
alyze the image with pre-trained medical knowledge.
The complete process can be finished while the med-
ical personnel is having a nice sleep, and a detailed
report will be shown on the user’s computer at the
end. However, in this research, we focus our method-
ology on the focus measurement instead of the whole
microscope project.
Most auto-focus methods are based on a sharpness
function that delivers a real-valued image quality es-
timate. However, a threshold has to be set to classify
whether the target image is blurry or not. Meanwhile,
the most commonly used method for filtering blurry
images is the Gradient-based operator or Laplacian-
based operator calculation of an image. As a result,
in our early pipeline design, there was only one com-
ponent: “calculate the sharpness value.” After facing
different problems, we devised a new pipeline solu-
tion for the microscope image focus measurement.
1 Sharpness Measurement: Using the second
derivative of an image, we could get the areas
of rapidly changing intensity in the image (Pech-
Pacheco et al., 2000). The Laplacian operator is
often used for edge detection. Furthermore, this
algorithm is based on the assumption that if a
picture has high variance, then it has a broader
frequency response range, representing a typical,
well-focused picture. But if the picture has a
slight variance, it has a narrower frequency re-
sponse range, which means that the number of
edges in the picture is low. As we know, the blur-
rier the picture is, the fewer it has edges. At the
beginning of our experiment, we thought it would
be straightforward to pick a clear image since
(a) (b)
Figure 1: Fixing impurities on the microscope makes the
program regard a blurry image as a clear image with several
sharp edges of impurities target.
Laplacian-based operators have been regarded as
the most effective way to get this job done. How-
ever, compared to the standard image in our daily
life, there were too many impurities in the micro-
scope image (Figure 1). Every impurity on the
image will be recognized as several rapidly chang-
ing intensity areas. This would make the Lapla-
cian transform value quite similar between pho-
tos since every image uses the same microscope
lenses, and there will be the same impurities in
the same position. The red circle in Figure 1(a)
shows the impurities in the image. Figure 1(b) is
the clear image of this set of images, which we
expected the program to pick.
However, we still needed information on the
sharpness value of the image. We then tried the
Gradient-based operator, which only calculates
the first derivative of the image. Formally, an im-
age gradient is defined as a directional change in
image intensity. Finally, we got a more accurate
result compared to the Laplacian-based operator.
Figure 2 illustrates the sharpness value calculated
with both Laplacian and gradient-based operators,
the clearest image in this set is the 35-th (image
ids). In this figure, the x-axis coordinate shows the
image of a set, which also stands for the different
distances between the microscope stage and the
microscope objective (descending power). The y-
axis corresponds to the sharpness value of both
Gradient-based and Laplacian-based operators.
2 Colorfulness Measurement: Although sharp-
ness seems to be the most efficient method for
blur detection, we found that since impurities are
black dots of various sizes when the image is be-
ing transformed into grayscale in the sharpness
calculation process, machines cannot tell the im-
purities or the cells are precisely the main tar-
gets it should focus on. This resulted in mak-
ing the wrong decision about a clear image. We
come up with a different aspect of blur detec-
tion in this case-Colorfulness calculation. The
whole point of the colorfulness calculation is that
we want to filter the total blur image with only
A Microscope Image Auto-Focus Method Based on Colorful-Gradient
695

Figure 2: Comparison between Gradient-based sharpness
value and Laplacian-based sharpness value.
impurities. These kinds of images share a com-
mon feature: they lack other colors except black
and white. Haslera and S
¨
usstrunk the colorfulness
calculation (Haslera and S
¨
usstrunk, 2003). First,
they split the image into RGB color values, R, G,
and B. Then, they immediately calculate
rg = R − G (3)
yb =
1
2R + 2G
− B (4)
Following up, they calculated both the standard
deviation of rg, σ
rg
, the standard deviation of yb,
σ
yb
, the mean value of rb, µ
rb
, and the mean value
of yb, µ
yb
, to fit the final Colorfulness function
Color f ulness f unction = σ
rgyb
+ 0.3 × µ
gryb
(5)
where
σ
rgyb
=
q
σ
2
rg
+ σ
2
yb
(6)
µ
rgyb
=
q
µ
2
rg
+ µ
2
yb
(7)
We set it as a double-check value which makes
sure that the high sharpness of a single image is
not just caused by the impurities on the micro-
scope lenses. Figure 3 shows the difference be-
tween adding colorfulness to the sharpness value
or not. The index of the image stands for the
microscope stage’s height, as when the index in-
crease, the stage height goes higher and higher
to the microscope objective. If the colorfulness
value is not considered, the program will pick the
second image as the clearest due to the impurity
problem. On the other hand, colorfulness did help
check this kind of mistake by making the correct
clear image have a higher score by adding a higher
colorfulness value on the sharpness value.
