
Leveraging Deep Neural Networks for Automatic and Standardised
Wound Image Acquisition
Ana Filipa Sampaio
1 a
, Pedro Alves
1 b
, Nuno Cardoso
1 c
, Paulo Alves
2, 3 d
, Raquel Marques
3 e
,
Pedro Salgado
4
and Maria Jo
˜
ao M. Vasconcelos
1 f
1
Fraunhofer Portugal AICOS, Porto, Portugal
2
School of Nursing Department, Universidade Cat
´
olica Portuguesa (UCP), Porto, Portugal
3
Centre for Interdisciplinary Research in Health, Instituto de Ci
ˆ
encias da Sa
´
ude, UCP, Porto, Portugal
4
F3M Information Systems, S.A, Portugal
Keywords:
Skin Wounds, Mobile Health, Object Detection, Deep Learning, Mobile Devices.
Abstract:
Wound monitoring is a time-consuming and error-prone activity performed daily by healthcare professionals.
Capturing wound images is crucial in the current clinical practice, though image inadequacy can undermine
further assessments. To provide sufficient information for wound analysis, the images should also contain
a minimal periwound area. This work proposes an automatic wound image acquisition methodology that
exploits deep learning models to guarantee compliance with the mentioned adequacy requirements, using a
marker as a metric reference. A RetinaNet model detects the wound and marker regions, further analysed by
a post-processing module that validates if both structures are present and verifies that a periwound radius of
4 centimetres is included. This pipeline was integrated into a mobile application that processes the camera
frames and automatically acquires the image once the adequacy requirements are met. The detection model
achieved mAP@.75IOU values of 0.39 and 0.95 for wound and marker detection, exhibiting a robust detection
performance for varying acquisition conditions. Mobile tests demonstrated that the application is responsive,
requiring 1.4 seconds on average to acquire an image. The robustness of this solution for real-time smartphone-
based usage evidences its capability to standardise the acquisition of adequate wound images, providing a
powerful tool for healthcare professionals.
1 INTRODUCTION
The prevalence of chronic wounds is high world-
wide, being considered a major public health prob-
lem. Chronic wounds are those that do not progress
through a normal and timely sequence of repair
(Werdin et al., 2009) and can include pressure, ve-
nous, arterial, diabetic ulcers, and others. The study
of (Martinengo et al., 2019) indicates a pooled preva-
lence of 2.21 per 1000 population for chronic wounds
of mixed etiologies, and the prevalence for chronic
leg ulcers was estimated at 1.51 per 1000 population.
In the United States, chronic wounds affect the qual-
a
https://orcid.org/0000-0003-1770-4429
b
https://orcid.org/0000-0002-0372-4755
c
https://orcid.org/0000-0001-5736-7995
d
https://orcid.org/0000-0002-6348-3316
e
https://orcid.org/0000-0002-6701-3530
f
https://orcid.org/0000-0002-0634-7852
ity of life of nearly 2.5% of the total population, hav-
ing a significant impact on healthcare (Sen, 2021). In
Portugal, the prevalence is 0.84 per 1000 population,
and patients with more than 80 years of age present a
prevalence of 5.86 (Passadouro et al., 2016).
Wound healing is a complex process that involves
several phases and proper monitoring of its evolution.
The rise of the digital health sector can help health-
care professionals properly monitor wound evolution
by storing clinical information in each monitorization,
including wound details and images. According to the
study of (Chen et al., 2020) current evidence suggests
that telemedicine has similar efficacy and safety with
conventional standard care of chronic wounds.
An essential step for proper wound documenta-
tion is wound image capturing (Mukherjee et al.,
2017),(Anisuzzaman et al., 2022b); however, poor
image quality can negatively influence later assess-
ments. Many of the approaches reported in the lit-
erature for automatised wound monitoring address
Sampaio, A., Alves, P., Cardoso, N., Alves, P., Marques, R., Salgado, P. and Vasconcelos, M.
Leveraging Deep Neural Networks for Automatic and Standardised Wound Image Acquisition.
DOI: 10.5220/0012031200003476
In Proceedings of the 9th International Conference on Information and Communication Technologies for Ageing Well and e-Health (ICT4AWE 2023), pages 253-261
ISBN: 978-989-758-645-3; ISSN: 2184-4984
Copyright
c
2023 by SCITEPRESS – Science and Technology Publications, Lda. Under CC license (CC BY-NC-ND 4.0)
253

the inadequate image quality using computer vision
methodologies to remove acquisition artefacts and
correct colour and perspective distortions in the cap-
tured images (Lu et al., 2017; Cui et al., 2019). Still,
the application of pre-processing strategies may be
insufficient for some tasks since it does not ensure
that all the relevant structures needed for an adequate
wound assessment (e.g. the periwound skin) are prop-
erly visible. Subsequently, efforts to enhance the im-
age quality at the acquisition step have been con-
ducted by either providing acquisition guidelines or
using semi-automated acquisition processes.
Many of the attempts to automatise the acquisi-
tion of wound images resort to object detection mod-
els, namely convolutional neural networks (CNNs)
to locate the wound bed in the captured image field.
