
Fundus Unimodal and Late Fusion Multimodal Diabetic Retinopathy
Grading
Sara El-Ateif
1a
and Ali Idri
1,2 b
1
Software Project Management Research Team, ENSIAS, Mohammed V University in Rabat, Morocco
2
AlKhwarizmi College of Computing, Mohammed VI Polytechnic University, Marrakech-Rhamna, Benguerir, Morocco
Keywords: Diabetic Retinopathy, Fundus, Multiclass, Late Fusion, Efficientnet, Swin Transformer,
Hybrid-EfficientNetB0-SwinTF.
Abstract: Diabetic Retinopathy (DR) is an eye disease with complications, if left untreated grow, split into four grades:
mild, moderate, severe, and proliferative. We propose to (1) compare and evaluate three different recently
used deep learning models: EfficientNet-B5, Swin Transformer, and Hybrid-EfficientNetB0-SwinTF (HES)
on the APTOS 2019 dataset’s fundus and early fused (EF) weighted gaussian blur fundus. (2) Evaluate three
fine-tuning and pre-processing schemes on the best model. And (3) choose the best model-scheme per
modality and perform late fusion on them to get the final DR grade. Results show that our best method, late
fusion HES model, results in F1-socre of 81.21%, accuracy of 81.83%, and AUC of 96.30%. We propose
using late fusion HES model in population-wide diagnosis to assist doctors in Morocco to reduce DR burden.
1 INTRODUCTION
Diabetic Retinopathy (DR) an eye disease caused by
diabetes, as of 2015, has affected 1 over 3 people
according to the Moroccan League for the Fight
against Diabetes. In 2018, the Moroccan Ministry of
Health and Social Protection announced that more
than 2 million citizens aged 18 and over are diabetic,
with 50% unaware of the disease, and an estimation
of 15,000 children affected. To detect such a disease,
doctors perform routine eye screening using Slit-lamp
– a tool that is rarely found in middle-income
countries. Recent advances in technology brought a
respectably accurate and affordable tool called digital
fundus photography (Begum et al., 2021). DR affects
a high number of citizens and it keeps growing as the
number of diabetic patients grows, diagnosing it early
helps avoid blindness and further complications (Teo
et al., 2021). The solution resides in a wide spread
diagnosis which is difficult to perform using
restricted resources. This fact pushed researchers to
investigate the use of artificial intelligence to help
increase and speed up the diagnostic process
(Sebastian et al., 2023). Most frequently used deep
learning (DL) models for DR grading (i.e.,
a
https://orcid.org/0000-0003-1475-8851
b
https://orcid.org/0000-1111-2222-3333
classification into no DR, mild, moderate, severe, or
proliferative DR) listed in (Sebastian et al., 2023),
between 2017 and 2022, are: ResNet, VGG,
InceptionV3, DenseNet, and EfficientNet based
models; with EfficientNet being more present in
2022. Using the APTOS 2019 dataset (APTOS)
(APTOS 2019 Blindness Detection | Kaggle, n.d.) of
fundus images for DR grading, (Shahriar Maswood et
al., 2020) train EfficientNet-B5 by optimizing the
Quadratic Weighted Kappa (QWK) score and Mean
Squared Error, and achieve an accuracy of 94.02%.
(Karki & Kulkarni, 2021) train EfficientNet (B1 to
B6) using different image resolutions, select the best
of these variants (i.e., B1, B2, B3, B5), and use a
weighted ensemble of these variants for the final
prediction to achieve a QWK of 0.924377. (Canayaz,
2022) train EfficientNet and DenseNet models for
feature extraction (results in 512 features). Then feed
these extracted features to the wrapper methods to
reduce the number of these features. Finally, the
newly chosen features are fed to the SVM and
Random Forest algorithms for classification. Using
the Kaggle DR dataset, (Lazuardi et al., 2020)
preprocess the fundus images using the Contrast
Limited Adaptive Histogram Equalization (CLAHE)
El-Ateif, S. and Idri, A.
Fundus Unimodal and Late Fusion Multimodal Diabetic Retinopathy Grading.
