
Skin Tone via Device-Independent Colour Space
Leah DeVos
1
, Gennadi Saiko
2a
and Alexandre Douplik
2,3 b
1
Department of Engineering, Toronto Metropolitan University, Toronto, Canada
2
Department of Physics, Toronto Metropolitan University, Toronto, Canada
3
iBest, Keenan Research Centre of the LKS Knowledge Institute, St. Michael’s Hospital, Canada
Keywords: Skin Tone, Melanin, Tissue Optics.
Abstract: Background: Skin colour is essential to skin and wound assessment as it brings valuable information about
skin physiology and pathology. An approach, which can help deconvolute and isolate various mechanisms
affecting skin colour, could be helpful to drive the rPPG utility beyond its current applications. Aim: The
present work aims to create a framework that links skin colour with melanin content. Material and methods:
The model consists of two parts. First, the model's core connects tissue chromophore concentrations with
changes in tissue reflectance. Seven-layer tissue models and Monte Carlo simulations were used to obtain the
tissue reflectance spectra. In the second step, the tissue reflectance is convoluted with the responsivity of a
sensor (tristimulus response in the case of the human eye) and the light source's emission spectrum. Results:
The model allows linking melanin content with skin colour. Conclusion: The model can be helpful for the
interpretation of the amplitudes of various components of the rPPG signal.
1 INTRODUCTION
Optical methods in the visible range have difficulties
extracting physiological parameters in subjects with
darker skin. Recent reporting identified potential skin
tone biases of PPG. For instance, Sjoding et al.
(Sjoding, 2020) investigated the occurrence of occult
hypoxemia across patients who self-identified as
White or Black, which is true oxygen saturation of
<88% given a PPG-quantified saturation from 92-
96% (i.e., a false negative from PPG on detection of
low saturation). They reported that occult hypoxemia
occurred in 12% of patients who self-identified as
Black, compared to 4% of patients who self-identified
as White.
Thus, it is plausible that current clinical datasets
are skewed toward subjects with lighter skin
complexion resulting in bias toward lighter skin
tones. Consequently, the validity of the immense
amount of accumulated clinical data may be
questionable. Thus, the research on the influence of
skin tone on optical physiological data (e.g., tissue
oxygenation) and algorithms considering/correcting
this impact are necessary.
a
https://orcid.org/0000-0002-5697-7609
b
https://orcid.org/0000-0001-9948-9472
Thus, the first step in this direction would be to
establish quantifiable metrics. However, clinically
used metrics (Fitzpatrick’s skin tones) are subjective,
and more objective models are required although to
be correlated with the Fitzpatrick’s scale to provide
consistency of the ‘results’ interpretation.
Thus, the critical step in that direction is using
objective (non-device specific) colour representation.
RGB, by far, is the most common colour space;
however, it suffers several drawbacks. The
International Commission on Illumination (CIE)
adopted the CIE XYZ colour space to overcome the
disadvantages of trichromatic additive colour spaces
like RGB. However, CIE XYZ space demonstrates
perceptual nonuniformity (MacAdams, 1942). In
adopting the CIELUV colour space, the CIE
attempted to address this concern (Colorimetry,
1986).
In previous work (Saiko, 2022) the skin colour
dependence on blood oxygenation and perfusion was
studied analytically. In the current work we aim to
investigate the influence of melanin content on skin
colour using Monte Carlo simulations. Ultimately
124
DeVos, L., Saiko, G. and Douplik, A.
Skin Tone via Device-Independent Colour Space.
DOI: 10.5220/0011748100003414
In Proceedings of the 16th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2023) - Volume 2: BIOIMAGING, pages 124-128
ISBN: 978-989-758-631-6; ISSN: 2184-4305
Copyright
c
2023 by SCITEPRESS – Science and Technology Publications, Lda. Under CC license (CC BY-NC-ND 4.0)
this framework can help extract additional
information from rPPG signals.
2 METHODS
The model conceptually consists of two parts. On step
one we calculate the tissue reflectance. On step two
we convolute the tissue reflectance with light source
spectrum and sensor response curves (tristimulus
response in the case of the human eye).
2.1 Tissue Reflectance
For this experiment, the spectrums of total and diffuse
reflectance at various percentages of melanin content
were simulated using the Monte Carlo method. To
achieve this, first a layer model was designed to
depict a computational model of human skin tissue.