The work in (Goyal et al., 2018) first presented an
algorithm to detect and locate diabetic foot ulcers
in real-time. The authors trained a Faster R-CNN
model with Inception-V2, obtaining a mean average
precision (mAP) of 91.8%. In (Faria et al., 2020), a
methodology for automated image acquisition of skin
wounds via mobile devices is presented. The applica-
tion of image focus validation combined with a detec-
tion deep neural network (DNN) for wound bed local-
isation (an SSDLite model with a MobileNetV2 back-
bone) achieved an mAP at a 0.50 intersection over
union (IOU) threshold (mAP@.50IOU) of 86.46%
and an inference speed of 119 milliseconds (ms)
for smartphone usage, ensuring simultaneous image
quality and adequacy in real-time. More recently, a
similar wound localiser for mobile usage was pre-
sented in (Anisuzzaman et al., 2022a). Two detection
DNNs, SSD with a VGG16 backbone and YOLOv3,
were trained in a private dataset of 1010 images of
venous, pressure and diabetic foot ulcers, achieving
mAP values of 86.4% and 93.9%, respectively. In
(Scebba et al., 2022), the authors trained a RetinaNet
model with three different feature extractors, all pre-
trained on the Common Objects in Context (COCO)
dataset. The best performance was achieved with the
MobileNet backbone, leading to a test mAP@.50IOU
of 0.762.
Despite relying mainly on private datasets for the
models’ development, most of these approaches also
resorted to a public database, Medetec (Medetec,
2014), to expand the training set (Faria et al., 2020)
and to assess the performance of their approach in
other datasets (Anisuzzaman et al., 2022a). How-
ever, even being used in many of the aforementioned
works, the Medetec database does not provide infor-
mation regarding the wound region location in its im-
ages (its usage was enabled by private annotations).
The high detection performances reported by
these approaches highlight the potential of DNNs for
the analysis of wound images. Yet, the majority of
these works only use it to narrow the wound region for
further processing by other algorithms, such as seg-
mentation or classification models. The exception is
the systems described in (Anisuzzaman et al., 2022a;
Faria et al., 2020), which integrate the trained wound
detectors in mobile wound acquisition applications.
Still, their frameworks do not use detection informa-
tion to analyse the adequacy of the captured images.
Thus, considering the importance of adequate
wound images and the lack of solutions that incorpo-
rate this validation in the image acquisition step, this
work presents an approach to standardise wound im-
age acquisition by automating the process while guar-
anteeing wound detection and periwound skin. For
this purpose, a new dataset of wound images with
reference markers was constructed and used to de-
velop a wound and marker detection model based on
a RetinaNet architecture with a MobileNetV2 back-
bone. This model was used as the basis for an image
adequacy validation module, responsible for ensur-
ing that the acquired images are compliant with the
requirements recommended by clinical experts: the
presence of the wound and marker regions and inclu-
sion of 4 centimetres (cm) of periwound skin in the
captured image. A mobile application incorporating
the proposed pipeline was implemented and tested on
several images, enabling a real-time, automated and
smooth process for image acquisition and assessment.
Therefore, this work advances the previous state-
of-the-art approaches by providing a detection model
for simultaneous wound and reference marker local-
isation, taking advantage of its potentialities to pro-
pose a simple yet effective strategy for validating
the adequacy of wound images, and integrating this
pipeline in a user-friendly mobile solution that aims
to facilitate monitoring procedures while ensuring the
quality of the wound images acquired.
2 METHODOLOGY
The proposed approach to automate the wound im-
age acquisition relies on the application of deep learn-
ing detection models for the correct localisation of
the wound bed and the reference marker in the field
of view, in association with a domain-adapted post-
processing procedure to verify the presence of all the
relevant structures - open wound, marker and peri-
wound skin. This strategy ensures that all the in-
formation needed for a complete visual assessment
of the wound status and the automated extraction of
wound properties from the image are present in the
ICT4AWE 2023 - 9th International Conference on Information and Communication Technologies for Ageing Well and e-Health
254

captured images, providing a useful tool for image
acquisition both for manual clinical monitorisation of
wounds and for automated assessment solutions.
For the development of this methodology, a novel
dataset - the Clinical Wound Support (CWS) dataset
- was constructed, as described in section 2.1. The
succeeding sections detail the hyperparameter tuning
process used to find the most promising model (sec-
tion 2.2.1), as well as the dataset preparation for the
training of the detection models (section 2.2.2), the
implementation of the adequacy validation module
(section 2.3) and the integration of the whole acqui-
sition pipeline in a mobile application (section 2.4).
2.1 Dataset Construction
The scarce datasets with reference markers available
in the literature are based on complex and expensive
markers and do not provide annotations regarding the
marker regions (Yang et al., 2016). Therefore, a novel
dataset comprising images of wounds and reference
markers was constructed. The images were acquired
by clinical professionals from nine Portuguese health-
care institutions using a smartphone application de-
veloped for this purpose using commercially available
adhesive reference markers
1
. These reference mark-
ers were selected due to their ready accessibility, ease
of usage and standardised size (2 × 2 cm).
The dataset comprises monitorisations of differ-
ent types of chronic wounds: diabetic foot ulcers,
pressure ulcers and leg ulcers (venous, arterial and
mixed), among others. Although that was not always
possible, in most cases, the wound was followed up
weekly over a 4-week period, resulting in a maximum
of 5 monitorisations per wound to capture varying
healing states of each type of wound. The resulting
images were annotated in terms of wound and marker
boundaries by three specialists in the field of wound
care, each responsible for a distinct subset of wound
images. The dataset distribution is presented in ta-
ble 1, and annotated examples are depicted in fig. 1.
Table 1: Composition of the acquired CWS dataset, in terms
of wounds, monitorisations and captured images.