DOI: 10.5220/0012048700003541
In Proceedings of the 12th International Conference on Data Science, Technology and Applications (DATA 2023), pages 219-226
ISBN: 978-989-758-664-4; ISSN: 2184-285X
Copyright
c
2023 by SCITEPRESS – Science and Technology Publications, Lda. Under CC license (CC BY-NC-ND 4.0)
219

and center-cropping steps and combine them with
progressive resizing training process using the
EfficientNet B4 and B5 architectures; and obtain an
accuracy of 83.88%. Recent work leverages the
robustness of vision transformer (ViT) models for DR
grading using different schemes (Gu et al., 2023; Sun
et al., 2021; Yao et al., 2022): (Sun et al., 2021)
propose a novel lesion-aware transformer (LAT)
using an encoder-decoder structure to jointly grade
and discover lesions. The novelty resides in using
weakly supervised lesion localization via the
transformer decoder, and lesion region importance
and diversity to learn specific lesion filters using only
image-level labels. On the Messidror-1 dataset, LAT
scored 96.3% on area under the curve (AUC). (Yao et
al., 2022) develop FunSwin, a Swin Transformer (Liu
et al., 2021) based model, and reduce data imbalance
by introducing data enhancements through rotation,
mirroring, CutMix (Yun et al., 2019) and MixUp
(Zhang et al., 2018). FunSwin scores an accuracy of
84.12% on the Messidor dataset. Gu et al. (Gu et al.,
2023) propose a model composed of two blocks:
feature extraction block (FFB) and a grading
prediction block (GPB). FFB uses the classical ViT
model to extract the features from the fundus images
with its fine-grained attention. The GBP module,
generates class-specific features using spatial
attention. The results on the DDR dataset (Li et al.,
2019) are an accuracy of 82.35%. In practice, to gain
further clarity, DR and fundus eye diseases diagnosis
require the use of other medical imaging modalities:
fluorescein angiography (FA), and Multicolor
imaging (MC). Few research works have been
developed to leverage some of these modalities
(Hervella et al., 2022; Song et al., 2021; Tseng et al.,
2020): (Tseng et al., 2020) propose two fusion
architectures similar to ophthalmologist’s diagnostic
process, late fusion and two-stage early fusion. Using
the lesion and severity classification models, the late
fusion approach combines these two models in
parallel using postprocessing; while the two-stage
early fusion combines them sequentially. These two
approaches are trained on three Taiwanese hospitals
datasets for DR severity grading, evaluated on the
Messidor-2 dataset and scored 84.29% in accuracy.
(Song et al., 2021) perform Multicolor DR detection
(DR, no DR) using the multimodal information
bottleneck network (MMIB-Net). The MMIB-Net
uses the information bottleneck theory to compute the
joint representation of different imaging modalities
collected using the Multicolor imaging tool a
confocal scanning laser ophthalmoscope (cSLO) that
generates 16 images (8 for each eye) composed of
Blue, Green, Infrared Reflectance and Combined-
Pseudo color fundus images. The proposed model
uses ResNet-50 as backbone and scored an accuracy
of 94.30% on private collected data. (Hervella et al.,
2022) perform DR grading using the fundus and FA
modalities, by first pre-training on the unlabeled
multimodal Isfahan dataset using their proposed
Multimodal Image Encoding (MIE) approach. Then,
using the fundus modality from a labeled dataset
(IDRiD and Messidor), the pre-trained model is fine-
tuned for DR grading. Finally, after pre-training and
fine-tuning, the neural network uses the fundus
modality to perform DR severity classification. On
IDRiD, MIE scores an accuracy of 65.05%.
From the studies listed previously, a trend of pre-
processing, use of large or private datasets, and
training of different architectures in parallel (model
ensembles or fusion) emerges. Meanwhile, for
multimodal models and datasets they are scarce and
in most cases the data is private; which could be
related to the high cost needed for collecting such
diversified modalities, storing them and ensuring
patients data privacy. Another new emerging trend
resides in the use of ViT models, due to their
robustness and efficient attention mechanisms. But
the classical ViT architectures require large amounts
of data and computing resources to train; enters Swin
Transformer, a ViT architecture optimized for
relatively small data and less demanding computing
resources. In this work we propose to leverage all of
the previously used powerful elements (i.e.,
preprocessing, relatively small dataset, ViT, and
multimodality) to train and compare the most recently
used EfficientNet architecture with Swin
Transformer and their hybrid combination: Hybrid-
EfficentNetB0-SwinTF (Henkel, 2021); using the
fundus, and the fusion of the Weighted Gaussian Blur
Fundus (WGBF) (El-Ateif & Idri, 2022) with the
fundus modality using the Discrete Wavelet
Transformation (Naik & Kunchur, 2020) to obtain the
early fused (EF) Fundus-WGBF modality. We
evaluate the performance of these three models on
this two different modalities using (1) six metrics:
accuracy, sensitivity, specificity, F1-score, and AUC;
along with (2) Scott-Knott Effect Size Difference
(SK-ESD) (Tantithamthavorn et al., 2019) statistical
test to cluster these models and find the best in terms
of F1-score; on (3) the APTOS dataset. Then we
further compare the best performing under different
settings: fine-tuning, and preprocessing. Finally, we
choose the most performant models per modality
(fundus and EF), fine-tune them, and combine them
using the late fusion approach.
In brief, the objective of this study is to evaluate
whether the integration of preprocessing techniques
DATA 2023 - 12th International Conference on Data Science, Technology and Applications
220
with multimodality and image fusion, trained on a
mid-sized dataset, could enhance the overall
performance.