3 CIE Lab Color Space: Combining colorfulness
and sharpness to calculate the focus value seems
Figure 3: Showing how the colorfulness value helped in the
situation of impurities problems.
Figure 4: A general look of a set of images with a color cast
problem.
to be the most accurate method to identify the
clear microscope image. However, the illuminator
under the stage can sometimes cause serious color
cast problems, as shown in Figure 4. Noticed that
it should be a set of images from blurry to non-
blurry, but several of them suddenly had a color
casting problem with a blue tone. Considering
that we were using colorfulness as one of our cen-
tral scoring values, the program may give a very
high score to the color cast image in the aspect
of colorfulness. As a result, we modified the col-
orfulness calculation function with CIE Lab color
space to check for the image’s color cast degree.
The distance between colors in the color space is
consistent with its perceived difference, so it is
more reasonable to detect the color cast image un-
der it.
4 Pipeline Processing: Finally, we designed a new
process to solve the microscope image autofocus,
considering that there will be undodgeable im-
purities on the lens and illuminator accidentally
causing the color cast problem. As Figure 5 shows
below, when an image is inputted to the opera-
tor, we will start calculating the sharpness value,
colorfulness value, and color cast score parallelly.
Afterward, we multiply the reciprocal number of
the color cast score by the colorfulness value and
ISAIC 2022 - International Symposium on Automation, Information and Computing
696

Figure 5: The overall workflow of the Color-gradient focus
value calculation.
add the original sharpness value on it to get the
final focus value of the colorful-gradient opera-
tor. Algorithm 1 shows the detail of our method.
To begin, three lists will be declared (Sharpness,
Colorful, ColorCast) for storing sharpness value,
colorfulness value, and color cast score, respec-
tively. Secondly, we read the image to the vari-
able “im” to start calculating the three main com-
ponents in three color-gradient functions (S, CF,
CC) with three sub-functions (SCal, CCal, CCCal).
After traversing the directory, we scale all the val-
ues with a min-max scaler and get the focus value
with the proposed function. Finally, the index of
the image with the highest focus value will return
as an output.
4 EXPERIMENTAL RESULTS
To have a better look at the colorful-gradient opera-
tor’s ability, we set up a few other operators to com-
pare the performance of picking the non-blurry image
from a set of images. The testing process included 18
directories of images, each of them containing multi-
ple images from blurry to non-blurry images, which
are all taken in real-life patients’ blood samples. For
these 18 cases of images, there are 12 cases taken on
microscopes with impurities on the lens, and 6 cases
are taken on a microscope with a clean lens. We com-
pare our method, color gradient (C
g
), with Gradient-
based (G
b
), Laplacian-based (L
b
), wavelet summary
(W
s
), wavelet variance (W
r
), DCT ratio (D
r
), and DCT
reduced ratio (D
rr
). As Table 1 shows below, the col-
orful gradient is doing a great job in the 12 cases with
impurities. The most explicit image in every case is
accurately picked by it. However, most of the other
operators could not have a stable performance while
facing impurities problems. On the other hand, when
it comes to cases without the interference of impuri-
Algorithm 1: Colorful-gradient auto-focus algorithm
(CGAF).
Input: A list of strings (path of every image
in the image directory), Input =
[path
1
, path
2
, path
3
, ··· , path
n
].
Output: A index of the most explicit image.
1 begin
2 Sharpness ← [];
3 Color f ul ← [];
4 ColorCast ← [];
5 len = length(Input);
6 while len > 0 do
7 im ← ImageToArray(Input[len − 1]);
8 Append SCal(im) to Sharpness;
9 Append CCal(im) to Color f ul;
10 Append CCCal(im) to ColorCast;
11 len ← len − 1;
12 end
13 SS ← MinMaxScaler(Shar pness);
14 CS ← MinMaxScaler(Color f ul);
15 CCS ← MinMaxScaler(ColorCast);
16 Focus ← ColorGradient(SS,CS,CCS);
17 return FindMaxValueIndex(Focus);
18 end
Table 1: The benchmark with impurity interference
# steps, s C
g
G
b
L
b
W
s
W
v
D
r
D
rr
s < 1 12 6 2 1 7 5 0
5 ≤ s < 15 0 3 3 1 1 5 5
15 ≤ s < 35 0 0 0 1 0 0 2
s ≥ 35 0 3 7 4 4 2 3
ties, as Table 2 showed, all of the operators can nearly
pick the clearest image by an error of about three steps
on average. We can still observe that the colorful-
gradient operator is very stable, regardless of whether
the impurities are interfering.