Wound
type
Pressure
ulcer
Venous
leg ulcer
Arterial
leg ulcer
Leg ulcer of
unknown
etiology
Diabetic
foot ulcer
Other
ulcer
types
Total
No. wounds 29 10 2 4 5 1 51
No. monitor. 61 36 7 18 17 6 145
No. images 65 52 8 20 17 6 168
2.2 Model Development
The wound and marker localisation module was de-
veloped using a RetinaNet detection architecture with
1
https://www.apli.com/en/product/18326
Figure 1: Illustrative examples of skin wound images and
respective ground truth localisation (open wound in cyan
and reference marker in green).
a MobileNetV2 backbone network. MobileNetV2
(Sandler et al., 2018) belongs to a family of CNNs
optimised for mobile deployment through the appli-
cation of lightweight filtering operations and an in-
verted residual structure, being able to provide com-
petitive performances with a reduced number of pa-
rameters and a lower computational complexity. Reti-
naNet is a one-stage detector that combines off-the-
shelf CNNs with a feature pyramid network to enable
the detection of objects at multiple scales and uses a
focal loss function to account for possible data imbal-
ances during training (Lin et al., 2018). The Tensor-
Flow Python implementation of the model was used,
resorting to the Object Detection API for model train-
ing and optimisation. This model was selected con-
sidering the need for deployment in a mobile scenario,
as it offers a good trade-off between detection per-
formance and inference time while being easily ex-
ported to a smartphone-compatible format (Tensor-
Flow Lite
2
). This was assessed based on the bench-
marks in (Huang et al., 2017; Ten, 2021).
2.2.1 Model Optimisation
The detection model configuration was optimised us-
ing a random search hyperparameter tuning process,
applied instead of grid search optimisation due to time
constraints. Six different batch sizes and learning rate
combinations were analysed. These two hyperparam-
eters were selected for tuning due to their high im-
pact on the training results. The corresponding values
were randomly sampled from adequate ranges based
on the recommendations of (Bengio, 2012). Batch
sizes of 8 and 16 were considered, as these provide
sufficient training instances in each learning itera-
tion while complying with the dynamic memory con-
straints of the hardware used. The learning rate val-
ues were sampled from a logarithmically scaled range
between 10
−6
and 0.01; a constant learning rate was
considered for all the iterations of each experiment.
Also, in view of the impact of the input image size on
the computational demands of the detection models
and the other processing steps, two image sizes were
assessed: 320 × 320 and 512 × 512 pixels. These hy-
perparameter combinations were tested for both input
2
https://www.tensorflow.org/lite
Leveraging Deep Neural Networks for Automatic and Standardised Wound Image Acquisition
255

Table 2: Hyperparameter values tested for the six experi-
ments conducted to optimise the detection model.
Experiment Batch size Learning rate Image size
1 8 7.56463E-03 320 / 512
2 8 1.12332E-05 320 / 512
3 16 5.59081E-04 320 / 512
4 8 6.57933E-05 320 / 512
5 8 1.14976E-04 320 / 512
6 16 7.56463E-03 320 / 512
sizes, resulting in the experiments shown in table 2.
A 3-fold cross-validation was followed for the hy-
perparameter tuning experiments, with details regard-
ing the data preparation being provided in the next
section. The evaluation of the tuning results was con-
ducted on the validation subsets of each fold, consid-
ering performance metrics appropriate for object de-
tection tasks - mAP and average recall (AR), com-
puted for IOU thresholds of 0.50 and 0.75 in the
case of mAP, and for a maximum number of 10 de-
tections for AR. A higher focus was attributed to
mAP@.75IOU because the higher IOU threshold im-
posed leads to a more representative metric of the
model’s ability to provide accurate regions of inter-
est; mAP@.50IOU was evaluated for the state-of-the-
art comparison (Goyal et al., 2018; Faria et al., 2020;
Anisuzzaman et al., 2022a; Scebba et al., 2022).
Each model was optimised by applying the Adam
optimisation algorithm, using the Huber regression
and focal cross-entropy functions for the localiza-
tion and classification losses, respectively. Although
each model was trained for 5000 epochs, during this
process, its validation performance was periodically
monitored, and the network parameters that yielded
the optimal validation mAP@.75IOU were consid-
ered as final for the respective experiment to pre-
vent overfitting. The network was initialised using
the parameters resulting from pre-training in the pub-
lic COCO dataset (Lin et al., 2014). All the non-
mentioned training conditions and hyperparameters
were set to default values provided with the model
implementation, as specified in (Con, 2022).
2.2.2 Data Preparation
To develop the model, 75% of the dataset was used
for training, with the remaining 25% being separated
as test data for the final model assessment. For the
hyperparameter tuning experiments, the training data
was split into three distinct folds of train and valida-
tion sets. The division of the data instances in the sev-
eral subsets was performed in a stratified manner, at
the level of the wounds and not the images, to ensure
that all images of each wound were kept in the same
subset and that the training and test subsets comprise
representative examples of each wound type, body
location and skin phototype. This process resulted
in a distribution of 98 training images from 29 dif-
ferent wounds and 70 test images from 22 wounds.
The images, already square as a result of their ac-
quisition process, were resized according to the input
size needed for the trained network. Geometric image
transformations, in particular 90
◦
, 180
◦
and 270
◦
ro-
tations, and vertical and horizontal flips, were applied
dynamically during training to augment the instances.
2.2.3 Final Model Training and Mobile
Deployment
The final model configuration was identified as the
one that resulted in the highest performance met-
rics. After the determination of the best experience
from hyperparameter tuning, the detection model was
re-trained using the whole training data considering
the corresponding hyperparameter values, for a fixed
number of epochs. The number of training epochs
was established as the average number of epochs
needed for the convergence of the models trained for
each cross-validation fold of the selected experiment.
The trained model parameters were then quantised
and optimised for mobile deployment using the Ten-
sorflow Lite framework. The best model of each input
size was deployed in a mobile device and compared
to assess its influence on the model’s inference speed
and its impact on the mobile acquisition workflow.