The remainder of this paper is organized as
follows: Section 2 presents the data used and its
preparation process. In section 3, we detail our
approach. Section 4 lists our results and their
discussion. Finally, section 5 concludes the paper and
lays out our future work.
2 DATA PREPARATION
The models are trained and evaluated on the APTOS
2019 Blindness Detection dataset with the publicly
available labels. The dataset contains fundus images
provided by Aravind Eye Hospital in India and is split
into 5 DR grades: (grade 0) No DR counts 1,805,
(grade 1) Mild counts 370, (grade 2) Moderate counts
999, (grade 3) Severe counts 193, and (grade 4)
Proliferative DR counts 295 fundus images. Note that
for the models training we split these reported
numbers into: train (80% of 2930: 2344), validation
(20% of 2930: 586) and test (20%: 732) sets for the
1
st
and 2
nd
training phases, as for the 3
rd
phase we use
only the train (80%: 2930) and test (20%: 732) sets;
while replicating the class imbalance by using scikit-
learn stratified k-fold method. Additionally, we resize
the fundus images to 512x512 and remove the black
background to leave the fundus only. Moreover, to
avoid overfitting we perform for the Hybrid-
EfficentNetB0-SwinTF and late fusion models the
following data augmentations: horizontal flip, 0.1
height and width shift range, 20° rotation, 0.1 shear
range, and 0.1 zoom range. Finally, for all the models,
we normalize the data by dividing the pixels by 255
to keep them into the range of 0 to 255.
In the following we detail how we generated the
second modality early fused (EF) Fundus-WGBF
modality and expand on the pre-processing
performed to experiment with performance
enhancement of the best classified models for the
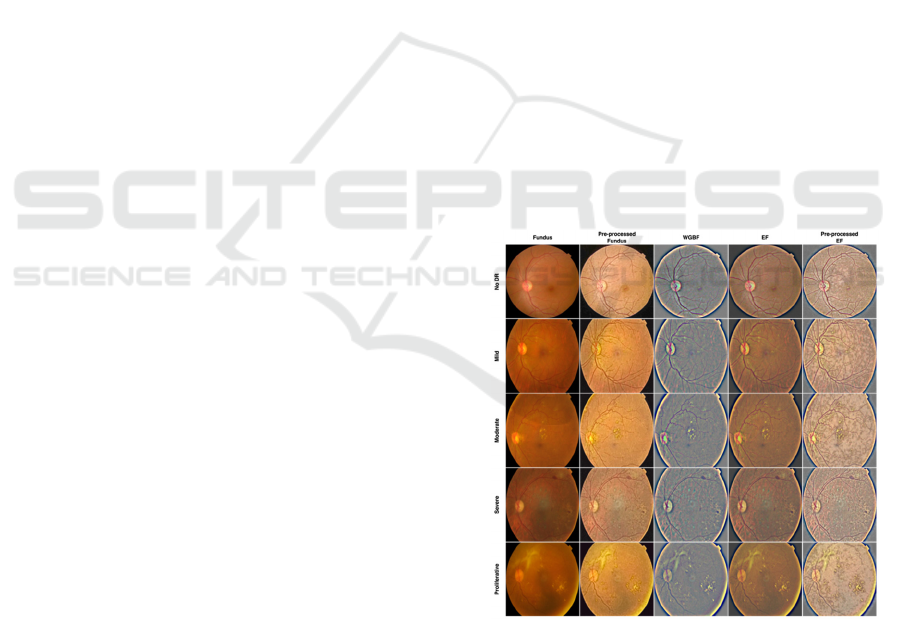
fundus and EF modalities. Figure 1 showcases all of
these processes by DR grade: fundus, its pre-
processing, WGBF, EF and its pre-processing.
2.1 Second Modality
To generate the early fused (EF) Fundus-WGBF
modality we at first generate the Weighted Gaussian
Blur Fundus (WGBF) from the fundus images using
the method followed in (El-Ateif & Idri, 2022): (1)
crop the image to remove the black background, (2)
resize the image to 512x512, and (3) apply the
Graham algorithm (Graham, 2015) that serves to
subtract the local average color and map 50% gray to
this local average so as to enhance the blood vessels
and DR lesions. After that we apply the Discrete
Wavelet Transformation (DWT) algorithm (Naik &
Kunchur, 2020) with the mean method using as input
the fundus and WGBF images of a respective patient
to obtain the early fused (EF) Fundus-WGBF
modality. The DWT helps capture the best aspect
from the fundus (color distribution and fundus
structure) and WGBF modalities (i.e., enhanced
blood vessels and DR lesions), respectively.
