
Using Analytical Methods and Simulation to Estimate the Magnitude
of Errors in Calculations for Recovery in Washed Red Blood Cells
John Blake
1,2
, Jason Acker
2
and Cherie Mastronardi
2
1
Department of Industrial Engineering, Dalhousie University, Halifax, NS, Canada
2
Centre of Innovation, Canadian Blood Services, Ottawa, ON, Canada
Keywords: Washed Red Blood Cells, Propagation of Error, Simulation.
Abstract: Canadian Blood Services produces a modified blood product, called washed red blood cells. RBCs are
washed to reduce potential transfusion reactions in vulnerable populations. Quality control standards require
that at least 75% of the red cells in a unit are retained through the washing process. However, field reports
suggest that cell recovery values greater than 100% can be observed. The purpose of this study is to analyse
the propagation of error in the washing process and to determine if values exceeding 100% are reasonable,
given the accuracy of the equipment in use. Employing analytical techniques and simulation methods, it was
found that recovery rates in excess of 100% are possible, but that any calculated value exceeding 102% is
unlikely and should be investigate for process errors.
1 BACKGROUND
Red blood cells (RBC) are cells that are responsible
for oxygenating a person’s cells. In general, most
patients receiving a transfusion are supplied with
production standard RBC. However, in patients with
potential for severe anaphylactic reactions, RBC are
washed to remove plasma, plasma protein, micro-
aggregates, cytokines, and unwanted antibodies from
a blood product (Hansen, Turner, Kurach, & Acker,
2015). Washed RBCs reduce the incidence of
unwanted, and potentially dangerous, transfusion
related reactions in certain vulnerable recipient
populations.
Canadian Blood Services (CBS) is the not-for-
profit agency responsible for the collection,
production, testing, and distribution of blood and
blood products in all of Canada, outside of the
Province of Quebec, which maintains its own agency
(Blake & Hardy, 2013). As a regulated blood agency,
CBS maintains an extensive quality control program
to ensure the viability of its products and to monitor
its processes. For example, quality control standards
dictate that production/distribution sites providing
washed RBCs to customers must perform a monthly
audit of their procedures to ensure that the equipment
and practices employed result in products with
acceptable characteristics. These standards dictate
that the amount of recovered red cells in the output
product must be ≥ 75% of the red cells in the input
product (Canadian Blood Services, 2021).
However, it has been observed in the field that in
some instances of washed process audit the
percentage of recovered cells identified in the output
product exceeded 100%. Since the percent recovery
is based on the number of red cells in the output bag
divided by the number of cells in the input bag and
cells cannot be added to the output product via the
washing process, ratios greater than 1.0 are physically
impossible and must, therefore, be due either to errors
in method or the accuracy of equipment used to
measure values used in the calculation.
2 OBJECTIVE
This study provides a method for evaluating the
degree of error associated with the accuracy of the
equipment used to measure parameters used in the
percent recovery calculation at Canadian Blood
Services and to estimate the range of error in practice.
The purpose of this study is to identify when a
calculated percent recovery can be considered
reasonable, given known or estimated, error in the
process and when a calculated value must be
340
Blake, J., Acker, J. and Mastronardi, C.
Using Analytical Methods and Simulation to Estimate the Magnitude of Errors in Calculations for Recovery in Washed Red Blood Cells.
DOI: 10.5220/0012097800003546
In Proceedings of the 13th International Conference on Simulation and Modeling Methodologies, Technologies and Applications (SIMULTECH 2023), pages 340-345
ISBN: 978-989-758-668-2; ISSN: 2184-2841
Copyright
c
2023 by SCITEPRESS – Science and Technology Publications, Lda. Under CC license (CC BY-NC-ND 4.0)

considered anomalous, indicating that a cause for the
exception must be identified.
3 METHOD
The range of potential values for percent recovery
was evaluated using analytical and simulation
methods. Monte Carlo simulation, either on its own
or combined with analytical methods, is a common
method for estimating error propagation in complex
systems. It has been used to estimate uncertainty in
digital elevation models for geospatial applications
(Temme, Heuvelink, Shoorl, & Claessens, 2009),
data corruption in high performance computing (Li,
et al., 2021) and air pollution modelling (Evans,
Cooper, & Kinney, 1984), amongst other
applications. Our method follows the same general
plan as Evans, Cooper and Kinney (1984), but
tailored for a process of washing red blood cells. We
believe that this is the first application of Monte Carlo
methods to support error propagation analysis in a red
cell washing process.