2.3 Image Adequacy Validation
The validation of the image adequacy was performed
based on the outputs of the detection model by as-
sessing if both the open wound and reference marker
were detected. In addition to the open wound, the
surrounding area, known as the periwound area, also
contains important information regarding the state of
the wound. Therefore, there is a need to ensure that
the captured image also contains this area. Following
experts’ recommendation, 4 cm in all directions of the
open wound border were considered.
Given the marker size of 2× 2 cm, the largest side
of the detected marker’s bounding box was used as
a reference, ensuring that, even if the marker is not
perpendicular to the camera, the side closer to 2 cm
will be used. Then, the verification is performed by
ensuring that there is a distance of at least 2 times
the reference is present around the four sides of the
wound’s bounding box.
ICT4AWE 2023 - 9th International Conference on Information and Communication Technologies for Ageing Well and e-Health
256

2.4 Mobile Application
The methodology presented in the previous subsec-
tions was deployed as an Android application running
on a smartphone with the aim of supporting health-
care professionals in wound monitoring procedures.
This application allows the automatic acquisition
of skin wound images in an easy and intuitive manner
while providing real-time feedback about the wound
bed and marker’s localisation and guaranteeing the
proper periwound area presence (see fig. 2).
Figure 2: Automatic wound image acquisition diagram.
The user is first guided to centre the skin wound
in the camera feed through a central target while the
wound and marker localisation module is running
(fig. 3 left). In the detection module, the raw im-
ages are cropped to a square at its centre with a height
equal to its total width and resized to the model’s in-
put size (pre-processing, see fig. 2). Once the de-
tection is complete, the user receives the indication
through the left icon of the screen, and the adequacy
validation module (post-processing block in fig. 2)
starts running (fig. 3 right). The detection and ad-
equacy validation modules keep running frame by
frame until either one frame fails the detection (there-
fore restarting the process) or the acquisition is suc-
cessfully completed. An image is automatically ac-
quired when 20 consecutive frames or all the frames
acquired in the span of 1250ms detect a wound and
a marker with a score of at least 50% each. In case
the developed modules fail, the user is always capa-
ble of manually acquiring an image by clicking on the
bottom right button on the screen. After the acquisi-
tion, to make sure the user did not move after the last
frame was processed, the acquired image is also veri-
fied with the detection and adequacy validation mod-
ules. In case of failure, the user has the option to retry
the acquisition or to still continue with the previously
acquired image.
3 RESULTS AND DISCUSSION
This section presents the results of the model optimi-
sation experiments, as well as an in-depth analysis of
the best-performing model in terms of detection per-
formance and mobile inference metrics.
Figure 3: Application screenshots of real-time wound local-
ization (left) and image adequacy validation (right).
3.1 Hyperparameter Tuning
To identify the most adequate hyperparameter com-
bination, the performance achieved in the differ-
ent experiments conducted for each image size was
compared in terms of mAP@.75IOU and AR@10,
for each detected class (open wound or reference
marker) and total. The average cross-validation
mAP@.75IOU of the hyperparameter tuning experi-
ments is presented in fig. 4. Although the AR@10
results were also taken into account in the com-
parative analysis of the six experiments, those val-
ues exhibited a similar tendency as the ones of
mAP@.75IOU and are not graphically represented.
Figure 4 shows that all experiments resulted in a sim-
ilar performance for the reference marker detection,
so the best hyperparameter combination was selected
based mostly on the wound detection ability provided.
Thus, through the analysis of the values reported for
the wound class, experiment 5 yielded the highest
mAP@.75IOU and AR@10 metrics for both image
sizes, so the associated hyperparameters were consid-
ered for the final detection model implementation.
For this training setup, the model trained on
the 320 × 320 images obtained cross-validation
mAP@.75IOU and AR@10 values of 0.46699 ±
0.01170 and 0.48186 ± 0.03115 for the wound class,
and 0.99010 ± 0.01400 and 0.83523 ± 0.00923 re-
garding the detection of the marker. Similarly,
the detection performance of the 512 × 512 model
resulted in mAP@.75IOU and AR@10 metrics of
0.46107 ± 0.04047 and 0.51709 ± 0.06306 for the
open wounds, and 0.99928 ± 0.00102 and 0.86706 ±
0.00871 for the marker class. Concerning the
cross-validation mAP@.50IOU for the open wound
class, the 320 × 320 network achieved an average of
0.66188 ± 0.05608, while the 512 × 512 model ob-
tained 0.78117 ± 0.11202; the mAP@.50IOU values