2.2 Pre-Processing
In previous work (Canayaz, 2022; Karki & Kulkarni,
2021; Lazuardi et al., 2020; Shahriar Maswood et al.,
2020), pre-processing the fundus images helped
improve significantly the studied models
performance by enhancing the images quality. To test
if the models trained replicate the same results, we
perform the following: For the fundus modality we
apply: (1) median blur (aperture=5), (2) gamma
correction (gamma=1.7), and (3) CLAHE with clip
limit equal to 2.0 and tile gride size of 80 by 80. For
the EF modality, we apply: (1) gamma correction
(gamma=1.5), and (2) CLAHE with clip limit equal
to 2.0 and tile gride size of 60 by 60.
Figure 1: Data samples of fundus, pre-processed fundus,
WGBF, EF, and pre-processed EF by DR grade.
3 EXPERIMENTAL SETUP
We train and evaluate three different models:
EfficientNet-B5 (ENetB5), Swin Transformer (STF),
Fundus Unimodal and Late Fusion Multimodal Diabetic Retinopathy Grading
221

and Hybrid-EfficientNetB0-SwinTF (HES); using the
fundus, and EF modalities. We perform this training
in three phases: Phase 1: We train using 5-fold
stratified cross validation (CV) on the train, and
validation sets EfficientNet-B5, Swin Transformer,
and Hybrid-EfficientNetB0-SwinTF for each of the
fundus and EF, respectively. Then we evaluate and
compare on the 5-fold CV test set; and pick the two
best models, based on the F1-score, figuring in 1
st
and
2
nd
cluster of SK-ESD. Phase II: The best models per
modality (i.e., Fundus HES, and EF HES) are then
compared and trained on the 5
th
fold based on
accuracy following four schemes for each modality:
(1) fine-tuning using weights saved in Phase I (FT0),
(2) fine-tuning using weights from Phase I while
unfreezing the last 20 layers of ENetB0 (FT1), (3)
fine-tuning using weights from Phase I while
unfreezing the last 20 layers of ENetB0 and freezing
STF blocks (FT2), (4) training on pre-processed
fundus and EF data. Phase III: We take the best from
these four schemes (i.e., FT2 Fundus (no pre-
processing train and test) and EF (pre-processed train
and test) HES model) and use the FT2 scheme to train
these two models in parallel and average their
predictions to get the final DR grade, to perform what
is called late or decision level fusion. Hereafter, we
detail the four models’ architectures with their
training and testing processes, the empirical process,
statistical methods and performance metrics used to
evaluate the models.
3.1 Modeling
We use the ImageNet pre-trained EfficientNet-B5
(Tan & Le, 2019) (ENetB5) from the EfficientNet
family. EfficientNet models are convolutional neural
networks with uniform scaling faculties of width,
depth, resolution using compound coefficients. In the
classification layer of this model, we use: Global
Average Pooling 2D, Dropout (0.5), Dense (1024
nodes, and ReLU as activation), and the output layer
composed of a Dense layer with 5 nodes and Softmax
as activation function. We freeze all of its layers apart
from the classification part and train using Adam
optimizer and categorical cross entropy as loss.
ENetB5 uses as input 456x456 images and batch size
of 32.
From the ViT family of models we use Swin
Transformer (Liu et al., 2021) (STF) a hierarchical
ViT using shifted windows scheme to improve self-
attention computation efficiency to non-overlapping
local windows and provide cross-window connection.
The model takes 224x224 in input shape, 4x4 in patch
size, 0.03 in dropout rate, 8 in number of heads, 96 in
embedding dimension, 1024 in number of nodes for
multilayer perceptron (MLP), 7 in window size, 1 in
shift size, 0.1 in label smoothing for the categorical
cross entropy loss, and batch size of 32.
The 3
rd
model we train is Hybrid-EfficientNetB0-
SwinTF (HES) (Henkel, 2021), see Figure 2,
composed of ImageNet pre-trained ENetB0 using the
AdvProp (Xie et al., 2020)–an adversarial training
approach used to prevent overfitting by treating
adversarial examples as additional examples–
weights in its head, STF in its body, and a Dense layer
with 5 nodes and Softmax activation as output. In the
classification head of STF we define: Global Average
Pooling 1D, Alpha Dropout (0.5), and Batch
Normalization. While for ENetB0 we define: Global
Average Pooling 2D, Alpha Dropout (0.5) and use
sparse categorical cross entropy as loss with no label
smoothing. The model takes as input 384x384 sized
images, batch size of 8, patch size of 2x2, dropout of
0.5, 8 in number of heads, embedding dimension of
64, 128 for MLP, 2 in window size, 1 in shift size and
24x24 STF image dimension. We retrieve
‘block6a_expand_activation’ output of the ENetB0
model to reduce the image size of fundus and EF from
384x384 to 24x24 to reduce features size and get a
better representation of DR grades. Both HES and
STF models use AdamW optimizer with 1e-3 in
learning rate, and 0.0001 in weight decay.