The operational calculation used to determine
percent recovery was analyzed and, using algebra
combined with assumptions regarding typical values,
the potential range of values was identified. A
sensitivity analysis was performed on the assumed
values. A simulation was then employed to evaluate
the likely range of errors and to confirm the analytic
results
.
3.1 Analytical Analysis
Percent recovery is a ratio of cells post-wash to cells
pre-wash. Since the number of cells in both the input
and output product bags cannot be measured directly,
they must be estimated. To estimate the number of
cells, the volume of product in a bag is multiplied by
the product hematocrit, or percent of a blood product
composed of red blood cells, as determined by a cell
analyzer, based on a small sample taken from the
product or an associated segment. Percent recovery
is thus calculated as:
% 𝑅𝑒𝑐𝑜𝑣𝑒𝑟𝑦 =
𝑣
ℎ
𝑣
ℎ
(1)
Where:
v
O
is the volume of the product (post-wash)
h
O
is the hematocrit of the product (post-wash)
v
R
is the volume of the product (pre-wash)
h
R
is the hematocrit of the product (pre-wash)
However, the volume of the input (pre-wash) and
output (post-wash) products also cannot be directly
measured. Instead, the volume is calculated by
multiplying the net weight of the product in the bag
by the specific gravity of blood as follows:
% Recover
y
=
n
sg
h
n
sg
h
(2)
Where:
n
O
is the net weight of the product (post-wash)
h
O
is the hematocrit of the product (post-wash)
n
R
is the net weight of the product (pre-wash)
h
R
is the hematocrit of the product (pre-wash)
sg
B
is the specific gravity of blood.
Since the term sg
B
appears in both the numerator
and denominator of we can simplify the calculation in
(2):
% Recover
y
=
n
h
n
h
(3)
The net weight of the product, both pre- and post-
wash is determined by weighing the product and the
container holding it and then subtracting from this
weight an assumed tare weight (i.e. the weight of the
empty container). If we define w
O
and w
R
to be the
gross weight (i.e., total weight of the product and bag)
of the output and input products respectively, and t
O
and t
R
to be the tare weights of the empty bags, then:
𝑛
=
(
𝑤
−𝑡
)
𝑛
=
(
𝑤
−𝑡
)
(3a)
and Equation (3) can be written as:
% Recovery (SOP) =
(
𝑤
−𝑡
)
ℎ
(
𝑤
−𝑡
)
ℎ
(4)
Where:
w
O
is the weight (measured) of the bag and blood
(post-wash)
w
R
is the weight (measured) of the bag and blood
(pre-wash)
t
O
is the tare weight (assumed) of the bag (post-
wash)
t
R
is the tare weight (assumed) of the bag (pre-
wash)
h
R
is the hematocrit (measured) of the product (pre-
wash)
h
O
is the hematocrit (measured) of the product
(post-wash)
Equation 4 is the calculation specified at CBS for
calculating percent recovery in washed RBCs.
However, this calculation assumes that all values are
known with certainty. In reality, of course, there are
Using Analytical Methods and Simulation to Estimate the Magnitude of Errors in Calculations for Recovery in Washed Red Blood Cells
341

errors in quantities measured due to accuracy
limitations of the equipment used to determine the
parameters of weight and hematocrit. Accordingly, if
one were to assume that the calculated value was
equal to the true value plus a randomly distributed
error term, then equation (4) becomes:
% Recover
y
=
(
w
+w
)
−
(
t
+t
)
(
h
+h
)
(
w
+w
)
−
(
t
+t
)
(
h
+h
)
(5)
Where:
w
O’
is the error in the weight of the bag and blood
(post-wash)
w
R’
is the error in the weight of the bag and blood
(pre-wash)
t
O
’
is the error in the tare weight of the bag (post-
wash)
t
R’
is the error in the tare weight of the bag (pre-
wash)
h
O’
is the error in the hematocrit of the product (post-
wash)
h
R’
is the error in the hematocrit of the product (pre-
wash)
Note that while the error is shown as additive in
Equation (5), it should be understood that the error
may be plus or minus from the true values and thus
the error quantities themselves are defined as real
numbers. With some algebra, the terms in (5) can be
re-arranged:
% Recover
y
=
(
w
−
t
)
h
+
(
w
−
t
)
h
+
(
w
−
t
)
h
+
(
w
−
t
)
h
(
w
−
t
)
h
+
(
w
−
t
)
h
+
(
w
−
t
)
h
+
(
w
−
t
)
h
(6)
Equation (6) shows that the % Recovery
calculation is comprised of three terms: a term
derived from the measured values, a term arising from
the errors in measurement and a mixed term that
depends both on the measured values and the errors
in measurement.
