
Differences and Relations Between Chrono-Biological and
Motor-Functional Characteristics of Infants
Jelena Marunica Karšaj
1
and Igor Gruić
2a
1
University Department of Rheumatology, Physical and Rehabilitation Medicine,
University Hospital Centre “Sestre milosrdnice”, Vinogradska cesta 29, 10000, Zagreb, Croatia
2
Faculty of Kinesiology University of Zagreb, Horvaćanski zavoj 15, 10 000, Zagreb, Croatia
Keywords: Muscle Tone, Neurodevelopment, Jaundice, Infants, Obstetric Mode of Delivery.
Abstract: Differences and relationships between chrono-biological (body weight-BW, body length-BL, gestational age-
GA) and motor-functional characteristics (e.g., muscle tone) of infants with relation to different obstetric
mode of delivery and jaundice were analysed. The assessment of muscle tone is an integral part of neuromotor
evaluation. The study included 179 infants of both genders (AS±SD: age 158,36±110,91 days; BW
3267,78±708,69 grams; and BL of 49,33±3,09 cm) due to muscle tone disorders with the presence of mild
and moderate neurodevelopmental disorders as a sequelae of immature brain impairment. Study revealed
statistically significant differences in chrono-biological variables depending upon the different obstetric mode
of delivery (BW, BL, and GA), as well upon neonatal jaundice (BW, BL, and possibly GA). Also, there is a
statistically significant correlation among chrono-biological variables (BW, BL, GA: 0,62-0,88). When
compared to infant’s age at first physiatrist examination (AFE), individually and combined with GA,
correlations imply importance of further inter-parametrial insights – in this case with relation to muscle tone
classified in 4 groups (normal-, hypo-, hyper-, and changing-). Findings confirm statistically significant
differences between infants differently categorized by muscle tone and infant’s AFE- among hypertonic and
hypotonic infants as well among hypertonic and alternating ones respectively. Although there are no
correlations between the AFE with BW, BL (with GA they are very little correlated – 0,19), there is an
indication that the existing categorization by tone demands more frequent or earlier 'screening' - embedded
into existing communication for a balanced development overall.
1 INTRODUCTION
The assessment of muscle tone is an integral part of
the routine neuromotor evaluation of new-borns,
infants and children. Observation of a fluctuation in
muscle tone aids in setting up a diagnosis, is used as
justification for therapeutic approach, and is
considered as an indicator of neurological change
(Kathrein, 1990). It is well-known that many infants
show one or two signs of atypical neuromotor
performance, and that, generally, only an aggregation
of multiple signs of atypical neuromotor performance
is associated with elevated risk of
neurodevelopmental disorders (Hadders-Algra et al.,
2010). We addressed the alteration of muscle tone in
infants and described the functional characteristics of
muscle tone as follows: lowered (hypotonia),
a
https://orcid.org/0000-0001-6680-8940
increased (hypertonia), changing muscle tone and
normal mostly of a central etiology which happens to
emerge antenatally, intrapartum, or postnatally.
Muscle tone alterations are often represented in the
so-called “risky children” (children who were
exposed to one or more risk factors for
neurodevelopmental disorders in their medical
history), and those can be an implication of a primary
disorder of the central nervous system (CNS) in terms
of a prior brain disturbances (Lindahl et al., 1988).
The changed muscle tone in terms of hypotonia,
hypertonia and changing muscle tone is considered to
be a symptomatic risk and it stands in need for the
proper and prompt habilitation treatment, even
though the spontaneous normalization is often
achievable (Lazić et al., 2011). Thus, obtaining a
comprehensive medical history is crucial in
Marunica Karšaj, J. and Grui
´
c, I.
Differences and Relations Between Chrono-Biological and Motor-Functional Characteristics of Infants.
DOI: 10.5220/0012268300003587
In Proceedings of the 11th International Conference on Sport Sciences Research and Technology Support (icSPORTS 2023), pages 237-246
ISBN: 978-989-758-673-6; ISSN: 2184-3201
Copyright © 2023 by SCITEPRESS – Science and Technology Publications, Lda. Under CC license (CC BY-NC-ND 4.0)
237

consolidation the extensive list of differential
diagnoses due to muscle tone alternations. Worldwide
shortly after birth, more than 80% of all term and late
preterm infants develop some extent of neonatal
jaundice or hyperbilirubinemia (Keren et al., 2008).
Many studies pointed out that the GA was
significantly associated to jaundice. It has been
described that the risk of jaundice significantly
heightens with lowering GA (Sarici et al., 2004).
Moderate jaundice turned out to be linked with a
significant increase in minor neuromotor disorder
throughout the infancy (Soorani-Lunsing et al.,
2001). Further inspections of muscle tone may be
assessed within sEMG approach (presented in
e.g.(Cifrek et al., 2021).
Bobaths’ methodology gave definition on muscle
tone as “the speed and the degree of resistance to
passive external manipulation of the extremities for a
period of time without interfering with length. It is a
function of the tactile, vestibular and proprioceptive
arrangement enabling an individual to preserve body
posture against the pull of gravity in a resting
position- static as well as the attainment of movement
patterns-dynamic” (Bobath K, 1984). So, tone is
defined as the resistance of muscles to stretch;
therefore, hypotonia is diminished resistance of
muscles to passive stretching (Crawford, 1992).
However, a more appropriate definition of hypotonia
is an impairment of the ability to sustain postural
control and movement against gravity. Thus, floppy
infants exhibit poor control of movement with
prolonged head lag within arm pull test,
procrastinated motor skills, and hypotonic motor
movement patterns. The atypical motor patterns
consist of alterations in postural control, increased
range of motion of joints, and inadequate stability and
movement biomechanics (Peredo et al., 2009). The
critical feature of hypotonia refers to head and neck
control. Mild or benign head lag is a common finding
in new-borns and generally resolves by itself;
however, the presence of severe relentless head lag
beyond three to four months of age typically points to
disorders related to hypotonia and muscle weakness
in infancy (Linder et al., 1998). On the other hand,
hypertonia is defined as an irregularly high resistance
at the time of externally imposed passive movement.