For the late fusion model, we use FT2 of HES of
fundus and EF, train them on train and test split (2930
train and 732 test) in parallel and average their
predictions to get the final output. But for EF HES we
use the SGD optimizer (learning rate of 1e-4) instead
of AdamW and train on pre-processed EF with their
respective weights.
All models are trained for 50 epochs, using reduce
learning rate on plateau and early stopping that
monitor the validation loss.
Figure 2: Hybrid-EfficientNetB0-SwinTF (HES) model
architecture.
DATA 2023 - 12th International Conference on Data Science, Technology and Applications
222

3.2 Empirical Process
The empirical process followed is split into three
phases:
1) Phase I: Cluster the fundus and EF three
models (ENetB5, STF, HES) using SK-ESD
based on F1-score to select the best model by
modality.
2) Phase II: Train and compare the best models
from (1) following FT0, FT1, FT2, pre-
processing schemes using accuracy as
performance metric.
3) Phase III: Pick the best approach from (2) for
fundus and EF respectively, train them in
parallel, average their predictions and report
sensitivity, specificity, precision, and F1-
score by DR grade and accuracy and weighted
average of one versus rest AUC.
3.3 Statistical Methods and
Performance Metrics
We hope you find the information in this template
useful in the preparation of your submission. In phase
I we trained and evaluated the three models using 5-
fold stratified CV, and clustered the models by
modality using Scott-Knott Effect Size Difference
(SK-ESD) (Tantithamthavorn et al., 2019) statistical
test based on F1-score. In phase II we reported the
accuracy values of the 5
th
fold. In phase III, we
reported the scores of six metrics for each DR grading
to evaluate the performance of the best models:
accuracy, sensitivity, specificity, precision, F1-score,
and weighted average one versus rest AUC score.
These first five metrics are defined in Eqs.1–5
respectively:
Accuracy =
(1)
Sensitivity = Recall =
(2)
Precision =
(3)
F1 = 2
(4)
where: TP: true positive. FP: false positive. TN: true
negative, and FN: false negative.
4 RESULTS AND DISCUSSION
In this section we lay out the results of phase I, II, and
III for the three models ENetB5, STF and HES (phase
I), HES fundus and EF trained using different fine-
tuning schemes and pre-processing (phase II), as well
as late fusion HES of best fundus and EF HES (phase
III). Figure 3 lays out the results of SK-ESD
clustering of all three trained models from phase I by
modality based on F1-score (to account for class
imbalance). Table 1 reports accuracy scores and loss
of phase II experiments. Table 2, 3 and 4 record the
results of best results from phase II (Table 2 and 3) of
FT2 HES and phase III (Table 4) late fusion HES
using precision, sensitivity, F1-score, and specificity
by DR grade. Table 5 lays out the comparison
between our best proposed model and state-of-the-art
trained on the APTOS dataset. In the following we
layout these results and discuss what they entail.
4.1 Unimodal Results
Figure 3 shows that the best ranking model according
to SK-ESD is Fund_HES or fundus Hybrid-
EfficentNetB0-SwinTF as it appears in the first
cluster. In second place we find the EF_HES or EF
Hybrid-EfficentNetB0-SwinTF model. Apart from
HES and ENetB5 models where the fundus
outperformed EF, for STF the EF modality
outperformed the fundus one. This result may be
related to the propriety of ViT models that are robust
to changes in images. In conclusion, for phase I, the
best ranking models are fundus HES and EF HES
with an F1-score of 78.62% and 76.53%,
respectively.
For phase II, we train fundus and EF HES models
using different schemes of fine-tuning and pre-
processing of fundus and EF by using saved weights
from phase I HES per modality. From Table 1, the
best approach reported in terms of accuracy and loss
is HES model trained using pre-processed EF with
Stochastic Gradient Descent (SGD) optimizer instead
of AdamW (Loshchilov & Hutter, 2019) and non-pre-
processed fundus with previously set AdamW
optimizer (as we previously trained it after pre-
processing and by changing to SGD optimizer and it
provided worse results) on the train set and evaluated
on the validation and test sets with an accuracy of
92.35% for fundus and 88.39% for EF. The change of
the optimizer helped improve the training process by
improving the research for optimal parameters by
slowly decreasing the learning rate through SGD. As
for EF pre-processing, it improved the DR lesions
detection by smoothing the features and equilibrating
the color distribution. Meanwhile, FT2 approach
helped train the ENetB0 module further for better
feature extraction using convolutions while keeping
the best representation from Swin blocks.
Fundus Unimodal and Late Fusion Multimodal Diabetic Retinopathy Grading
223

Figure 3: Fundus and EF SK-ESD F1-score results, with
Fund referring to the fundus modality and EF referring to
the early fused Fundus-WGBF DWT modality.