Figure 1: Classification of terms in % recovery calculation.
Because there are mixed terms in Equation (6), it
is not possible to obtain an absolute estimate of
experimental error; the weight and the hematocrit of
the product pre- and post-wash influence the percent
recovery calculation and so no absolute error can be
calculated analytically.
However, it is possible to provide an estimate of
the average magnitude of error that might be
expected by assuming typical values for the required
parameters. See Table 1 for data used in this analysis,
which was obtained from a sample of washed red
blood cells at a Canadian Blood Services production
centre.
3.2 Data
Table 1: Values for % Recovery Calculation.
Parameter Assumed
Value
Source
w
O
351.375 g Sample of 8 washes
from collection centre A
t
O
89 g Assumed tare weight of
output
b
ag
h
O
0.7820 Sample of 8 washes
from collection centre A
w
R
401.75 Sample of 8 washes
from collection centre A
t
R
35 Assumed tare weight of
collection
b
ag
h
R
0.6589 Sample of 8 washes
from collection centre A
w'
O
+
/
-1g
m
Accuracy of scale
t'
O
+
/
-1 g
m
Accuracy of scale
h'
O
+/- 0.006 Based on a sample of 20
washes.
w'
R
+
/
-1g
m
Accuracy of scale
t'
R
+
/
-1 g
m
Accuracy of scale
h'
R
+/- 0.012 Based on a sample of 20
washes.
3.3 Sensitivity Analysis
The value of h
O
listed in Table 1 is derived from
sample wash data provided by the collection centre A.
However, if one were to assume 100% recovery, the
necessary hematocrit for the output product can be
calculated as follows. If 100% recovery is achieved,
then:
(
𝑤
−𝑡
)
ℎ
=
(
𝑤
−𝑡
)
ℎ
(7)
and thus, the h
O
that would be necessary to achieve
100% recovery can be calculated as:
ℎ
=
(
𝑤
−𝑡
)
ℎ
(
𝑤
−𝑡
)
(8)
For example, using the average values listed in Table
1, the h
O
Perfect
that would be associated with 100%
recovery can be calculated as 0.9210.
To calculate the maximum value that could be
observed in Equation (6), given the typical values
listed in Table 1 it is assumed that the error terms
listed in the equation take on the signs listed in Table
Measurement
Term
Error
Term
Mixed
Term
SIMULTECH 2023 - 13th International Conference on Simulation and Modeling Methodologies, Technologies and Applications
342

2. The resulting values of % recovery can be found
in Table 3.
Table 2: Error Sign Necessary to Maximize % Recovery
Calculation.
Error Parameter Si
g
n
w'
O
+
t'
O
-
h'
O
+
w'
R
-
t'
R
+
h'
R
-
Table 3: Expected and Maximum Values of Percent
Recovery Calculations.
Assume h
O
from Data
Assume h
O
Perfect
Expected value 84.91% 100.00%
Maximum value 89.81% 105.57%
Difference 4.91% 5.57%
From Table 3 it can be observed that the
difference between the expected value of the
calculation (i.e., the value if all errors are 0) and the
maximum value of the calculation (i.e., the value if
all errors contribute towards maximizing Equation
(6)) is between 4.91% and 5.57%. Of course, this
value depends on the actual weights of the products
pre- and post-wash. To give an idea of the range of
potential difference between expected and maximum
values in the percent recovery calculation, a
sensitivity analysis was conducted on the assumed
product weight and hematocrit values used in the
calculation. See Tables 4 and 5.
Table 4: Sensitivity Analysis Product Weight.
Product Weight -20% Product Weight
+20%
Assume
h
O
Assume
h
O
Perfect
Assume
h
O
Assume
h
O
Perfect
Expected
value
79.61% 100.00% 88.30% 100.00%
Maximum
value
84.57% 105.95% 93.16% 105.35%
Difference 4.96% 5.95% 4.86% 5.35%
Table 5: Sensitivity Analysis Product Hematocrit.