Increased muscle tone is usually characterized by stiff
and inflexible muscles which happen to be hardly
stretched, difficulty in moving from one position to
another, involuntary crossing of lower extremities
when lifted vertically, abrupt tremors or jerks which
aggravate during periods of stress, coordination
deficiency and delay in motor skills maturation. It is
associated with opisthotonus which is assigned to
aberrant axial extension and arching of the trunk
produced by excessive contractions of the paraspinal
musculature (Sanger et al., 2003). Recurrence-wise,
hypertonia is less common in infants than hypotonia
(Sanger et al., 2003).
Final goal of this research is to analyse and
recognize differences between infants differently
categorized by muscle tone and infant’s AFE
(normal-, hypo-, hyper-, and changing-), correlations
among chrono-biological variables, differences in
chrono-biological variables depending upon the
different obstetric mode of delivery and neonatal
jaundice.
2 METHODS
2.1 Sample of Entities
The sample of entities is comprised of 179 infants in
age of 158,36±110,91 days at first physiatrist
examination (as inclusion criterion), with BW
3267,78±708,69 grams and BL of 49,33±3,09 cm,
without special exclusion criterion.
2.2 Sample of Variables
Anthropometric measures and data from antenatal,
intrapartum and postnatal history were obtained on
the first visit either based upon parental report or
collected from documented medical history. Sample
of variables is comprised of 3 independent (grouping)
variables– tone (normal-, hypo-, hyper-, changing),
jaundice (yes/no), birth delivery technique
(spontaneously throughout birth canal/Caesarean
section-CS), and 5 item battery of dependent
variables, 4 basic and one derived – BW (grams), BL
(cm), GA (days), AFE (days), GA and first
examination summed (days).
2.3 Experimental Design
A series of comparisons of (two) independent
variables (groups) were performed. The set of
dependent variables covers chrono-biological
characteristics of entities at birth, with age when first
physiatrist examination was added. The set of
grouping variables was determined by obstetric
modes of delivery, jaundice, and muscle tone. The
study included infants of both genders, aged 0-24
months, who were examined by a physiatrist due to
the muscle tone disorders with the presence of mild
and moderate neurodevelopmental signs and
symptoms as a sequelae of immature brain lesion. The
K-BioS 2023 - Special Session on Kinesiology in Sport and Medicine: from Biomechanics to Sociodynamics
238

modality of habilitation was prescribed
corresponding to infant's clinical indication. Due to
experience follow up shows those infants
predominantly accomplished normal muscle tone and
typical development in further childhood. Our study
is the result of the continuous monitoring of the
development assessed by physical examination of
infants who were at risk due to their antenatal,
intrapartum and postnatal medical history.
Monitoring was carried out methodically, and a
physiatrist examination was conducted in the
outpatient polyclinic of the University Department of
Rheumatology, Physical Medicine, and
Rehabilitation.
While assessing muscle tone, an infant should be
alert and relaxed but not sobbing. Extremity tone is
readily assessed by externally imposed passive
movements. Muscle tone is clinically evaluated by
observation, and palpation during passive
examination of the range of movement of
appendicular and peripheral skeleton. Observation
gives insight into both the global posture of the body
and the acquirement and maintenance of the
antigravity position in the supine and prone position
of an infant. Besides examination is performed
separately in the nuchal, truncal region and on the
upper and lower extremities. In the neck, muscle tone
is evaluated when testing resistance during passive
rotation of the head to the right and left side. The
muscle tone in the shoulder girdle is done by
anteflexion with elevation and adduction of the flexed
arm to the opposite shoulder (“scarf sign”); on the
upper extremities’ pronation and supination, flexion
of the wrist, opening of the hands. Passive examining
of the flexion and lateral flexion of the truncal region
is also involved. Hip flexion, hip abduction and
adduction, knee extension and flexion and foot
dorsiflexion are passively examined on the lower
extremities. Besides the engagement of locomotor
system to assess muscle tone, inevitably are observed
the expression of the infant's face, making eye
contact, social smile and cry, the way of reacting to
auditory, visual stimulus and qualitatively how infant
initiates and performs movement (Goo et al., 2018).
Truncal and nuchal muscle tone may be best
examined using arm traction test, horizontal and
vertical suspension tests. In assessing arm traction
test in supine position no head lag is expected after
the age of three months. On vertical suspension, a
healthy infant should maintain the head perpendicular
and mid-line without descending through the
examiner’s hands. On horizontal suspension, the
infant should maintain a straight back with the head
upright and extremities in flexion position. In
comparison, hypotone infants may wrap over the
examiner’s arms (Leyenaar et al., 2005).
2.4 Data Processing
Descriptive statistics were presented via parameters
of central tendency and dispersion, followed by K-S
tests for distribution normality. Even with existence
of a certain frame for inferential statistics, all analyses
follow nonparametric rules, due to consistency
towards experimental design and results leading to
conclusions based on the nature of analysed and
hypothesized phenomena.
Spearman Rank Correlations among variables and
Mann-Whitney U Test for comparisons of two
independent variables were assessed in TIBCO
Software Inc., ver 14.0.0.15. Power analyses and
sample size calculations were performed via TIBCO
Software Inc., ver 14.0.0.15., and G*Power version
3.1.9.6. Univeresity Kiel, Germany.
2.5 Ethical Issues
Research was approved by Ethical Committee of
University Hospital Centre “Sestre milosrdnice”;
Vinogradska cesta 29, 10000 Zagreb, Croatia, ICH
GCP and Helsinki Declaration; C: 003 06/22-03/003;
No: 251-29-11-22-01-7.
3 RESULTS
Table 1 Descriptive statistics and normality
distribution test (in Appendices).
Table 2 Spearman Rank Order Correlations (in
Appendices).
In addition to basic information about BW and
BL, GA at birth for processing the general health of
infants, obstetric mode (spontaneous, CS), jaundice
(has/none), Apgar score (Casey et al., 2001), which
represents the 10-point score has been used to assess
the condition and prognosis of new-borns worldwide
for the past 70 years, succession of previous
pregnancies and born children, etc. are also
registered.
Table 3 Descriptive statistics analysis according
to obstetric mode of delivery and jaundice (in
Appendices).