Table 1: Accuracy in % (loss) results for HES model on
fundus and EF modalities, respectively.
*PrepEF stands for pre-processed EF and SGD references to
using SGD optimizer.
4.2 Late Fusion Results
In phase III, we retrained the fundus HES (weighted
F1-score = 76%) and EF HES (weighted F1-score =
77%) on the train and test sets (the validation set was
added to the train set) on the 5
th
fold so as to get the
respective weights to train the late fusion model. The
late fusion HES model consists of training in parallel
HES fundus (with AdamW) and HES pre-processed
EF (with SGD) using these new weights and
following the FT2 scheme. Finally recuperating the
predictions from FT2-HES-Fundus and FT2-HES-
PrepEF and averaging them to get the final DR
grading. As exposed in Table 2, 3 and 4, the F1-score
of late fusion HES equal to 81.21%
(accuracy=81.83%, AUC=96.30%) is better than the
average F1-score of phase I (on Train: Test) fundus
and EF HES equal to 76.50% (accuracy=76.03%,
AUC=94.55%) and average F1-score of phase II-FT2
fundus and PrepEF equal to 60.96% (accuracy =
80,46%, AUC=89,81%). Table 2 and 3 show that
each model II-FT2 HES of fundus and EF modalities
compensated for the weaknesses of the other model
through the late fusion approach. Although, the scores
for the mild, severe, and proliferative are still below
70%; but based on the number of samples available
for these classes (74/732, 39/732, 59/732,
respectively) the provided results are promising.
Table 2: Metrics results of FT2 fundus HES model per DR
grade.
Table 3: Metrics results of FT2 pre-processed EF HES
model per DR grade.
Table 4: Metrics results of late fusion HES model per DR
grade.
4.3 Comparison with State-of-the-Art
In terms of accuracy compared to state-of-the-art
model (Canayaz, 2022) that used nature-inspired
wrappers with EfficientNet and scored an accuracy of
96.32%, our best model late fusion HES accuracy is
less than ~14.49% on the APTOS dataset. The
difference in performance could be due to the
interesting method presented by (Canayaz, 2022)
along with the different pre-processing approach
Phase Fundus
HES (%)
EF HES
(%)
II-Pre-processing on Train:
Val: Test
72.40
(
2.06
)
73.50
(
2.16
)
II-FT0 on Train: Val: Test
73.50
(
2.55
)
86.75
(
1.14
)
II-FT1 on Train: Val: Test
89.10
(1.05)
87.84
(0.93)
II-FT2 on Train: Val: Test
92.35
(0.46)
86.75
(0.87)
II-FT2 (PrepEF with SGD*)
on Train: Val: Test
92.35
(
0.46
)
88.39
(
0.69
)
I on Train: Test
76.23
(1.88)
75.82
(1.75)
II-FT2 (PrepEF with SGD*)
on Train: Test
81.83
(1.53)
79.09
(0.77)
DR grade
Precision
(%)
Specificity
(%)
F1-
score
(%)
Sensitivity
(%)
No DR 97.52 97.57 97.79 98.06
Mil
d
43.09 89.36 53.81 71.62
Moderate 77.14 94.00 63.72 54.27
Severe 27.78 92.50 36.04 51.28
Proliferative 67.65 98.37 49.46 38.98
Weighted
average
80.35 95.56 76.90 76.23
DR grade
Precision
(%)
Specificity
(%)
F1-
score
(%)
Sensitivity
(%)
No DR 64.45 46.63 78.21 99.45
Mil
d
17.21 84.65 21.43 28.39
Moderate 100.00 100.00 04.90 02.51
Severe 25.00 96.97 20.90 17.95
Proliferative 45.00 98.37 22.78 15.25
Weighted
average
65.67 71.83 45.02 54.78
DR grade
Precision
(%)
Specificity
(%)
F1-
score
(%)
Sensitivity
(%)
No DR 96.75 96.77 97.80 98.90
Mil
d
51.37 91.94 61.20 75.68
Moderate 77.35 92.31 73.68 70.35
Severe 52.94 98.85 32.14 23.10
Proliferative 64.29 97.03 62.61 61.02
Weighted
average
81.94 95.20 81.21 81.70
DATA 2023 - 12th International Conference on Data Science, Technology and Applications
224

undertaken (i.e., masks created according to a
tolerance value). As for the second-best model
(Shahriar Maswood et al., 2020), that train to
optimize the QWK score and Mean Squared Error, it
outperformed our model by 11.50%. From these
results we conclude that leveraging preprocessing,
multimodality, and image fusion does not result into
model performance improvement as would be
expected. But, as transformer models are more robust
in comparison with convolution models, further
development and validation on real world datasets is
still needed for further conclusions.