Product Hematocrit -
20%
Product Hematocrit
+20%
Assume
h
O
Assume
h
O
Perfect
Assume
h
O
Assume
h
O
Perfect
Expected
value
84.91% 100.00% 84.91% 100.00%
Maximum
value
90.80% 106.68% 89.17% 104.84%
Difference 5.89% 6.68% 4.26% 4.84%
From Tables 3-5 it may be observed that the
maximum error in the percent recovery ranges from
4.26% to 5.95% across the sensitivity analysis. Error,
moreover, increases inversely to increases in both
product weight and product hematocrit. Thus, the
smaller the value of either weight or hematocrit, the
larger the potential for error in the calculation.
Finally, it should be noted that the percent recovery
calculation is more sensitive to errors in the
determination of hematocrit than product weight.
Measurement error for percent recovery is
normally distributed, since repeated measurements
made of the same quantity are, by definition,
normally distributed (Miller & Miller, 1988).
Further, for the maximum errors as listed in Tables 3-
5 to be observed it is necessary that all errors be in the
correct direction and at their extreme values at the
same time. If one assumes that individual errors are
independent of one another, the probability of seeing
all errors at their extreme value and with the requisite
sign to maximize the total error is unlikely, but
calculable under the assumption of normality. Thus,
while the maximum values listed in Tables 3-5 are
possible, they may not be likely values. To estimate
the likely range of values that could be observed,
given the typical values assumed in Table 1, a
simulation reproducing the measurement process was
run. The simulation assumes that that errors in weight
measurement (w
O
’, w
R
’, t
O
’, t
R
’, h
O
’, h
R
’) are
uniformly distributed since these values are
dependent on the accuracy of the equipment used to
take measures. The magnitude of the errors in
measurement of weight was assumed to be +/-1 gram,
based on the accuracy of the scales (i.e. the number
of significant digits displayed by the equipment).
Hematocrit errors were calculated from sample data
such that the observed error would have a mean of 0
and a standard deviation that would yield a coefficient
of variation (CV=σ/µ) equal to 0.0080 for h
R
’ and
0.0186 for h
O
’. The estimates of coefficient of
variation were derived from a sample of 20 washes