Not only can the colorful gradient handle the im-
purities cases, but it can also deal with a color cast
that often happens in microscope images. One of
the 12 cases with impurities interference also has a
severe color casting problem, as shown in Figure 5
(Notice that to have an easier understanding of the
color cast problem, we did not show all of the images
in Figure 6. We picked images with an interval of
three started from the fourth image). A standard mi-
croscope image of blood cells should be purple due
to the dye fusing in the sample for more straight-
Table 2: The benchmark without impurity interference
# steps, s C
g
G
b
L
b
W
s
W
v
D
r
D
rr
s < 1 5 3 1 0 1 0 0
5 ≤ s < 5 1 3 5 6 5 6 5
s ≥ 5 0 0 0 0 0 0 1
A Microscope Image Auto-Focus Method Based on Colorful-Gradient
697
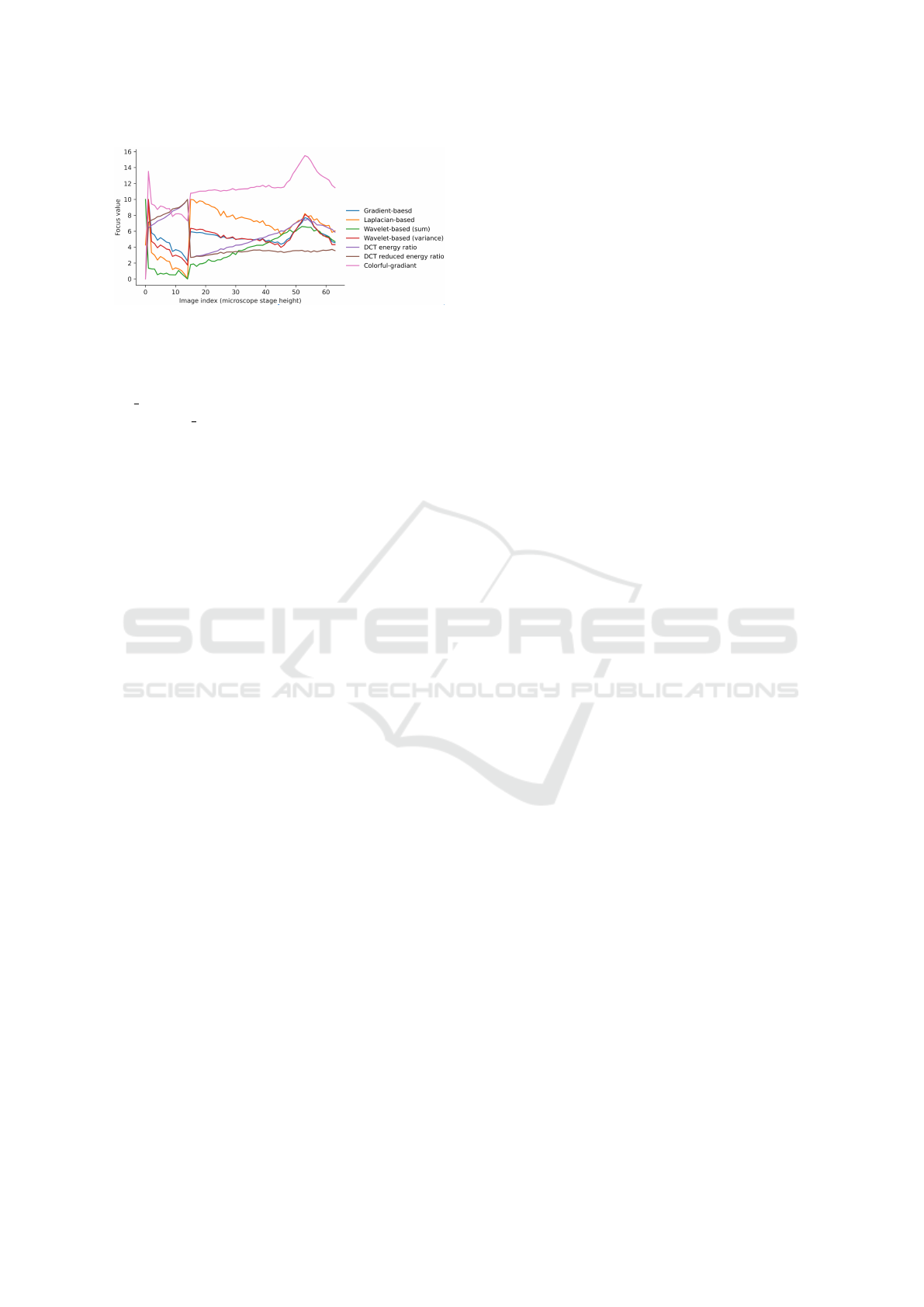
Figure 6: Benchmark comparison between other operators
and the Color-gradient operator.
forward observation. However, we can see several
photos in the set have a color casting to blue (exam-
ple 1 ∼ 5.jpg), which made all of the operators pick
the example 2.jpg, which is the wrong one with the
color cast problem. Figure 6 is a line chart of all the
operators calculating this color cast case. Because of
the blue color casting, all lines suddenly peak when
the image index is number 2. Even if the process fi-
nally meets the actual clear image, number 52 in the
set, the peak can only be a local maximum instead of
a global maximum for all the other operators, leading
to a wrong answer. Once again, the colorful gradient
operator is still the only one that survives in this color
casting case.