The obstetric mode of delivery as a previous
decision of the physician, and manifested neonatal
jaundice as a subsequently established condition,
show different statistical strength for distinguishing
infants by the results of the measured BW and BL,
GA and therefore have impact on decision concerning
Differences and Relations Between Chrono-Biological and Motor-Functional Characteristics of Infants
239
age at which the infant should be included in
habilitation treatment.
Different disorders from normal tone pattern are
shown through results in the observed variables in the
Table 3.
Table 4 Descriptive statistical analysis according
to muscle tone (in Appendices).
According to the average AFE
(Mean±SD:158,36±110,91 for the whole sample,
Mean±SD:151,34±127,59 for normal tone) within
hypertonic infants a deviation is presented
(Mean±SD: 115,67±51,86), which cumulatively with
GA (Mean±SD:387,275±55,3798) presents valid
information to be furtherly analysed.
Table 5 Mann-Whitney U Test by variable
obstetric mode of delivery (in Appendices).
Table 6 Mann-Whitney U Test by variable
jaundice (in Appendices).
Table 8 Mann-Whitney U Test by variable muscle
tone (between groups 1 and 3) (in Appendices).
BW
Box plo t b
y
Gr ou p
Variable: body weig ht
Median
25%-75%
Min -Ma x
01
obstetr i c mode of delivery
1000
1500
2000
2500
3000
3500
4000
4500
5000
body wei ght
BL
Bo x plo t b
y
Group
Variabl e: body leng th
Median
25%-75%
Min -Ma x
01
obstetri c mode of deli very
38
40
42
44
46
48
50
52
54
56
58
60
body l ength
GA
Box plot b
y
Group
Variable: g estational age
Median
25%-75%
Min -Max
01
obstetr i c mode of del ivery
20
40
60
80
100
120
140
160
180
200
220
240
260
280
300
320
gestational age
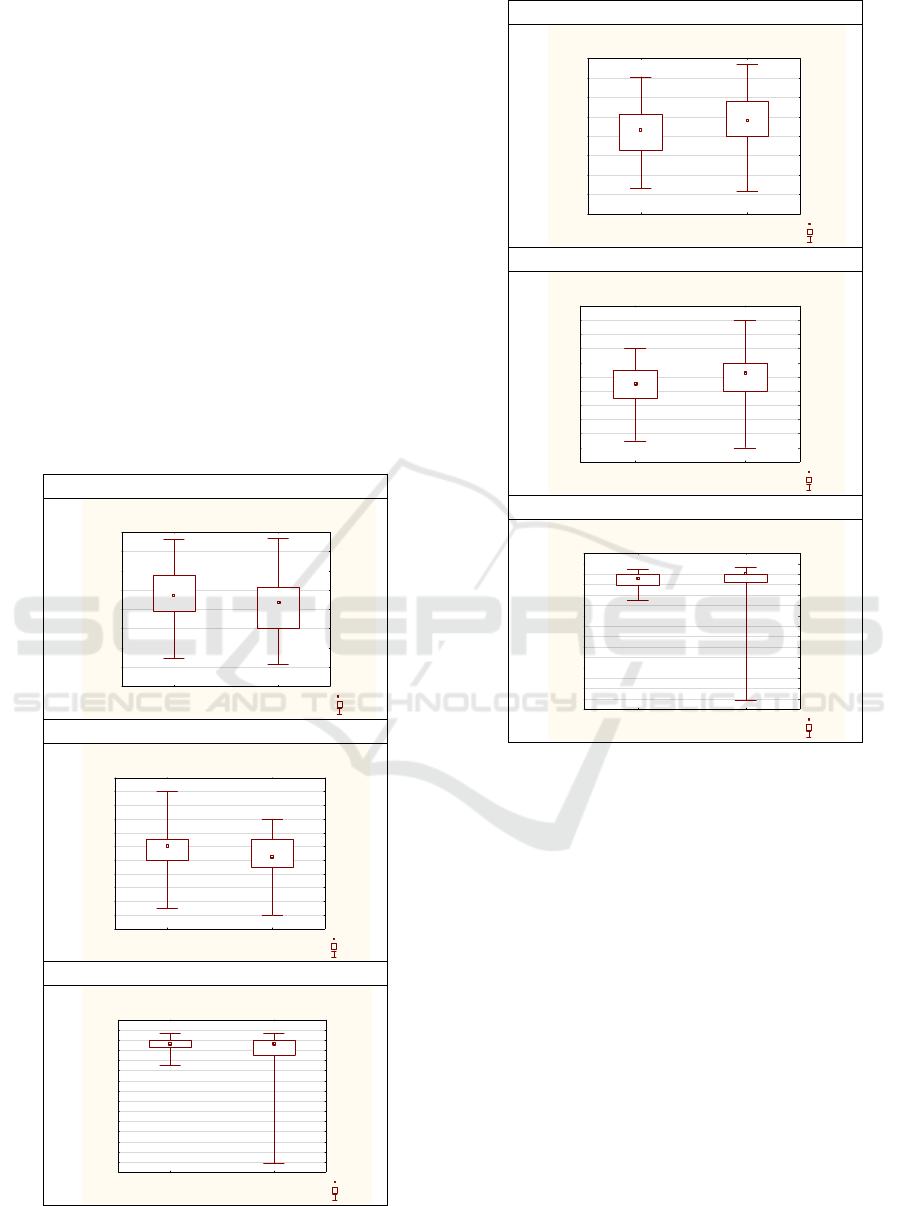
Figure 1: Boxplot by Group (obstetric mode of delivery).
BW
Bo x pl ot b
y
Gr ou p
Variabl e: body weig ht
Median
25%-75%
Min -Ma x
10
jaundi ce
1000
1500
2000
2500
3000
3500
4000
4500
5000
body weight
BL
Box p lot b
y
Gr ou p
Variable: body l ength
Median
25%-75%
Mi n- Ma x
10
jaundice
38
40
42
44
46
48
50
52
54
56
58
60
body l ength
GA
Box p lot b
y
Gr ou p
Variable: gestational age
Median
25%-75%
Mi n- Ma x
10
jaundi ce
20
40
60
80
100
120
140
160
180
200
220
240
260
280
300
320
gestational age
Figure 2: Boxplot by Group (jaundice).