using the standard operating procedure. Since
hematocrit error is related to the product mass,
coefficient of variation, rather than standard deviation
is used for simulation calculations.
Based on an average hematocrit of 0.6589 for a
pre-washed unit and 0.7820 for a post-washed unit,
error estimates of 0.012 for the pre-wash
measurement and 0.0063 for the post-wash
measurement can be calculated. Using these values,
a simulation was then executed for a total of 500,000
replications and the resulting percent recovery was
recorded. The simulation yielded the following
results:
Using Analytical Methods and Simulation to Estimate the Magnitude of Errors in Calculations for Recovery in Washed Red Blood Cells
343
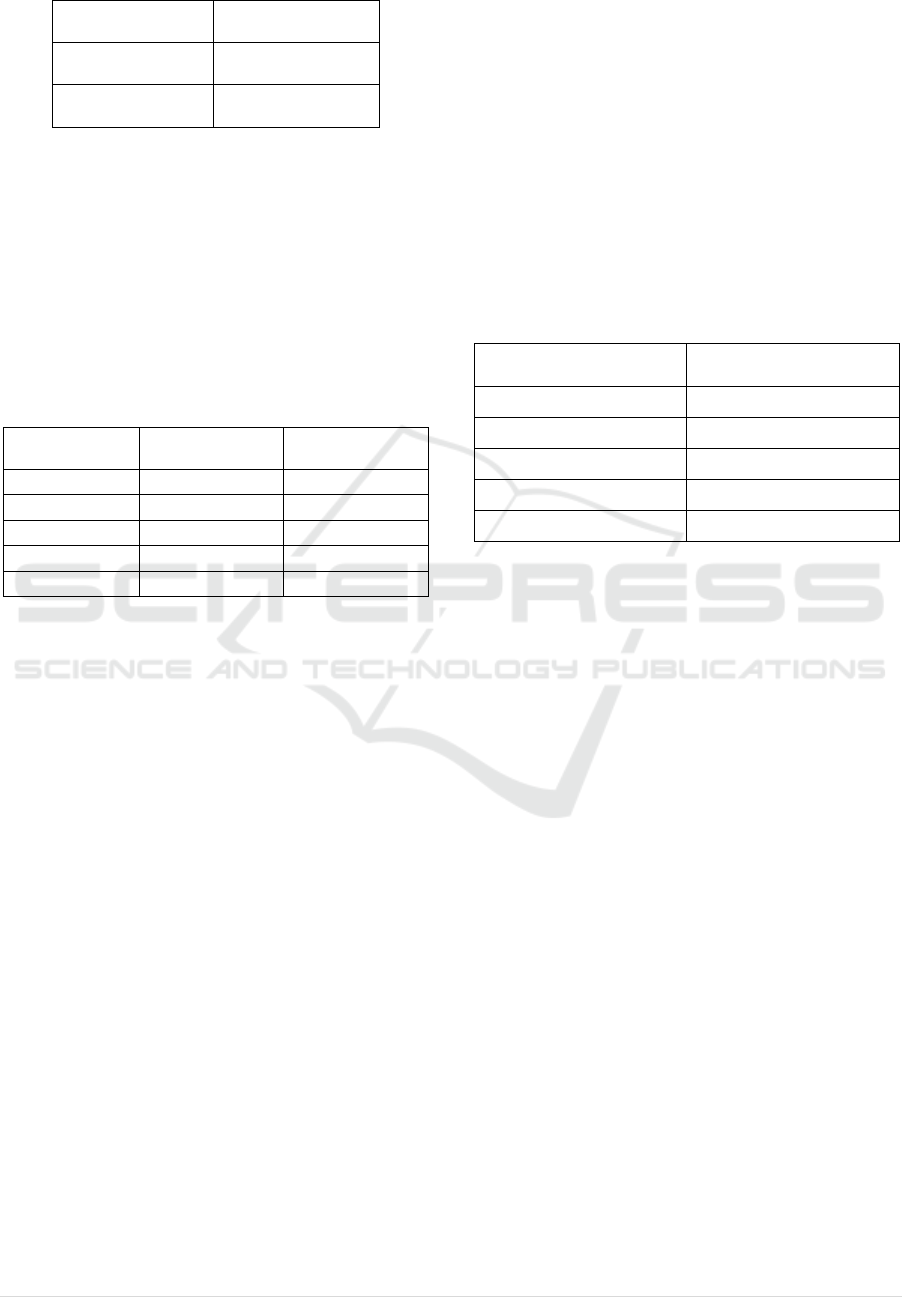
Table 6: Simulation Output.
Maximum
% Recover
y
88.04%
Average
% Recover
y
84.91%
Standard
Deviation 1.03%
The simulation validates the maximum error
calculations made in Table 3-5 (i.e., the range is ~
85% +/-3%). However, the simulation also provides
information on the likelihood of seeing extreme
values. Since errors from repeated measurements are
normally distributed and the observed standard
deviation over the 500,000 replications of the
simulation was 1.03%, the likelihood of seeing an
error of a particular magnitude can be estimated from
the properties of a normal distribution as follows:
Table 7: Probability of Observing Error of a Particular Size.
Magnitude of
Erro
r
Probability Error
<= Value
Probability Error
>= Value
1.03% 84.134% 15.866%
1.69% 95.002% 4.998%
2.06% 97.725% 2.275%
3.09% 99.865% 0.135%
4.12% 99.997% 0.003%
Accordingly, it may be seen that while the
maximum error possible could be as large as 5.57%,
error values exceeding +/- 1.69% are unlikely. Thus,
calculated percent recovery calculations exceeding
101.69% are not likely to be due to random
fluctuations in measurement and other sources of
error should be suspected in such situations.
It is also possible to extrapolate from Table 7 a
lower tolerance limit for the percent recovery
calculation. Since current quality standards dictate
that the amount of recovered red cells in the output
product must be ≥ 75% of the cells in the input
product, there may be an advantage in setting a lower
tolerance level for the wash process above 75%.