Then using that model, Monte Carlo simulations were
run for the wavelength spectrum of 400-1000 nm with
5 nm increments, for melanin concentrations of 1, 2,
4, 6, 8, 16, and 32% respectively.
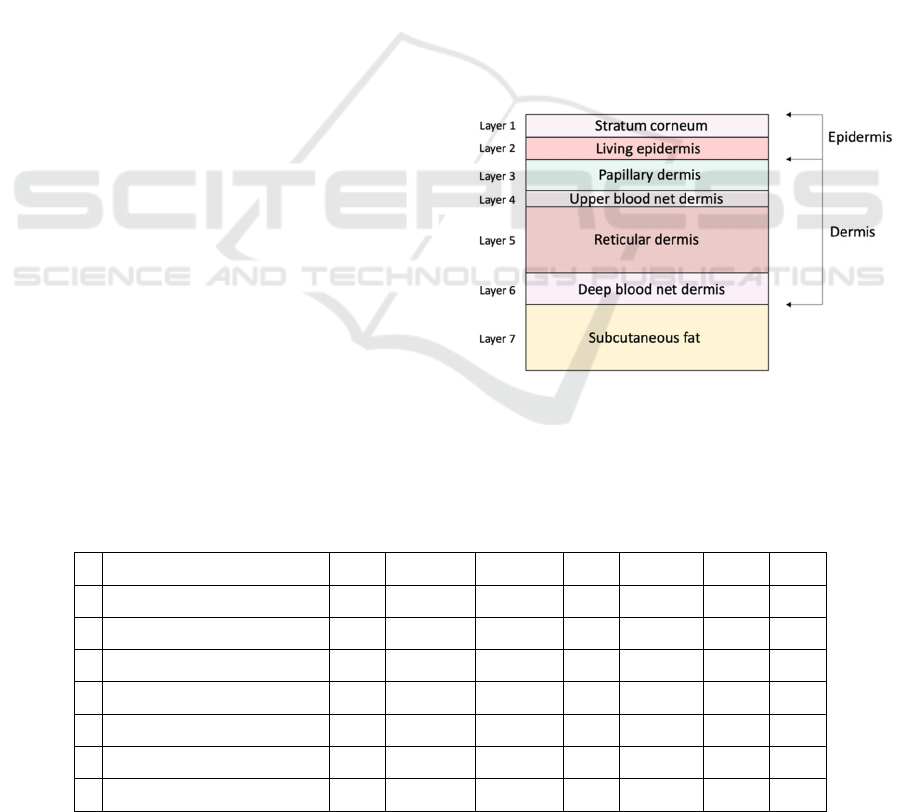
2.1.1 Tissue Model
The computational modelling of skin tissue is based
on the consideration that skin is a three-dimensional
half-infinite medium divided into several layers with
varying optical properties (Wang, Jacques, & Zheng,
1995). The layers considered in this experiment are
the stratum corneum, the living epidermis, the
papillary dermis, the upper blood net dermis, the
reticular dermis, the deep blood net dermis, and the
subcutaneous fat. The top two layers (stratum
corneum and living epidermis) comprises the
bloodless epidermal layer. Stratum corneum is the
first layer and is approximately 20 μm thick, it is
composed of flattened dead cells mainly containing
keratin (Meglinsky & Matcher, 2001). The second
layer is the living epidermis and is mainly composed
of living cells including, dehydrated cells, laden cells
with keratohyalin granules, columnar cells, melanin
dust, small melanin granules and melanosomes
(Meglinsky & Matcher, 2001). This layer is approx.
80 μm thick. The dermis has the inhomogeneous
distribution of the blood vessels and skin capillaries
within the skin (Meglinsky & Matcher, 2001). To
emulate this complexity, we split the dermis layer into
four sublayers, the papillary dermis (150 μm thick),
the upper blood net dermis (80 μm thick), the reticular
dermis (1500 μm thick) and the deep blood net dermis
(170 μm thick). The last layer considered is the
subcutaneous fat. We approximated it as 6 mm thick.
The physical organisation of these layers can be
visualised in Figure 1. Table 1 demonstrates the order
of these layers as well as some of their optical and
physical properties. The values for layer thickness
and optical properties are an approximation and
would vary slightly between in vivo subjects.
Figure 1: Skin layers for Monte Carlo simulations.
Table 1: Layer settings for λ = 700 nm and 1% melanin concentration, where n = refractive index,
μ
a
= absorption coefficient,
μ
s
= scattering coefficient, g = scattering anisotropy, d = layers thickness, C
b
=blood volume fraction and C
w
= water volume
fraction.