for the marker class are approximately equal to the
Leveraging Deep Neural Networks for Automatic and Standardised Wound Image Acquisition
257
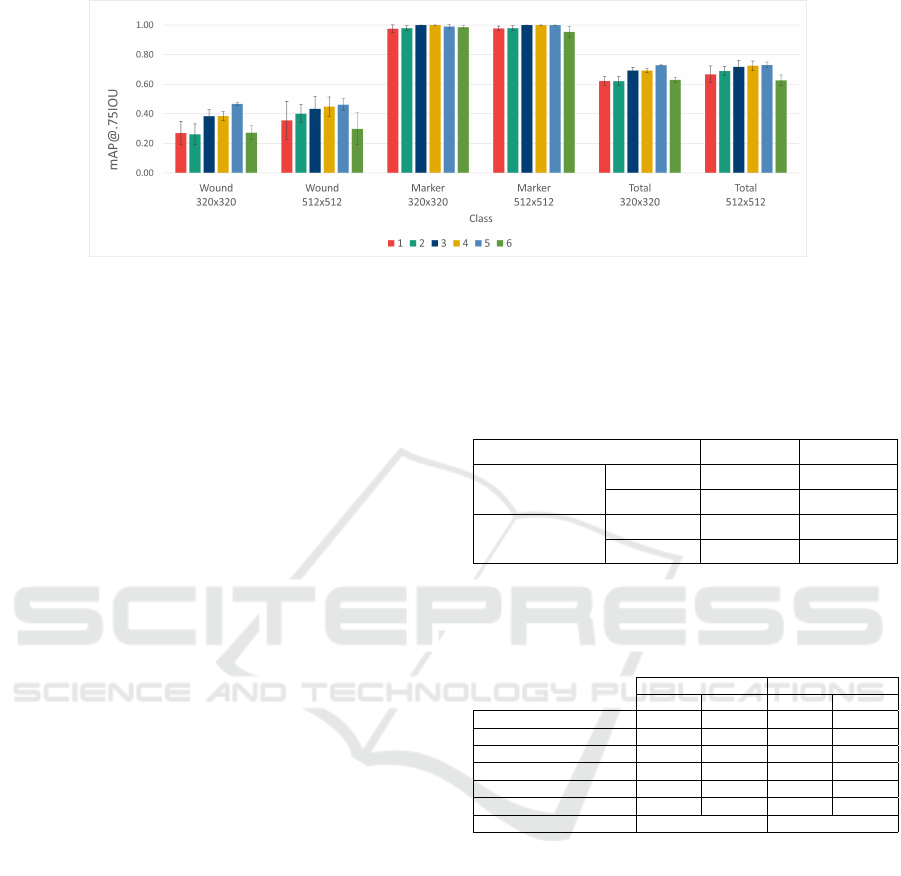
Figure 4: Average class-wise 3-fold cross-validation mAP@.75IOU results obtained with the different hyperparameter set-
tings tested in each experiment for the two image sizes analysed. The represented values were obtained as the average
validation results for the three cross-validation splits. The total mAP was determined by averaging the results of the two
classes (wound and marker). The error bars correspond to the standard deviation over all splits.
mAP@.75IOU values reported. Both models exhibit
superior performance for marker detection in relation
to the open wound class, possibly due to the simplic-
ity of the marker shape and the lower variability of
the marker’s regions in the dataset. Moreover, de-
spite providing good performance for the identifica-
tion of wounds in the images (materialised in the high
mAP@.50IOU values achieved), the models demon-
strated an impaired ability to accurately distinguish
the region of interest corresponding to the whole open
wound, as indicated by the lower mAP obtained when
imposing a stricter IOU threshold of 0.75.
3.2 Selection of the Best Model
Table 3 shows the test results of the final RetinaNet
detection model for each class after being re-trained
on the whole training set with the optimal hyperpa-
rameter set-up (batch size of 8 and learning rate of
1.14976 × 10
−4
). Comparing the test metrics with
the cross-validation results presented in the previ-
ous section, we can observe that there was a general
decline in the models’ performance, both in terms
of detection accuracy and sensitivity (reflected by
the mAP@.50IOU and AR@10 metrics, correspond-
ingly), even though this deterioration was less se-
vere in the case of the marker class. This difference
may be due to the occurrence of overfitting during
model training or to the variability of properties in
the training and test set images. Moreover, the dis-
crepancy between the cross-validation and test perfor-
mances was more expressive for the 320 × 320 model.
Therefore, despite the similar cross-validation results
demonstrated by the models trained with the two im-
age sizes, in the test set the 512 × 512 demonstrated
considerably higher detection robustness.
To assess the applicability of each detection model
for real-time usage, both models were deployed on
Table 3: Class-wise detection performance of the best mod-
els, re-trained in the overall training set and evaluated using
the test data from the CWS dataset.
Evaluated metric/ Image size 320 × 320 512 × 512
mAP@.75IOU
Wound 0.23712 0.39141
Marker 0.92079 0.95050
AR@10
Wound 0.36563 0.47031
Marker 0.79412 0.83971
Table 4: Processing times (in milliseconds) of the differ-
ent steps of the image acquisition module using the best-
performing models associated with each input image size,
assessed in mid and high-range (MR, HR) smartphones.
320 × 320 512 × 512
MR HR MR HR
Total time per frame 132.00 103.43 393.52 274.79
Pre-processing 1.21 1.28 1.39 1.47
Detection inference 130.26 101.53 391.41 272.76
Adequacy validation 0.00 0.00 0.06 0.02
Total acquisition time 1313.98 1296.13 1400.63 1394.67
Avg. no. frames per acq. 12 12 5 6
Model size 10.9 MB 11.2 MB
two mobile devices (mid and high range), namely Xi-
aomi Poco X3 NFC and Samsung S21. Since the ac-
quisition of each image involves the analysis of sev-
eral camera frames by the application, the time re-
quired by each processing step - the pre-processing
to get compatible image shape and dimensions, the
detection model’s inference and the validation of the
image structures’ adequacy (open wound, reference
marker and periwound area) - was examined for all
the frames analysed, along with the total acquisition
time and the average number of frames processed for
each image acquired. The results of the mobile as-
sessment are presented in table 4. This evaluation was
carried out by using the developed image acquisition
application to capture images of the test set images.
From the times presented for each step, it is pos-
sible to verify that the inference time of the detection
ICT4AWE 2023 - 9th International Conference on Information and Communication Technologies for Ageing Well and e-Health
258
model is the most time-consuming step of the acqui-
sition process, being the major contributor to the time
taken to analyse each frame and, subsequently, for the
whole image acquisition flow. Despite the higher in-
ference times required by the 512 × 512 model (in re-
lation not only to the 320 × 320 model but also to the
approach described in (Faria et al., 2020)), it is still
able to provide a responsive and smooth acquisition
process in both high and mid-range devices, with a
sufficient amount of evaluated frames in the stipulated
acquisition time. This, in combination with its supe-
rior detection performance in the test set, motivated
its selection as the final model to be deployed in the
final acquisition application.