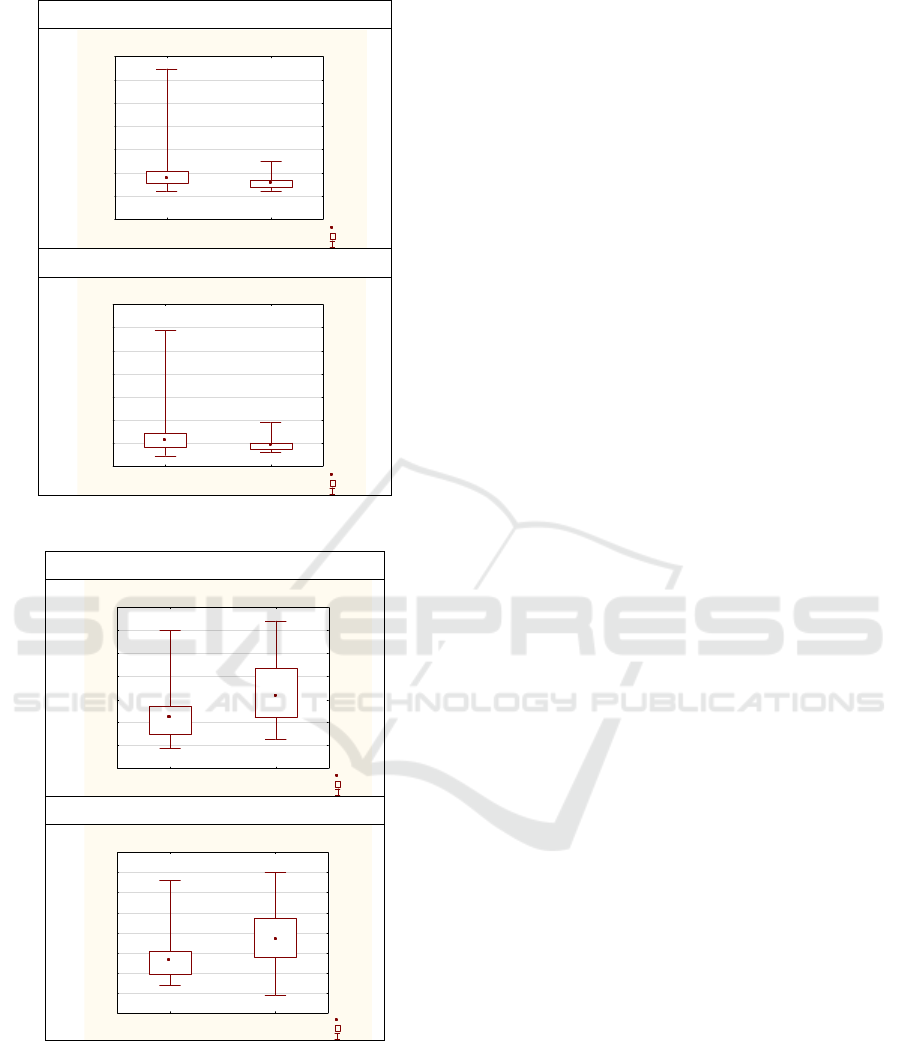
Within series of Mann-Whitney U Tests by
variable tone, among all groups 0-3 (0: hypo-,
1:hyper-, 2:normal-; 3:changing), relevant findings
are presented in table 7 and graph 3 (between groups
0 and 1), and Table 8 and Graph 4 (between groups 1
and 3).
Table 7 Mann-Whitney U Test by variable muscle
tone (between groups 0 and 1) (in Appendices).
4 DISCUSSION AND
CONCLUSIONS
Infants with hypotonia, hypertonia and changing
muscle tone are frequently associated with adjuvant
issues concerning developmental milestones and they
pose challenges for clinicians because they may
appear be the contributing sign of both benign and
serious conditions.
K-BioS 2023 - Special Session on Kinesiology in Sport and Medicine: from Biomechanics to Sociodynamics
240
AFE
B oxplot by Group
Variable: a
g
e at 1st
p
h
y
siat ric ex aminat ion
Median
25% -75%
Min-Max
01
muscle tone
-200
0
200
400
600
800
1000
1200
age at 1st physiatric examination
GA+AFE
B oxpl ot by Group
Variable:
g
est at ion a
g
e*a
g
e at 1st
p
h
y
.exam.
Median
25% -75%
Min-Max
01
muscle tone
200
400
600
800
1000
1200
1400
1600
gest at ion age*age at 1st phy. exam.
Figure 3: Boxplot by Group (muscle tone).
AFE
B oxplot by Group
Variable: a
g
e at 1st
p
h
y
siat ric examinat ion
Median
25% -75%
Min-Max
13
muscle tone
0
50
100
150
200
250
300
350
age at 1st physiat ric examination
GA+AFE
B oxplot by G roup
Variable:
g
est at ion a
g
e*a
g
e at 1st
p
h
y
.exam.
Median
25% -75%
Min-Max
13
muscle tone
250
300
350
400
450
500
550
600
650
gest at ion age*age at 1st phy. exam.
Figure 4: Boxplot by Group (muscle tone).
At first glance, the magnitude of the differential
diagnosis, the oddness of associated disturbances, and
the ongoing advances in diagnostic interpretation and
management may appear overwhelming (Leyenaar et
al., 2005). In prospective observational study infants
born by prelabour CS were compared with a group of
spontaneously born infants. Follow-up assessments
were performed at four and twelve months. Prelabour
CS (n = 66) had significantly lower results in „Ages
and Stages Questionnaire”; adverse
neurodevelopmental outcomes in infants born by
prelabour CS may be apparent already a few months
after birth (Zaigham et al., 2020). Taiwan Birth
Cohort Study was designed to assess the
developmental trajectories of 24 200 infants born in
2005. Their results implied that the association
between CS birth and infant’s neurodevelopmental
disorders was significantly influenced by GA. Infants
born by CS had significant increases in
neurodevelopmental disorders (20% compared with
infants born by spontaneous delivery) (Chen et al.,
2017). In this Swedish population-based birth cohort
study of more 1.1 million infants, births via planned
or intrapartum CS were associated with a moderately
increased risk of neurodevelopmental disorders in
infants, but these associations were attenuated after
adjusting for familial factors (Zhang et al., 2021). In
the representative sample of 4621 singleton infants
the objective of analyses was to determine whether
there are independent effects of BW and GA on motor
and social development (MSD) and the magnitude of
effects. Low BW status and preterm delivery are
associated independently with small, but measurable,
delays in MSD through early childhood and should be
considered along with other known risk factors for
development delays in determining the need for
developmental evaluation (Hediger et al., 2002). In
Irish study within the national sample represented of
73,662, infants born by elective or emergency CS
may face a delay in cognitive and motor development
at age of nine months (Khalaf et al., 2015). In a
systematic review there were 28 studies of small for
gestational (SGA), with a total of 7861 SGA and 91
619 control appropriate for gestational age (AGA)
babies, and three studies of fetal grow restriction
(FGR), with a total of 119 FGR and 49 control AGA
babies. The findings of the study demonstrate that
among infants born at term, being SGA is associated
with lower scores on neurodevelopmental outcomes
compared to AGA controls (Arcangeli et al., 2012).