# Skin Layer
n
μ
a
(cm
-1
)
μ
s
(cm
-1
)
g
d (mm)
C
b
C
w
1 Stratum corneum
1.33
0.0012
285.71
0.9
0.02
0
0.2
2 Living epidermis
1.33
2.2162
285.71
0.85
0.05
0
0.2
3 Papillary dermis
1.37
0.0207
183.52
0.8
0.15
0.004
0.65
4 Upper blood net dermis
1.4
0.0881
183.52
0.9
0.08
0.02
0.65
5 Reticular dermis
1.4
0.0207
183.52
0.76
1.5
0.004
0.65
6 Deep blood net dermis
1.4
0.1723
183.52
0.95
0.17
0.04
0.65
7 Subcutaneous fat
1.44
0.1266
183.52
0.8
6
0.03
0.05
Skin Tone via Device-Independent Colour Space
125

2.1.2 Optical Settings in Layer Model
The values of the optical properties set in this
experiment were found both from existing literature
as well as derived from optical equations. Retrieved
from literature were the values for scattering
anisotropy which was set to 0.9 and the values for the
refractive index which ranged from around 1.34-1.53
depending on the tissue layer (Moço, Stuijk, & de
Haan, 2018). This experiment ran simulations for a
wavelength range of 400 to 1000 nm for each
different concentration of melanin. For each
wavelength and skin configuration the scattering and
absorption coefficients for both the epidermal and the
dermal layers was adjusted. The optical properties
represented in Table 1 correspond to a wavelength of
700 nm and a concentration of melanin of 1%. The
equations used to derive the values for the scattering
and absorption coefficients of the dermis and
epidermis were retrieved from work by Jacques et al
(S. Jacques, T. Li & S. Prahl, 2019).
Absorption Coefficient,
μ
a
The same equation is used to calculate the absorption
coefficient of both the epidermis and the dermis, but
the calculations differ in which input values were
included or omitted. For example, when calculating
the absorption coefficient of the epidermis, the
volume fraction of melanosomes had to be considered
while it did not for the absorption coefficient of the
dermis and was set to zero. For the dermis, differing
from the epidermis, in calculating the absorption
coefficient, the average volume fraction of blood had
to be included. The equation used to calculate the
absorption coefficients was:
𝜇
= 𝐶
(𝑆𝑂2𝜇
.
+
(
1 −𝑆𝑂2
𝜇
.
)+
𝐶
𝜇
.
+ 𝐶
𝜇
.
+ 𝐶
𝜇
.
(1
)
Where: C
b
= blood volume fraction, SO2 =
oxygen saturation of hemoglobin, C
w
= water volume
fraction, C
f
= fat volume fraction, Cm = volume
fraction of melanosomes,
μ
a.HbO2
= absorption
coefficient of oxygenated hemoglobin,
μ
a.RHb
=
absorption coefficient of deoxygenated hemoglobin,
μ
a.fat
= absorption coefficient of fat,
μ
a.water
=
absorption coefficient of water, and
μ
a.melanosome
=
absorption coefficient of melanosomes.
Scattering Coefficient,
μ
s
Tissue scattering is described as a summation of
Rayleigh and Mie Scattering. To calculate the
scattering coefficient first the reduced scattering
coefficient, which describes the diffusion of photons
in a random walk, needs to be calculated. After the
reduced scattering coefficient has been calculated, it
can be incorporated with the anisotropy to calculate
the scattering coefficient. The main difference
between the scattering coefficient of the epidermis
versus the dermis is that dermal scattering is
described in terms of the relative contributions of Mie
and Rayleigh scattering due to collagen fibres while
epidermal scattering is relative to scattering due to
keratin fibres (Jacques, 1998). Again, the same
equations were used to calculate the scattering
coefficient of the dermis and the epidermis differing
only by the values of the input parameters. The
equations used were as follows:
𝜇
= 𝜇
.
(𝑓
+
(
1 −𝑓
)
) (2)
𝜇
=
(3)
Where 𝜇
= reduced scattering coefficient, 𝜇
=
scattering coefficient, 𝜇
.
= reduced scattering
coefficient at 500 nm, 𝑓
= fraction of Rayleigh
scattering at 500 nm, 𝑓
= fraction of Mie scattering
at 500 nm, 𝑏
= scatter power for Mie scattering,
λ
is
the wavelength in [nm] and g is the anisotropy of
scattering.