3.3 Final Model Assessment
To allow the comparison with the detection ap-
proaches described in the state of the art and men-
tioned in section 1, the mAP@.50IOU metric com-
puted for the wound class was also analysed. Compar-
ing the cross-validation mAP@.50IOU of the devel-
oped model (0.78117±0.11202) with the correspond-
ing performances reported in (Goyal et al., 2018;
Faria et al., 2020) (0.918 and 0.865), it is possible to
verify that, despite the lower metric value achieved,
it is associated with a similar magnitude order. In
contrast, the obtained test metric (0.66829) exhibits
a greater deviation in relation to the test performances
of (Anisuzzaman et al., 2022a; Scebba et al., 2022)
(0.939 and 0.762), which might be due to the pres-
ence of borderline wound cases in the test set, further
discussed below. Still, the direct comparison of these
metrics does not provide a fair analysis of the differ-
ent models’ performance since they were evaluated in
different datasets, whose information is not available.
Moreover, the models proposed in other works were
solely focused on the detection of the open wound
area, while this work provides a model that is able
to detect directly both the open wound and reference
marker. Hence, future experiments should be con-
ducted to enable a more even comparison, by training
or at least evaluating the models in the same dataset.
In addition, to validate the detection ability of
the network selected as the final model, its predic-
tion results in the test images were analysed in detail.
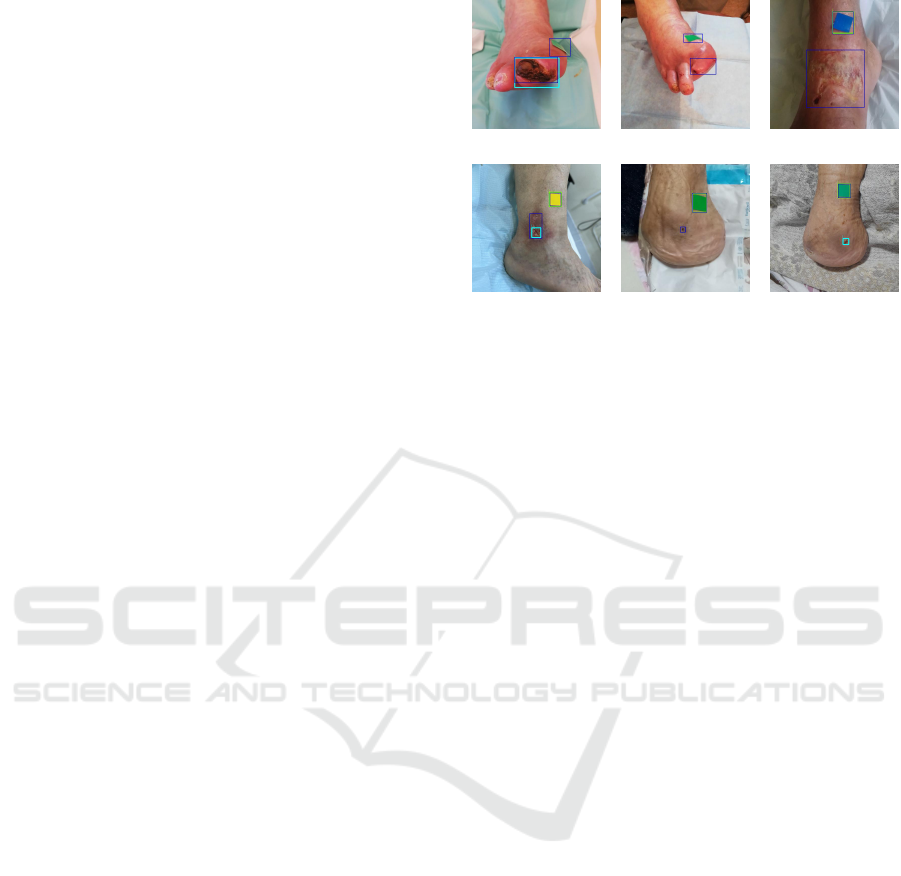
Figure 5 comprises instances for which the detection
of the model was not as satisfactory, whereas fig. 6
presents examples of correct detections.
The inspection of the test predictions shows that
the model’s detection ability is flawed under very
specific acquisition conditions, providing insights re-
garding the possible causes of the inferior test perfor-
mance displayed. Particularly, it demonstrated diffi-
(a) (b) (c)
(d) (e) (f)
Figure 5: Examples of test images that highlight the perfor-
mance limitations of the 512 × 512 model. These include
images acquired in a skewed perspective (a and b), superfi-
cial wounds (c and d) and wounds in the open/healed tran-
sition (e and f). The ground truth annotations are marked
in dark blue, while light blue and green boxes outline the
wound and marker regions detected by the network.
culty in correctly detecting the marker and/or wound
region when the image is captured from a skewed per-
spective, a common occurrence in diabetic foot ulcer
cases, as showed in figs. 5a and 5b. In some cases
of more superficial leg ulcers, especially with hetero-
geneous lighting conditions and the presence of re-
flections, the model is not capable of detecting the
wound region (fig. 5c), or provides only a partial de-
tection that does not include the whole region of in-
terest (fig. 5d), which might affect the verification of
the periwound area in the photo and be an obstacle to
the usage of this information to further extract wound
properties in automated systems. Notwithstanding,
these limitations can be overcome in the acquisition
moment by using adequate lighting and adjusting the
smartphone’s positioning in relation to the wound.