Developmental changes in the immature central
nervous system have a large impact on the expression
of atypical motor development. It may happen that a
lesion of the developing brain results in neuromotor
dysfunction in infancy but is followed by a typical
developmental outcome. The reverse may also occur
e.g., an apparently typical development in the early
phases of infancy may be followed by the
development of worst neurodevelopmental outcome-
cerebral paralysis (Hadders-Algra, 2004). In infancy,
Differences and Relations Between Chrono-Biological and Motor-Functional Characteristics of Infants
241

atypical motor development may be conveyed by a
delay in the acquiring of developmental milestones
(which may be associated to impaired selection of
neurons), by mild, moderate or severe alteration in
muscle tone (velocity-dependent resistance to
stretch), by a persistence of infantile reflexes (e.g., the
Moro or Galant reflex), and by a reduced variation in
motor repertoire. The following manifestation may be
the most explicit expression of an early lesion of the
brain (Hadders-Algra, 2000), (Prechtl, 2001) whereas
the other signs may be the outcome of a lesion of the
brain but also may be complementary to other types
of adversities during early phase of development,
such as low-risk preterm delivery (Kostović & Judaš,
2007). Results of this study go in line with previous
findings regarding differences in BM (p<0,01), BL
(p<0,02), and GA (p<0,01), for CS vs spontaneously
born infants (Table 5).
Neurodevelopmental maturation may be impacted
by the ability of bilirubin to promote alterations in
synaptogenesis, neuritogenesis, and neurogenesis,
notably in the premature infant. These clinical
manifestations are characterized by the following
domains: neuromotor implications; muscle tone
abnormalities; hyperexcitable neonatal reflexes;
variety of neurobehavior manifestations, expression
and language articulation irregularities; and evolving
cluster of central processing abnormalities, such as
sensorineural, hearing, and visuomotor dysfunctions
(Bhutani & Johnson-Hamerman, 2015). In the study
among two groups of 20 infants’ moderate jaundice
is clearly associated with an increase in minor
neurologic disorder throughout the infancy in terms
of mild irregularities in muscle tone in combination
with indicative postural and reflex dysfunction at the
age of one year (Soorani-Lunsing et al., n.d.). Thirty-
nine term infants with moderate jaundice shortly after
delivery, were assessed and compared to 36 infants
born at term who did not develop neonatal jaundice.
The results of this prospective study demonstrate no
significant differences in neurodevelopmental
outcome parameters with respect to moderate
jaundice between the study groups at age of 3.
(Heimler & Sasidharan, 2010). The Dutch study
which enrolled 43 healthy term infants showed that
up to 18 months of age, term infants with moderate
degrees of hyperbilirubinemia have rates of minor
neurologic dysfunction similar to those of
comparison infants. (Soorani-Lunsing et al., n.d.).
The findings of I Soorani-Lunsing et al., study, which
used sensitive measures for neurodevelopmental
outcome, are in line with the results of three previous
studies among 41324 singleton infants that indicated
that moderate jaundice (bilirubin<342 µmol/l) in
healthy term infants does not affect subsequent
neuromotor outcome (Newman & Klebanoff, 1993).
The subtler symptoms of bilirubin-induced
neurodevelopmental dysfunction (BIND) may be
under-recognized and contribute to increased risk of
motor impairment such as developmental
coordination disorder and learning disabilities
(Johnson & Bhutani, 2011). It has been assumed that
preterm infants may be more predisposed to develop
hearing impairments, whereas on the other hand term
infants may more recurrently develop cerebral palsy
with associated motor disorders and cerebellar
impairment, due to the timing of the bilirubin toxicity
in relation to the developing brain area (Shapiro,
2003). Mild kernicterus may manifest with motor
symptoms including dystonia with or without
athetosis and mild gross motor delays such as late
developmental milestones such as age of initiation of
walking. These infants generally can ambulate well
on their own later in childhood and can speak with
some clarity. Infants exposed to lower TB levels, not
severe enough to cause kernicterus, may have mild
damage to the basal ganglia and cerebellum that may
manifest as mild hypotonia, lack of coordination, or
generalized clumsiness – not severe enough to be
classified as a specific movement disorder (Rose &
Vassar, 2015). However, the influence of exposure to
low-moderate levels of total bilirubin on the
developing CNS is not well understood. Further
analysis is needed to identify the range of motor
impairments that may result from neonatal jaundice,
to perceive the interplay between perinatal risk
factors and bilirubin toxicity, and to develop
enhanced neuroprotective treatment for motor
disorders related to jaundice (Bhutani & Johnson-
Hamerman, 2015). Numerous retrospective studies
have tried to support or refute the relationship of
neonatal jaundice with neurodevelopmental
outcomes. A specific objective in understanding this
correlation has been the use of differing measures of
neurodevelopment. Although neonatal jaundice is
quite common, affecting 60%-80% of new-borns
overall (Chou et al., 2003), observational data have
implicated neonatal jaundice with an elevated risk of
later NDI, though these findings have not always been
reproduced in following studies (Wusthoff & Loe,
2015).
Results of this study also go in line with previous
findings regarding jaundice (Table 6) and differences
in BM (p<0,01), BL (p<0,01), and, with limited
statistical power, in GA (p=0,00).