2.1.3 Monte Carlo Simulations of Skin
Reflectance
For this experiment the Monte Carlo for Multi-
Layered media (MCML) program by L. Wang & S.
L. Jacques was used to provide a realistic model of
light propagation in biological tissue (Wang &
Jacques, 1992). In essence, the Monte Carlo method
describes the transport of an infinitely narrow photon
beam perpendicularly incident on a multi-layered
tissue (Wang et al, 1995). Running Monte Carlo
simulations generates a variety of output results but
the output of interest for this experiment was the total
and diffuse reflectance. To achieve these results, first
an input file was generated to specify the simulation.
This input file was generated in MATLAB using the
function create_MCML_input_file that sets up the
layer model used to describe the multi-layered tissue
the simulation is being performed on (Akerstam &
Andersson-Engels, 2011). An example of this layer
model can be seen in Table 1. In the input file the
number of incident photons to be used is also
declared, for this experiment the amount of photons
set was 100000. Once the input file has been
generated, the input file is fed to the MCML program,
and the simulation is run. Once the simulation is
finished an output file is generated with the results.
The MATLAB program getmcml.m was used to read
BIOIMAGING 2023 - 10th International Conference on Bioimaging
126

the generated output file and interpret the results
(Wang & Jacques, 1992). These results include the
diffuse and specular reflectance, which when
combined provides the total reflectance. This process
was repeated for a wavelength range of 400 to 1000
nm at different volume fractions of melanosomes.
Using these results, the total reflectance and diffuse
reflectance versus wavelength was plotted for each
volume fraction of melanosomes of interest.
2.2 Light Source
It should be noted that while the colour representation
for additive colour schemas (emissive case) can be
considered absolute, it is not the case for subtractive
colour schemas (reflection and transmission), where
the response needs to be convoluted with the spectral
power distribution of the illuminant. Thus, perceived
colour in a subtractive colour scheme is light source
dependent.
CIE standard illuminant E was used as the light
source in our simulations.
2.3 Tristimulus Colour Space
The human eye and typical imaging systems interpret
colours using three colour channels. Thus, in step 2,
we need to aggregate the tissue reflectance spectra
into three-channel responses. The CIE XYZ colour
space encompasses all colour sensations visible to a
person with average eyesight using the CIE's colour
matching functions (
𝑥
(
𝜆
)
,𝑦
(
𝜆
)
,𝑧
(
𝜆
)
), which quantify
the chromatic response of the average observer. The
CIE 1931 colour space defines the tristimulus values
denoted by X, Y, and Z. In the case of the subtractive
colour schema (reflection and transmission) for the
known light source spectral distribution I(l), the
tristimulus values can be found as
𝑋
=
𝐾
𝑁
𝑅
(
𝜆
)
𝐼
(
𝜆
)
𝑥
(
𝜆
)
𝑑𝜆
(4
)
𝑌 =
𝐾
𝑁
𝑅
(
𝜆
)
𝐼
(
𝜆
)
𝑦
(
𝜆
)
𝑑𝜆
(5
)
𝑍 =
𝐾
𝑁
𝑅
(
𝜆
)
𝐼
(
𝜆
)
𝑧
(
𝜆
)
𝑑𝜆
(6
)
Here N=
I
(
λ
)
y
(
λ
)
dλ
, R is the tissue
reflectance, and K is the scaling factor. The XYZ
colour space can be transformed into commonly used
RGB colour space by a simple linear transformation
(multiplication on a 3x3 matrix).
However, the CIE XYZ colour space allows
decomposition into two parts: brightness and
chromaticity. The CIE XYZ colour space was
deliberately designed so that the Y parameter is also a
measure of the luminance of a colour. That allows the
representation of each colour on 2D colour space
using normalization
𝑥 =
𝑋
𝑋
+
𝑌
+
𝑍
(7
)
𝑦 =
𝑌
𝑋
+
𝑌
+
𝑍
(8
)
The chromatic coordinates (x,y) can be
transformed into chromatic coordinates (u',v') in the
CIELUV colour space (Colorimetry, 1986), which
has certain advantages over the CIE XYZ colour
space (namely, perceptual uniformity):
𝑢
=
4𝑥
−2𝑥+12
𝑦
+3
(9
)
𝑣
=
9𝑦
−2𝑥+12
𝑦
+3
(10
)
3 RESULTS
In the first step, we generated the tissue's simulated
reflectance spectrum in the 400-1000 nm range for
different melanin content (1, 2, 4, 6, 8, 16, and 32%,
respectively). The results of the MC simulations are
depicted in Figure 2.