Table 5: State-of-the art comparison with best resulting
model using the APTOS dataset.
5 CONCLUSION AND FUTURE
WORK
In this work we proposed to train and compare three
different models: EfficientNetB5, Swin Transformer,
and Hybrid-EfficientNetB0-SwinTF according to
three phases and using different schemes (fine-tuning
and pre-processing) for the fundus and early fused
fundus with its preprocessing. The best models from
fundus and early fused modality were trained in
parallel and their predictions were averaged to predict
the final DR grade as a late fusion process. Although
the results were promising with an accuracy of
81.83%, compared to state-of-the-art (Canayaz,
2022) (accuracy=96.32%), our late fusion model still
needs fine-tuning. In future work, we aim to improve:
the data quality by introducing a more diverse and
grades enriched dataset from different hospitals, the
pre-processing process by proposing a dynamic
approach (changes depending on image quality), and
optimization of the late fusion approach by reducing
the numbers of parameters; and perform further
validations on local datasets.
ACKNOWLEDGEMENTS
The authors would like to express their gratitude for
the support provided by Google Ph.D. Fellowship.
REFERENCES
APTOS 2019 Blindness Detection | Kaggle. (n.d.).
Retrieved November 1, 2021, from https://
www.kaggle.com/c/aptos2019-blindness-
detection/overview/description
Begum, T., Rahman, A., Nomani, D., Mamun, A., Adams,
A., Islam, S., Khair, Z., Khair, Z., & Anwar, I. (2021).
Diagnostic accuracy of detecting diabetic retinopathy
by using digital fundus photographs in the peripheral
health facilities of Bangladesh: Validation study. JMIR
Public Health and Surveillance, 7(3). https://
doi.org/10.2196/23538
Canayaz, M. (2022). Classification of diabetic retinopathy
with feature selection over deep features using nature-
inspired wrapper methods. Applied Soft Computing,
128, 109462. https://doi.org/10.1016/j.asoc.2022.
109462
El-Ateif, S., & Idri, A. (2022). Single-modality and joint
fusion deep learning for diabetic retinopathy diagnosis.
Scientific African, 17, e01280. https://doi.org/10.
1016/j.sciaf.2022.e01280
Graham, B. (2015). Kaggle Diabetic Retinopathy Detection
competition report. 1–9.
Gu, Z., Li, Y., Wang, Z., Kan, J., Shu, J., & Wang, Q.
(2023). Classification of Diabetic Retinopathy Severity
in Fundus Images Using the Vision Transformer and
Residual Attention. Computational Intelligence and
Neuroscience, 2023, 1–12. https://doi.org/10.1155/
2023/1305583
Henkel, C. (2021). Efficient large-scale image retrieval
with deep feature orthogonality and Hybrid-Swin-
Transformers. 1–5. http://arxiv.org/abs/2110.03786
Hervella, Á. S., Rouco, J., Novo, J., & Ortega, M. (2022).
Multimodal image encoding pre-training for diabetic
retinopathy grading. Computers in Biology
and Medicine, 143. https://doi.org/10.1016/j.
compbiomed.2022.105302
Karki, S. S., & Kulkarni, P. (2021). Diabetic retinopathy
classification using a combination of EfficientNets.
2021 International Conference on Emerging Smart
Computing and Informatics, ESCI 2021, Figure 2, 68–
72. https://doi.org/10.1109/ESCI50559.2021.9397035
Lazuardi, R. N., Abiwinanda, N., Suryawan, T. H., Hanif,
M., & Handayani, A. (2020). Automatic diabetic
retinopathy classification with efficientnet. IEEE
Region 10 Annual International Conference,
Proceedings/TENCON, 2020-Novem, 756–760.
https://doi.org/10.1109/TENCON50793.2020.9293941
Li, T., Gao, Y., Wang, K., Guo, S., Liu, H., & Kang, H.
(2019). Diagnostic assessment of deep learning
algorithms for diabetic retinopathy screening.
Information Sciences, 501, 511–522. https://doi.org/10.
1016/j.ins.2019.06.011
Liu, Z., Lin, Y., Cao, Y., Hu, H., Wei, Y., Zhang, Z., Lin,
S., & Guo, B. (2021). Swin Transformer: Hierarchical
Vision Transformer using Shifted Windows.
Proceedings of the IEEE International Conference on
Computer Vision
, 9992–10002. https://doi.org/10.
1109/ICCV48922.2021.00986
Model
Modality Accuracy
(%)
(Canayaz, 2022) Fundus 96.32
(Shahriar Maswood et al.,
2020)
Fundus 93.33
Late Fusion HES (proposed) PrepEF 81.83
Fundus Unimodal and Late Fusion Multimodal Diabetic Retinopathy Grading
225

Loshchilov, I., & Hutter, F. (2019). Decoupled weight
decay regularization. 7th International Conference on
Learning Representations, ICLR 2019.