Within current research, transversal contextual
insights are also necessary to empower understandings
of the main findings. E.g. when analysing the
K-BioS 2023 - Special Session on Kinesiology in Sport and Medicine: from Biomechanics to Sociodynamics
242

succession of the child in a family (especially between
second and forth) statistically significant differences
for BW p<0,01, BL p<0,01 and AFE p<0,02 may be
pointed out, as well with regard to succession of
pregnancy (first and second) for BW p<0,001, BL
p<0,001, and with limited statistical power to GA
p<0,022). In line with statistically significant
differences in chrono-biological variables depending
upon the different obstetric modes of delivery (Table
5, Graph 1), as well upon neonatal jaundice (Table 6,
Graph 2) importance of correlations among chrono-
biological variables is also introduced (Table 2) -
statistically significant for BW, BL&GA: 0,62-0,88).
When broadened with and compared to infant’s AFE,
individually and combined with GA (Table 2),
correlation matrix (SB: 0,15-0,95) implies the
importance of further inter-parametrical insights – in
this case with relation to muscle tone classified in 4
groups (normal-, hypo-, hyper-, and changing-).
The most relevant information for the
understanding goal of this research regarding
representative sample is that - infants with diagnosed
increased muscle tone appear to be much earlier
referred from primary care to a physiatrist
(AS±SD:115,68±51,86 days, in Table 4, compared to
average total AS±SD:158,36±110,91 days, in Table
1). The main findings confirm statistically significant
differences between infants differently categorized by
muscle tone and infant’s AFE - among hypertonic and
hypotonic (AFE: p=0,00; GA+ AFE: p<0,01; Table 7;
Graph 3) as well among hypertonic and changing tone
respectively (AFE: p=0,00; GA+ AFE: p<0,01; Table
8; Graph 4).
Conclusively - although there are no correlations
between the AFE of the infant with BW, BL (and with
GA they are very little correlated - 0.19; Table 2),
based on previously presented and discussed findings,
it seems that there is a quantitative and measurable
indication that the existing categorization by muscle
tone indicates the need for more frequent or earlier
short 'screening'. It should be embedded into existing
official protocols and communication by which it is
possible to intervene early enough towards a balanced
overall health of the infant.
The main limitation of the study is not
predominantly within a variability of clinical practices,
but due to different patterns of reference of infants with
diagnosed increased muscle tone - from primary care
to a physiatrist. Also, future studies must be adequately
powered to examine neurodevelopmental impairments
due to immature brain lesion among preterm infants
separately from term infants. The next step within
neurodevelopmental disorders would be stratification
obstetric mode of delivery in terms of spontaneous or
instrumental vaginal delivery, elective or urgent
caesarean section and accompanying severity of
manifested neonatal jaundice.
REFERENCES
Arcangeli, T., Thilaganathan, B., Hooper, R., Khan, K. S.,
& Bhide, A. (2012). Neurodevelopmental delay in
small babies at term: a systematic review. Ultrasound in
Obstetrics & Gynecology : The Official Journal of the
International Society of Ultrasound in Obstetrics and
Gynecology, 40(3), 267–275. https://doi.org/
10.1002/UOG.11112
Bhutani, V. K., & Johnson-Hamerman, L. (2015). The
clinical syndrome of bilirubin-induced neurologic
dysfunction. Seminars in Fetal & Neonatal Medicine,
20(1), 6–13. https://doi.org/10.1016/J.SINY.2014.
12.008
Bobath K, B. B. (1984). The neuro-developmental treatment.
In Scrutton D editor(s). Management of the Motor
Disorders of Children with Cerebral Palsy. (pp. 6–18).
Casey, B. M., McIntire, D. D., & Leveno, K. J. (2001). The
Continuing Value of the Apgar Score for the
Assessment of Newborn Infants. New England Journal
of Medicine, 344(7), 467–471. https://doi.org/10.1056/
NEJM200102153440701
Chen, G., Chiang, W. L., Shu, B. C., Guo, Y. L., Chiou, S.
T., & Chiang, T. L. (2017). Associations of caesarean
delivery and the occurrence of neurodevelopmental
disorders, asthma or obesity in childhood based on
Taiwan birth cohort study. BMJ Open, 7(9).
https://doi.org/10.1136/bmjopen-2017-017086
Chou, S. C., Palmer, R. H., Ezhuthachan, S., Newman, C.,
Pradell-Boyd, B., Maisels, M. J., & Testa, M. A.
(2003). Management of hyperbilirubinemia in
newborns: measuring performance by using a
benchmarking model. Pediatrics, 112(6 Pt 1), 1264–
1273. https://doi.org/10.1542/PEDS.112.6.1264
Cifrek, M., Gruić, I., & Medved, V. (2021). Kinesiological
Electromyography. In Measurement and Analysis of
Human Locomotion (pp. 171–218). Springer, Cham.
https://doi.org/10.1007/978-3-030-79685-3_9
Crawford, T. O. (1992). Clinical evaluation of the floppy
infant. Pediatric Annals, 21(6), 348–354. https://
doi.org/10.3928/0090-4481-19920601-06
Goo, M., Tucker, K., & Johnston, L. M. (2018). Muscle tone
assessments for children aged 0 to 12 years: a systematic
review. Developmental Medicine and Child Neurology,
60(7), 660–671. https://doi.org/10.1111/DMCN.13668
Hadders-Algra, M. (2000). The neuronal group selection
theory: promising principles for understanding and
treating developmental motor disorders. Develop-
mental Medicine and Child Neurology, 42(10), 707–
715. https://doi.org/10.1017/S0012162200001316
Hadders-Algra, M. (2004). General movements: A window
for early identification of children at high risk for
developmental disorders. The Journal of Pediatrics,
145(2 Suppl). https://doi.org/10.1016/J.JPEDS.2004.05.
017
Differences and Relations Between Chrono-Biological and Motor-Functional Characteristics of Infants
243

Hadders-Algra, M., Heineman, K. R., Bos, A. F., &
Middelburg, K. J. (2010). The assessment of minor
neurological dysfunction in infancy using the Touwen
Infant Neurological Examination: strengths and
limitations. Developmental Medicine & Child
Neurology, 52(1), 87–92. https://doi.org/10.1111/
J.1469-8749.2009.03305.X
Hediger, M. L., Overpeck, M. D., Ruan, W. J., & Troendle,
J. F. (2002). Birthweight and gestational age effects on
motor and social development. Paediatric and Perinatal
Epidemiology, 16(1), 33–46. https://doi.org/10.1046/
j.1365-3016.2002.00393.x
Heimler, R., & Sasidharan, P. (2010). Neurodevelopmental
and audiological outcome of healthy term newborns with
moderately severe non-haemolytic hyperbilirubinemia.