Figure 2: The skin diffuse reflectance spectrum as a
function of the melanin content (1, 2, 4, 6, 8, 16, and 32%,
respectively).
In step 2, the generated spectra were convoluted with
CIE's colour matching functions and light source
spectrum to obtain values X, Y, and Z using Eqs. 4-6.
We approximated the CIE XYZ colour-matching
functions by a sum of Gaussian functions (Wyman et
al., 2013). CIE standard illuminant E was used as the
light source.
Skin Tone via Device-Independent Colour Space
127

Then using Eqs.7 and 8, x and y were obtained. The
result of tissue colour simulations in (x,y) colour
space is presented in Fig 3 as a function of the
melanin content (1, 2, 4, 6, 8, 16, and 32%,
respectively).
Figure 3: The simulated tissue colour in chromaticity
diagrams (CIE XYZ colour space) as a function of the
melanin content (1, 2, 4, 6, 8, 16, and 32%, respectively).
The respective transformation into CIELUV colour
space using Eqs. 9-10 is depicted in Fig 4 as a
function of the melanin content (1, 2, 4, 6, 8, 16, and
32%, respectively).
Figure 4: The simulated tissue colour in chromaticity
diagrams (CIELUV colour space) as a function of the
melanin content (1, 2, 4, 6, 8, 16, and 32%, respectively).
4 CONCLUSIONS
In summary, we proposed a simple approach where
the realistic tissue reflectance spectrum generated
using the multi-layer Monte Carlo model is onvoluted
with CIE's colour-matching functions and ambient
light spectrum to obtain tristimulus values in XYZ
colour space. The proposed approach allows for
quantitative analysis of the influence of tissue
chromophores on tissue colour.
ACKNOWLEDGEMENTS
The authors acknowledge funding from NSERC
Alliance (Douplik & Saiko), NSERC Discovery
(Douplik), NSERC RTI (Douplik), and Ryerson
Health Fund (Douplik).
REFERENCES
Akerstam E. & Andersson-Engels S. (2011). “Monte Carlo
Simulations of Light Transport in Tissue. Department
of Physics, Lund University. https://www.atomic
.physics.lu.se/fileadmin/atomfysik/Biophotonics/Educ
ation/Tissue_Optics_-_Computer_Exercise_-_MC.pdf
Colorimetry, 2nd ed, CIE publication 15.2, (Central Bureau
CIE, Vienna, 1986)
Jacques S. (1998). “Skin Optics”. Oregon Medical Laser
Center News.
https://omlc.org/news/jan98/skinoptics.html
Jacques S., Li T., & Prahl S. (2019). mcxyz.c. Monte Carlo
Light Scattering Programs.
https://omlc.org/software/mc/mcxyz/index.html
MacAdams D.L., (1942) Visual sensitivities to color
differences in daylight. J OSA. 32(5) 247–274
Meglinsky, I. V., & Matcher, S. J. (2001). Modelling the
sampling volume for skin blood oxygenation
measurements. Medical & Biological Engineering &
Computing, 39(1), 44-50. doi:10.1007/BF02345265
Moço, A. V., Stuijk, S., & de Haan, G. (2018). New insights
into the origin of remote PPG signals in visible light and
infrared. Scientific Reports, 8(1), 8501-15.
doi:10.1038/s41598-018-26068-2
Saiko G (2022) How skin color depends on tissue
oxygenation, Adv Exp Med Biol (submitted)
Sjoding, MW et al. (2020) Racial bias in pulse oximetry
measurement. New England Journal of Medicine
383(25): 2477-2478
Wang L. & Jacques S. L. (1992). “(MCML) Monte Carlo
for Multi-Layered media”. Monte Carlo Light
Scattering Programs. https://omlc.org/software/mc/
Wang, L., Jacques, S. L., & Zheng, L. (1995). MCML—
Monte Carlo modeling of light transport in multi-
layered tissues. Computer Methods and Programs in
Biomedicine, 47(2), 131-146. doi:10.1016/0169-
2607(95)01640-F
Wyman C; Sloan PP; Shirley P. (2013) Simple Analytic
Approximations to the CIE XYZ Colour Matching
Functions. J Comp Graph Tech. 2 (2): 1-11
BIOIMAGING 2023 - 10th International Conference on Bioimaging
128