The model also misdetected wounds that were re-
cently healed or close to healing, by incorrectly de-
tecting the scab region in fig. 5f as an open wound
and by missing the detection of the small wound re-
gion in fig. 5e. It is worth highlighting that these cases
are ambiguous even for trained clinical experts, so this
limitation is not critical for a practical scenario.
In spite of failing in some of the described circum-
stances, the model presented a satisfactory detection
performance in a varied set of conditions, being able
to detect both the wound and marker in images ac-
quired from a skewed perspective (fig. 6c) and in-
consistent lighting (such as the shadows present in
fig. 6d). It demonstrated a consistent detection ability
for the multiple wound types and body locations of
the dataset - observable in the detections for hip pres-
sure ulcers (figs. 6a, 6d and 6e), leg ulcers (fig. 6b)
Leveraging Deep Neural Networks for Automatic and Standardised Wound Image Acquisition
259

(a) (b) (c)
(d) (e) (f)
Figure 6: Examples of test images with correct predictions
generated by the 512 × 512 model in varied settings. These
comprise images from different wound types and locations
(hip pressure ulcers in a, d and e, leg ulcer in b and di-
abetic foot ulcer in c), and images with healed wounds (f).
The ground truth annotations are marked in dark blue, while
light blue and green boxes outline the wound and marker re-
gions detected by the network.
and diabetic foot ulcers (fig. 6c) -, while showing a
reduced number of false positives, as it is possible
to observe by the lack of wound area detections in
images without open wounds (fig. 6f). Remarkably,
notwithstanding the low representation of higher pho-
totypes in the dataset, it also maintained a good detec-
tion ability for varying skin phototypes (displayed by
the heterogeneous types of skin in fig. 6), evidencing
its robustness and suitability for usage in clinical sce-
narios.
Thus, regardless of the apparently superior perfor-
mances reported in the literature, the developed model
was able to locate the wound and reference marker
regions consistently for different image acquisition
conditions. Its combination with the image valida-
tion module and the integration of this pipeline in a
mobile-based solution make this the first methodol-
ogy (to the authors’ knowledge) to exploit the poten-
tial of detection architectures to ensure wound image
adequacy in the acquisition process, providing a real-
time acquisition system that captures images with suf-
ficient information for the analysis of wound healing
by human experts and for the development of auto-
mated wound characterisation algorithms.
4 CONCLUSIONS
This work presented a pipeline for the automation
of wound image acquisition and adequacy validation,
which leverages the potential of deep detection neural
networks to guarantee that relevant skin regions are
captured while providing a metric reference, both el-
ements of great utility to support further wound anal-
ysis tasks.
A deep neural network - RetinaNet with a Mo-
bileNetV2 backbone - was used to detect the open
wound region and a reference marker, to be used as
a metric reference. This model was developed using
a new dataset of wounds and markers of diverse types
and characteristics. Two models associated with dif-
ferent image sizes of 320 × 320 and 512 × 512 were
tuned and deployed in mobile devices. Their compari-
son showed that the 512 × 512 model offers a superior
detection performance while still providing inference
times suitable for real-time image acquisition, being
selected as the final model. Although this model
yielded detection metrics slightly inferior to those re-
ported in the literature for similar models (evaluated
in other datasets), it demonstrated a satisfactory abil-
ity to detect wounds and markers in a variety of ac-
quisition conditions, evidencing its robustness.
The developed detection model was used as the
basis for an image adequacy validation module, re-
sponsible for ensuring the presence of the reference
marker, wound area and recommended 4 cm radius of
periwound skin (validated using the marker’s metric
reference) in the captured picture. A mobile appli-
cation incorporating both modules was implemented
and tested for the acquisition of several wound im-
ages. This assessment showed that the proposed ap-
proach contributed to the acquisition of adequate im-
ages through an easily usable interface that stream-
lines the acquisition process. Nonetheless, the peri-
wound radius verification could be improved by get-
ting a more rigorous marker segmentation and using
the resulting sides or diagonal as a metric reference
instead of its bounding box, as well as resorting to
perspective correction methods to account for possi-
ble image distortions.
In spite of the identified refinement possibilities,
the integration of the developed detection network
with the image adequacy validation module provides
a powerful tool to assist wound image acquisition, en-
abling its standardisation. Consequently, it facilitates
the acquisition of more adequate images, with the rep-
resentation of all the structures relevant for wound
healing assessment, offering an invaluable asset for
both the visual inspection by clinical experts and the
automated wound properties extraction in more so-
phisticated systems. Although its accuracy in the
wound region identification should be improved for
the latter application scenario (through the expansion
of the dataset with private or public images or the ex-
ploration of new detection architectures), the robust-
ness demonstrated in varied acquisition settings, com-
ICT4AWE 2023 - 9th International Conference on Information and Communication Technologies for Ageing Well and e-Health
260

bined with its user-friendly, responsive mobile inter-
face, proved it is suitable for usage by clinical profes-
sionals in real-world scenarios.
ACKNOWLEDGEMENTS
This work was done under the scope of Clini-
calWoundSupport - ”Wound Analysis to Support
Clinical Decision” project (POCI-01-0247-FEDER-
048922), according to Portugal 2020 is co-funded
by the European Regional Development Fund from
European Union, framed in the COMPETE 2020.
The authors would like to thank Paula Teixeira from
Unidade Local de Sa
´
ude de Matosinhos for the col-
laboration in the data annotation process.