Naik, R. B., & Kunchur, P. N. (2020). Image Fusion Based
on Wavelet Transformation. International Journal of
Engineering and Advanced Technology, 9(5), 473–477.
https://doi.org/10.35940/ijeat.D9161.069520
Sebastian, A., Elharrouss, O., Al-Maadeed, S., &
Almaadeed, N. (2023). A Survey on Deep-Learning-
Based Diabetic Retinopathy Classification.
Diagnostics, 13(3), 345. https://doi.org/10.3390/
diagnostics13030345
Shahriar Maswood, M. M., Hussain, T., Khan, M. B., Islam,
M. T., & Alharbi, A. G. (2020). CNN Based Detection
of the Severity of Diabetic Retinopathy from the
Fundus Photography using EfficientNet-B5. 11th
Annual IEEE Information Technology, Electronics and
Mobile Communication Conference, IEMCON 2020,
147–150. https://doi.org/10.1109/IEMCON51383.202
0.9284944
Song, J., Zheng, Y., Wang, J., Zakir Ullah, M., & Jiao, W.
(2021). Multicolor image classification using the
multimodal information bottleneck network (MMIB-
Net) for detecting diabetic retinopathy. Optics Express,
29(14), 22732. https://doi.org/10.1364/oe.430508
Sun, R., Li, Y., Zhang, T., Mao, Z., Wu, F., & Zhang, Y.
(2021). Lesion-Aware Transformers for Diabetic
Retinopathy Grading. Proceedings of the IEEE
Computer Society Conference on Computer Vision and
Pattern Recognition, 10933–10942. https://doi.org/
10.1109/CVPR46437.2021.01079
Tan, M., & Le, Q. V. (2019). EfficientNet: Rethinking
Model Scaling for Convolutional Neural Networks.
36th International Conference on Machine Learning,
ICML 2019, 2019-June, 10691–10700. http://arxiv.
org/abs/1905.11946
Tantithamthavorn, C., McIntosh, S., Hassan, A. E., &
Matsumoto, K. (2019). The Impact of Automated
Parameter Optimization on Defect Prediction Models.
IEEE Transactions on Software Engineering, 45(7),
683–711. https://doi.org/10.1109/TSE.2018.2794977
Teo, Z. L., Tham, Y.-C., Yu, M., Chee, M. L., Rim, T. H.,
Cheung, N., Bikbov, M. M., Wang, Y. X., Tang, Y., Lu,
Y., Wong, I. Y., Ting, D. S. W., Tan, G. S. W., Jonas,
J. B., Sabanayagam, C., Wong, T. Y., & Cheng, C.-Y.
(2021). Global Prevalence of Diabetic Retinopathy and
Projection of Burden through 2045. Ophthalmology,
128(11), 1580–1591. https://doi.org/10.1016/j.ophtha.
2021.04.027
Tseng, V. S., Chen, C.-L., Liang, C.-M., Tai, M.-C., Liu, J.-
T., Wu, P.-Y., Deng, M.-S., Lee, Y.-W., Huang, T.-Y.,
& Chen, Y.-H. (2020). Leveraging Multimodal Deep
Learning Architecture with Retina Lesion Information
to Detect Diabetic Retinopathy. Translational Vision
Science & Technology, 9(2), 41. https://doi.org/10.
1167/tvst.9.2.41
Xie, C., Tan, M., Gong, B., Wang, J., Yuille, A. L., & Le,
Q. V. (2020). Adversarial examples improve image
recognition.
Proceedings of the IEEE Computer Society
Conference on Computer Vision and Pattern
Recognition, 816–825. https://doi.org/10.1109/CVPR
42600.2020.00090
Yao, Z., Yuan, Y., Shi, Z., Mao, W., Zhu, G., Zhang, G., &
Wang, Z. (2022). FunSwin: A deep learning method to
analysis diabetic retinopathy grade and macular edema
risk based on fundus images. Frontiers in Physiology,
13(July), 1–9. https://doi.org/10.3389/fphys.2022.
961386
Yun, S., Han, D., Chun, S., Oh, S. J., Choe, J., & Yoo, Y.
(2019). CutMix: Regularization strategy to train strong
classifiers with localizable features. Proceedings of the
IEEE International Conference on Computer Vision,
2019-Octob, 6022–6031. https://doi.org/10.1109/ICCV
.2019.00612
Zhang, H., Cisse, M., Dauphin, Y. N., & Lopez-Paz, D.
(2018). MixUp: Beyond empirical risk minimization.
6th International Conference on Learning
Representations, ICLR 2018 - Conference Track
Proceedings, 1–13.
DATA 2023 - 12th International Conference on Data Science, Technology and Applications
226