Journal of Paediatrics and Child Health, 46(10), 588–
591. https://doi.org/10.1111/J.1440-1754.2010.01800.X
Johnson, L., & Bhutani, V. K. (2011). The Clinical
Syndrome of Bilirubin-Induced Neurologic
Dysfunction. Seminars in Perinatology, 35(3), 101–
113. https://doi.org/10.1053/j.semperi.2011.02.003
Kathrein, J. E. (1990). Interrater reliability in the
assessment of muscle tone of infants and children.
Physical and Occupational Therapy in Pediatrics, 10(1),
27–41. https://doi.org/10.1080/J006V10N01_04
Keren, R., Luan, X., Friedman, S., Saddlemire, S., Cnaan,
A., & Bhutani, V. K. (2008). A comparison of
alternative risk-assessment strategies for predicting
significant neonatal hyperbilirubinemia in term and
near-term infants. Pediatrics, 121(1). https://doi.org/
10.1542/PEDS.2006-3499
Khalaf, S. Y. A., O’Neill, S. M., O’Keeffe, L. M.,
Henriksen, T. B., Kenny, L. C., Cryan, J. F., &
Khashan, A. S. (2015). The impact of obstetric mode of
delivery on childhood behavior. Social Psychiatry and
Psychiatric Epidemiology, 50(10), 1557–1567.
https://doi.org/10.1007/S00127-015-1055-9
Kostović, I., & Judaš, M. (2007). Transient patterns of
cortical lamination during prenatal life: do they have
implications for treatment? Neuroscience and
Biobehavioral Reviews, 31(8), 1157–1168.
https://doi.org/10.1016/J.NEUBIOREV.2007.04.018
Lazić, L., Spalević, M., Zlatanović, D., Stanković, A., &
Marinković, O. (2011). Habilitation treatment of
hypertonia in newborns and infants. Acta Medica
Medianae, 50(1), 22–25. https://doi.org/10.5633/
AMM.2011.0104
Leyenaar, J. A., Camfield, P., & Camfield, C. (2005). A
schematic approach to hypotonia in infancy. Paediatrics
& Child Health, 10(7), 397–400. https://doi.org/10.
1093/PCH/10.7.397
Lindahl, E., Michelsson, K., Helenius, M., & Parre, M.
(1988). NEONATAL RISK FACTORS AND LATER
NEURODEVELOPMENTAL DISTURBANCES.
Developmental Medicine & Child Neurology, 30(5),
571–589. https://doi.org/10.1111/J.1469-8749.1988.
TB04795.X
Linder, N., Tsur, M., Kuint, J., German, B., Birenbaum, E.,
Mazkereth, R., Lubin, D., Reichman, B., & Barzilai, A.
(1998). A simple clinical test for differentiating
physiological from pathological head lag in full-term
newborn infants. European Journal of Pediatrics, 157(6),
502–504. https://doi.org/10.1007/S004310050863
Newman, T. B., & Klebanoff, M. A. (1993). Neonatal
hyperbilirubinemia and long-term outcome: another
look at the Collaborative Perinatal Project. Pediatrics,
92(5), 651–657. https://doi.org/10.1542/peds.92.5.651
Peredo, D., Review, M. H.-P. in, & 2009, undefined.
(2009). The Floppy InfantEvaluation of Hypotonia.
Publications.Aap.Org. https://doi.org/10.1542/pir.30-
9-e66
Prechtl, H. F. R. (2001). General movement assessment as
a method of developmental neurology: new paradigms
and their consequences. The 1999 Ronnie MacKeith
lecture. Developmental Medicine and Child Neurology,
43(12), 836. https://doi.org/10.1017/S00121622010 015
29
Rose, J., & Vassar, R. (2015). Movement disorders due to
bilirubin toxicity. Seminars in Fetal & Neonatal
Medicine, 20(1), 20–25. https://doi.org/10.1016/
J.SINY.2014.11.002
Sanger, T. D., Delgado, M. R., Gaebler-Spira, D., Hallett,
M., & Mink, J. W. (2003). Classification and definition
of disorders causing hypertonia in childhood. Pediatrics,
111(1), e89–e97. https://doi.org/10.1542/PEDS.111.1 .E
89
Sarici, S. Ü., Serdar, M. A., Korkmaz, A., Erdem, G., Oran,
O., Tekinalp, G., Yurdakök, M., & Yigit, S. (2004).
Incidence, course, and prediction of hyperbilirubinemia
in near-term and term newborns. Pediatrics, 113(4),
775–780. https://doi.org/10.1542/PEDS.113.4.775
Shapiro, S. M. (2003). Bilirubin toxicity in the developing
nervous system. Pediatric Neurology, 29(5), 410–421.
https://doi.org/10.1016/J.PEDIATRNEUROL.2003.09
.011
Soorani-Lunsing, I., Woltil, H. A., & Hadders-Algra, M.
(2001). Are moderate degrees of hyperbilirubinemia in
healthy term neonates really safe for the brain?
Pediatric Research, 50(6), 701–705. https://doi.org/
10.1203/00006450-200112000-00012
Soorani-Lunsing, I., Woltil, H., Research, M. H.-A.-P., &
2001, undefined. (n.d.). Are moderate degrees of
hyperbilirubinemia in healthy term neonates really safe
for the brain? Nature.Com.
Wusthoff, C. J., & Loe, I. M. (2015). Impact of bilirubin-
induced neurologic dysfunction on neurodevelopmental
outcomes. Seminars in Fetal and Neonatal Medicine,
20(1), 52–57. https://doi.org/10.1016/j.siny.2014.12.003
Zaigham, M., Hellström-Westas, L., Domellöf, M., &
Andersson, O. (2020). Prelabour caesarean section and
neurodevelopmental outcome at 4 and 12 months of age:
an observational study. BMC Pregnancy and Childbirth,
20(1). https://doi.org/10.1186/S12884-020-03253-8
Zhang, T., Brander, G., Mantel, A., Kuja-Halkola, R.,
Stephansson, O., Chang, Z., Larsson, H., Mataix-Cols,
D., & Fernández De La Cruz, L. (2021). Assessment of
Cesarean Delivery and Neurodevelopmental and
Psychiatric Disorders in the Children of a Population-
Based Swedish Birth Cohort. JAMA Network Open,
4(3). https://doi.org/10.1001/jamanetworkopen.2021.