REFERENCES
(2021). TensorFlow 2 Detection Model Zoo. https://
github.com/tensorflow/models/blob/master/research/
object detection/g3doc/tf2 detection zoo.md.
(2022). Configuration settings for the mod-
els of the tensorflow object detection API.
https://github.com/tensorflow/models/tree/master/
research/object detection/configs/tf2.
Anisuzzaman, D., Patel, Y., Niezgoda, J. A., Gopalakr-
ishnan, S., and Yu, Z. (2022a). A mobile app for
wound localization using deep learning. IEEE Access,
10:61398–61409.
Anisuzzaman, D., Wang, C., Rostami, B., Gopalakrishnan,
S., Niezgoda, J., and Yu, Z. (2022b). Image-based
artificial intelligence in wound assessment: A system-
atic review. Advances in Wound Care, 11(12):687–
709.
Bengio, Y. (2012). Practical recommendations for gradient-
based training of deep architectures. In Neural net-
works: Tricks of the trade, pages 437–478. Springer.
Chen, L., Cheng, L., Gao, W., Chen, D., Wang, C., Ran, X.,
et al. (2020). Telemedicine in chronic wound man-
agement: systematic review and meta-analysis. JMIR
mHealth and uHealth, 8(6):e15574.
Cui, C., Thurnhofer-Hemsi, K., Soroushmehr, R., Mishra,
A., Gryak, J., Dom
´
ınguez, E., Najarian, K., and
L
´
opez-Rubio, E. (2019). Diabetic wound segmenta-
tion using convolutional neural networks. In 2019
41st Annual International Conference of the IEEE En-
gineering in Medicine and Biology Society (EMBC),
pages 1002–1005. IEEE.
Faria, J., Almeida, J., Vasconcelos, M. J. M., and Rosado, L.
(2020). Automated mobile image acquisition of skin
wounds using real-time deep neural networks. In Med-
ical Image Understanding and Analysis: 23rd Confer-
ence, MIUA 2019, Liverpool, UK, July 24–26, 2019,
Proceedings 23, pages 61–73. Springer.
Goyal, M., Reeves, N. D., Rajbhandari, S., and Yap, M. H.
(2018). Robust methods for real-time diabetic foot
ulcer detection and localization on mobile devices.
IEEE journal of biomedical and health informatics,
23(4):1730–1741.
Huang, J., Rathod, V., Sun, C., Zhu, M., Korattikara, A.,
Fathi, A., Fischer, I., Wojna, Z., Song, Y., Guadar-
rama, S., et al. (2017). Speed/accuracy trade-offs for
modern convolutional object detectors. In Proceed-
ings of the IEEE conference on computer vision and
pattern recognition, pages 7310–7311.
Lin, T., Goyal, P., Girshick, R., He, K., and Dollar, P.
(2018). Focal loss for dense object detection. IEEE
Transactions on Pattern Analysis and Machine Intel-
ligence, 42(2):318–327.
Lin, T.-Y., Maire, M., Belongie, S., Hays, J., Perona, P.,
Ramanan, D., Doll
´
ar, P., and Zitnick, C. L. (2014).
Microsoft coco: Common objects in context. In Euro-
pean conference on computer vision, pages 740–755.
Lu, H., Li, B., Zhu, J., Li, Y., Li, Y., Xu, X., He, L., Li,
X., Li, J., and Serikawa, S. (2017). Wound intensity
correction and segmentation with convolutional neu-
ral networks. Concurrency and computation: practice
and experience, 29(6):e3927.
Martinengo, L., Olsson, M., Bajpai, R., Soljak, M., Upton,
Z., Schmidtchen, A., Car, J., and J
¨
arbrink, K. (2019).
Prevalence of chronic wounds in the general popula-
tion: systematic review and meta-analysis of observa-
tional studies. Annals of epidemiology, 29:8–15.
Medetec (2014). Wound database. data re-
trieved from http://www.medetec.co.uk/files/
medetec-image-databases.html.
Mukherjee, R., Tewary, S., and Routray, A. (2017). Di-
agnostic and prognostic utility of non-invasive multi-
modal imaging in chronic wound monitoring: a sys-
tematic review. Journal of medical systems, 41:1–17.
Passadouro, R., Sousa, A., Santos, C., Costa, H., and
Craveiro, I. (2016). Characteristics and prevalence of
chronic wounds in primary health care. Journal of the
Portuguese Society of Dermatology and Venereology,
74(1):45–51.
Sandler, M., Howard, A., Zhu, M., Zhmoginov, A., and
Chen, L.-C. (2018). Mobilenetv2: Inverted residu-
als and linear bottlenecks. In Proceedings of the IEEE
conference on computer vision and pattern recogni-
tion, pages 4510–4520.
Scebba, G., Zhang, J., Catanzaro, S., Mihai, C., Dis-
tler, O., Berli, M., and Karlen, W. (2022). Detect-
and-segment: A deep learning approach to automate
wound image segmentation. Informatics in Medicine
Unlocked, 29:100884.
Sen, C. K. (2021). Human wound and its burden: updated
2020 compendium of estimates. advances in wound
care, 10(5):281–292.
Werdin, F., Tennenhaus, M., Schaller, H.-E., and Ren-
nekampff, H.-O. (2009). Evidence-based manage-
ment strategies for treatment of chronic wounds.
Eplasty, 9.
Yang, S., Park, J., Lee, H., Kim, S., Lee, B.-U., Chung,
K.-Y., and Oh, B. (2016). Sequential change of
wound calculated by image analysis using a color
patch method during a secondary intention healing.
PloS one, 11(9):e0163092.
Leveraging Deep Neural Networks for Automatic and Standardised Wound Image Acquisition
261