Doing so would reduce the likelihood that an
unacceptable unit would incorrectly be assumed to
meet the quality standard. Consider, for instance, a
unit that is found to have exactly 75% recovery, post-
wash. Based on the assumption that errors are
normally distributed, there is only a 50% chance that
the unit actually achieves the quality standard and
thus a 50% chance that the unit will be incorrectly
labelled as positive proof of the quality standard. (A
95% prediction interval would suggest a true range
between 72.7% and 77.9%, with 50% of all
observations falling below the nominal target
threshold.) Accordingly, if the minimum observation
for declaring a sample acceptable were to be
increased, a corresponding decrease in false positives
could be expected. Table 8 shows the expected
probability of a false positive for a given
measurement of percent recovery, under the
assumption of a measurement process with a
normally distributed error of N(0,0.0103). For
instance, if the nominal QC cut-off value was
increased to 77.78%, only 2.3% of the completed
units would fail to have the requisite minimum
requirement of 75% of the pre-wash cells preserved
through the washing process.
Table 8: Probability of False Positive, Given a Measured %
Recovery.
Measured % Recover
y
Probability of False
Positive
75.0% 50.0%
75.93% 15.9%
76.53% 5.0%
77.78% 2.3%
78.81% 0.1%
4 CONCLUSION
Errors in the accuracy of the equipment used to
measure the necessary parameters to estimate percent
recovery in washed red cells can reasonably give rise
to calculated values more than 100%. It was
determined that no absolute figure for accuracy could
be given that would be applicable in all cases, since
the error terms in the calculation interact with both
the product weight and hematocrit of the pre- and
post-wash products. However, using average values
obtained from a sample of data provided by collection
centre A, it was determined that values between
4.91% and 5.57% more than the true value of percent
recovery are possible, given the accuracy of the scales
and cell analyzers used to estimate parameters. Thus,
it is possible that all calculated values of percent
recovery less than 105.57% could potentially be
valid. However, since process errors are normally
distributed in aggregate, the more the value deviates
from the expected value, the lower the likelihood of
the event being truly due to error in machine accuracy
and the greater the probability that other factors (i.e.,
process or operator error) may be involved. Using a
simulation to estimate the likely range of errors it was
noted that all calculated values of percent recovery in
washed red blood cells exceeding 101.69% should be
SIMULTECH 2023 - 13th International Conference on Simulation and Modeling Methodologies, Technologies and Applications
344

regarded as suspicious. Similarly, it was determined
that, if QC minimums were increased to 77.78%,
errors in processes that provided less than a 75% yield
could be identified more often. See Figure 2 for a
diagram of the acceptable bounds for percent
recovery calculations.
Figure 2: Process limits derived from the simulation and the
analytical results.
We conclude by noting that information taken
from this study was used to inform standard operating
procedures used at Canadian Blood Services when
conducting quality control audits for washed red
cells.
REFERENCES
Blake, J., & Hardy, M. (2013). Using simulation to design
a blood supply network in the Canadian Maritime
provinces. Journal of Enterprise Information
Management, 26(1/2), 119-134.
Canadian Blood Services. (2021, January). Canadian Blood
Services Circular of Information. Retrieved March 28,
2023, from Canadian Blood Services:
https://www.blood.ca/sites/default/files/1000104991_2
021-01-25.pdf
Evans, J., Cooper, D., & Kinney, P. (1984). On the
propagation of error in air pollution measurements.
Environmental Monitoring and Assessment, 4, 139-
153.
Hansen, A., Turner, T., Kurach, J., & Acker, J. (2015).
Quality of red blood cells washed using a second wash
sequence on an automated cell processor. Transfusion,
55(10), 2415-2421.
Li, Z., Menon, H., Mohror, K., Bremer, P.-T., Livant, Y., &
Pascucci, V. (2021). Understanding a program's
resiliency through error propagation. In E. Petrank
(Ed.), PoPP '21: Proceedings of the 26th ACM
SIGPLAN Symposium on Principles and Practice of
Parallel Programming (pp. 362-373). Association for
Computing Machines.
Miller, J., & Miller, J. (1988). Basic Statistical Methods for
ANlaytical Chemistry Part I. Statistics of Repeated
Measurements: A Review. Analyst, 113, 1351-1356.
Temme, A., Heuvelink, G., Shoorl, J., & Claessens, L.
(2009). Geostatistical Simulation and Error
Propagation in Geomorphometry. Developments in Soil
Science, 33(1), 121-140.
Using Analytical Methods and Simulation to Estimate the Magnitude of Errors in Calculations for Recovery in Washed Red Blood Cells
345