The final simulation with python code files is all
open sources on the internet. Please refer to the
URL: https://github.com/Pockylee/Colorful-gradient
for a deeper understanding of the colorful-gradient
operator algorithm.
5 CONCLUSIONS
We proposed a new focus measurement operator con-
sidering three aspects: sharpness, colorfulness, and
color cast. Unlike most sharpness operators nowa-
days, colorful-gradient cannot only do the regular im-
age focus measurement job well, but it can also easily
deal with microscopy cases. According to the real-
life data collected from medical institutes, we found
the two main factors of making microscopy images
harder to do focus measurement. First, the impurity
on the lens is an undodgeable problem, especially in
medical institutions located in remote areas. Second,
the color cast problems are caused by the illuminator
of a conventional microscope. After all, we tested the
operators with real-life data and constructed a bench-
mark to show the difference between the colorful-
gradient operator and others. With the focus algo-
rithm we propose, we can perform excellently on the
microscope image focus detection cases. We had al-
ready implemented this algorithm onto the automated
microscope system. However, the current algorithm is
not fast enough to make a real-time adjustment with
the microscope stage adjust wheel. We can only take
one photo on every slight rotation (step) of the adjust-
ment wheel; after gathering all of the images of the
sample, we send them to the server to find the perfect
height for the microscope stage. As a result, we can
start from a higher height instead of taking the photo
from the bottom, which wastes much time.
REFERENCES
Baina, J. and Dublet, J. (1995). Automatic focus and
iris control for video cameras. In Fifth International
Conference on Image Processing and its Applications,
pages 232–235.
Blixt, L., Bogdanovic, G., Buggert, M., Gao, Y., Hober,
S., Healy, K., Johansson, H., Kjellander, C., Mravina-
cova, S., Muschiol, S., Nilsson, P., Palma, M., Pin, E.,
Smith, C. I. E., Stromberg, O., Chen, M. S., Zain, R.,
Hansson, L., and
¨
Osterbor, A. (2022). Covid-19 in
patients with chronic lymphocytic leukemia: clinical
outcome and b- and t-cell immunity during 13 months
in consecutive patients. Leukemia, 36(2):476–481.
Haslera, D. and S
¨
usstrunk, S. (2003). Measuring colour-
fulness in natural images. In SPIE - the International
Society for Optical Engineering, volume 5007, pages
87–95.
Lee, S.-Y., Yoo, J.-T., Kumar, Y., and Kim, S.-W. (2009).
Reduced energy-ratio measure for robust autofocus-
ing in digital camera. IEEE Signal Processing Letters,
16(2):133–136.
Nayar, S. K. and Nakagawa, Y. (1994). Shape from focus.
IEEE Transactions on Pattern Analysis and Machine
Intelligence, 16(8):824–831.
Pech-Pacheco, J. L., Cristobal, G., Chamorro-Martinez, J.,
and Fernandez-Valdivia, J. (2000). Diatom autofocus-
ing in brightfield microscope: a comparative study. In
the 15th International Conference on Pattern Recog-
nition (ICPR-2000), volume 3, pages 314–317.
Pertuz, S., Puig, D., and Garcia, M. A. (2013). Analysis of
focus measure operators for shape-from-focus. Pat-
tern Recognition, 46:1415–1432.
Shen, C.-H. and Chen, H. H. (2006). Robust focus measure
for low-contrast images. In 2006 Digest of Technical
Papers International Conference on Consumer Elec-
tronics, number 69-70.
Tong, X., Cheng, A., Yuan, X., Zhong, X., Wang, H., Xu,
W. Z. X., and Li, Y. (2021). Characteristics of periph-
eral white blood cells in covid-19 patients revealed.
BMC Infectious Diseases, 21(1):1236.
Yang, G. and Nelson, B. J. (2003). Wavelet-based autofo-
cusing and unsupervised segmentation of microscopic
images. In 2003 IEEE/RSJ International Conference
on Intelligent Robots and Systems (IROS 2003) (Cat.
No.03CH37453), pages 2143–2148.
ISAIC 2022 - International Symposium on Automation, Information and Computing
698