0837n.
K-BioS 2023 - Special Session on Kinesiology in Sport and Medicine: from Biomechanics to Sociodynamics
244

APPENDIX
Table 1: Descriptive statistics and normality distribution test.
All (N=179) Mean±SD
Minimu
m
Maximu
m
Skewness Kurtosis
Normality
BW
3267,77±708,69 1575,00 4850,00 -0,16 -0,31
K-S d=0,06, p> 0.20;
BL
49,33±3,09 40,00 58,00 -0,38 0,50
K-S d=0,13, p<0,01
GA
268,46±23,05 38,00 294,00 -5,84 55,57
K-S d=0,17, p<0,01
GA+ AFE
426,83±114,58 256,00 1375,00 3,73 26,22
K-S d=0,15, p<0,01
AFE
158,36±110,91 18,00 1095,00 3,94 28,49
K-S d=0,16, p<0,01
Table 2: Spearman Rank Order Correlations.
BW
BL GA AFE GA+AFE
BW
1,00 0,87* 0,67* 0,04 0,19*
BL
0,87* 1,00 0,61* 0,02 0,15*
GA
0,67* 0,61* 1,00 0,18 0,39*
AFE
0,04 0,02 0,18* 1,00 0,95*
GA +AFE
0,19* 0,15* 0,39* 0,95* 1,00
*Marked correlations are significant at p <,05.
Table 3: Descriptive statistics analysis according to obstetric mode of delivery and jaundice.
Mean±SD
0(spontaneous): (n=113) 1(CS): (n=66) 0(no jaundice): (n=92 1(jaundice): (n=87)
BW 3381,48±670,93* 3073,09±734,01* 3409,39±713,59* 3118,02±675,69*
BL 49,75±3,031 48,61±3,07* 49,89±3,29 48,74±2,75
GA 272,03±13,92 262,38±32,57 270,50±28,28 266,32±15,62
GA+ AFE 428,58±124,58 423,83±95,92* 428,39±127,82 425,18±99,42
AFE 156,56±122,32 161,45±88,84 157,89±125,11 158,86±94,33
*Normally distributed results K-S test: d, for p> .20.
Table 4: Descriptive statistical analysis according to muscle tone.
Mean±SD 0:hypo (n=88) 1:hyper (n=40) 2:normal (n=23) 3:changing (n=28)
BW
3248,52±694,44* 3297,70±741,39* 3398,57±831,78* 3178,12±611,63*
BL
49,28±3,22 49,20±3,12 49,96±3,23 49,14±2,52*
GA
266,47±29,09 271,60±14,94 271,00±16,74 268,21±13,81
GA + AFE
442,89±133,53 387,28±55,38* 422,35±135,14* 436,57±80,76*
AFE
176,42±129,90 115,68±51,86* 151,35±127,59* 168,36±75,28*
*Normally distributed results K-S test: d, for p> .20.
Differences and Relations Between Chrono-Biological and Motor-Functional Characteristics of Infants
245

Table 5: Mann-Whitney U Test by variable obstetric mode of delivery.
Rank Sum
Group 1
Rank Sum
Group 2
U
Z
p
-value
Zad
j
uste
d
p
-value
Valid N
Group 1
Valid N
Group 2
BW
11056,00 5054,00 2843,00 2,64 0,01 2,65 0,01 113 66
BL
10931,50 5178,00 2967,50 2,26 0,02 2,29 0,02 113 66
GA
10987,50 5122,50 2911,50 2,44 0,01 2,48 0,01 113 66
*AFE
9874,50 6235,50 3433,50 -0,88 0,37 -0,88 0,38 113 66
*GA+AFE
10164,50 5945,00 3723,50 -0,01 0,99 -0,014 0,99 113 66
*Interpretations are limited due to results of power/sample size analyses/calculations.
Table 6: Mann-Whitney U Test by variable jaundice.
Rank Sum
Group 1
Rank Sum
Group 2
U
Z
p
-value
Z
ad
j
uste
d
p
-value
Valid N
Group 1
Valid N
Group 2
BW
6896,00 9214,00 3068,00 -2,69 0,01 -2,69 0,01 87 92
BL
6854,50 9255,50 3026,50 -2,81 0,01 -2,83 0,01 87 92
GA
6769,50 9340,50 2941,50 -3,06 0,00 -3,10 0,00 87 92
*AFE
7885,00 8225,00 3947,00 0,16 0,88 0,16 0,87 87 92
*GA+AFE 7722,500 8387,50 3894,50 -0,31 0,76 -0,31 0,76 87 92
*Interpretations are limited due to results of power/sample size analyses/calculations.
Table 7: Mann-Whitney U Test by variable muscle tone (between groups 0 and 1).
Rank
Sum Group 1
Rank
Sum Group 2
U
Z
p
-value
Zad
j
uste
d
p
-value
Valid N
Group 1
Valid N
Group 2
2*1sided
exact p
AFE
6329,50 1926,50 1106,50 3,36 0,00 3,36 0,00 88 40 0,00
GA+ AFE 6213,00 2043,00 1223,00 2,76 0,01 2,76 0,01 88 40 0,01
Table 8: Mann-Whitney U Test by variable muscle tone (between groups 1 and 3).
Rank
Sum Group 1
Rank
Sum Group 2
U
Z
p
-value
Z
ad
j
uste
d
p
-value
Valid N
Group 1
Valid N
Group 2
2*1sided
exact p
AFE
1128,00 1218,00 308,00 -3,130,00 -3,14 0,00 40 28 0,00
GA+AFE 1158,00 1188,00 338,00 -2,760,01 -2,76 0,01 40 28 0,01
K-BioS 2023 - Special Session on Kinesiology in Sport and Medicine: from Biomechanics to Sociodynamics
246
