
AN ADAPTIVE REGION GROWING SEGMENTATION FOR
BLOOD VESSEL DETECTION FROM RETINAL IMAGES
Md. Alauddin Bhuiyan, Baikunth Nath and Joselito Chua
Computer Science and Software Engineering, The University of Melbourne
Melbourne, Australia 3010
Keywords: Medical Image, Blood Vessel, Adaptive Region Growing technique, Gradient Operator, Segmentation.
Abstract: Blood vessel segmentation from the retinal images is extremely important for assessing retinal
abnormalities. A good amount of research has been reported on blood vessel segmentation, but significant
improvement is still a necessity particularly on minor vessel segmentation. As the local contrast of blood
vessels is unstable (intensity variation), especially in unhealthy retinal images, it becomes very complicated
to detect the vessels from the retinal images. In this paper, we propose an edge based vessel segmentation
technique to overcome the problem of large intensity variation between major and minor vessels. The edge
is detected by considering the adaptive value of gradient employing Region Growing Algorithm, from
where parallel edges are computed to select vessels. Our proposed method is efficient and performs well in
detecting blood vessels including minor vessels.
1 INTRODUCTION
Automatic detection of blood vessels in the retinal
images can help physicians with diagnosing ocular
diseases, patient screening, clinical study, etc. For
instance, a patient may exhibit discoloration of the
optic nerve, or a narrowing of the blood vessels in
the retina. An ophthalmologist (a medical doctor
specialized in the structure, function, and diseases of
the human eye) uses this information to diagnose the
patient, as having for instance Coats' disease or a
central retinal artery occlusion. A common
procedure to examine the eye health is passing
through the procedure of retinal imaging. An optical
camera (for instance, Mydriatic and non-mydriatic
retinal cameras) is used to see through the pupil of
the eye to the rear inner surface of the eyeball. A
picture is taken showing the optic nerve, fovea,
surrounding vessels, and the retinal layer. The
ophthalmologist can then reference this image while
considering any observed findings.
The most effective treatment for many eye
related diseases is the early detection through regular
screening. Assessment of the characteristics of
vessels in the retina plays an important role in
medical diagnoses. For these tasks measurements are
needed of e.g., vessel width, colour, reflectivity,
tortuosity, abnormal branching, or have the
occurrence of vessels of a certain width. Blood
vessel appearance can provide information on
pathological changes caused by some diseases
including diabetes, hypertension, and
arteriosclerosis. Changes in retinal vasculature, such
as haemorrhages, angiogenesis; increases in vessel
tortuosity, blockages and arteriolar-venular diameter
ratios are important indicators of, for example,
diabetic retinopathy, and retinopathy of prematurity
and cardiovascular risk. Information about blood
vessels in retinal images can be used in grading
disease severity or as part of the process of
automated diagnosis of diseases (Hoover et al.
2000).
The automatic detection of blood vessels is very
important as ophthalmologist can potentially screen
larger populations for vessel abnormalities. In
contrast, manual delineation of vessels becomes
tedious or even impossible when the number of
vessels in an image is large or when a large number
of images are acquired. Furthermore, a detection and
segmentation of the vascular tree seems to be the
most appropriate representation for the entire retinal
image due to three following reasons. Firstly, it
maps the whole retina. Secondly, it does not move
except in a few diseases. Finally, it contains enough
404
Alauddin Bhuiyan M., Nath B. and Chua J. (2007).
AN ADAPTIVE REGION GROWING SEGMENTATION FOR BLOOD VESSEL DETECTION FROM RETINAL IMAGES.
In Proceedings of the Second International Conference on Computer Vision Theory and Applications - IFP/IA, pages 404-409
Copyright
c
SciTePress

information for the localization of some anchor
points (Chanwimaluang and Fan 2003).
Automated retinal segmentation is complicated
by the fact that the width of the retinal vessels can
vary from large to very small, and that the local
contrast of vessel is unstable, especially in unhealthy
retinal images (Li et al. 2006). Although a large
amount of research (Martinez-Perez et al. 1999;
Zana and Klein 1999; Hoover et al. 2000; Jiang and
Mojon 2001; and Li et al. 2006) has been published
for the detection of blood vessels, a huge
improvement in detection procedures remains a
necessity for the detection of small vessels
(branches).
In this paper we propose a novel vessel
segmentation technique based on vessel edges. The
proposed method employs the adaptive region
growing segmentation algorithm to overcome the
complexity of edge segmentation, as we cannot trace
them with a fixed intensity or gradient direction. The
gray values of the vessels in the retinal image are
changeable throughout the entire image and gradient
direction is not constant due to curvilinear structure
of the vessels. Based on these phenomena, it is
appropriate to apply the Adaptive Region Growing
(ARG) algorithm to detect the edges of vessels.
After segmenting the edges, we locate the parallel
edges based on gradient direction considering pixel
position of each region. These parallel edges are
considered as primary vessels and facilitate to
remove the noise and other objects. Finally, we map
the original retinal image based on pixel positions of
that segmented gradient image to detect the blood
vessels.
The rest of the paper is organized as follows:
section 2 presents a brief background and review of
the related segmentation based literature and section
3 discusses our proposed Adaptive Region Growing
segmentation based blood vessel detection method.
Section 4 provides preliminary results and
discussion. Finally, conclusions and future research
directions are drawn in section 5.
2 BACKGROUND LITERATURE
Previous methods for blood vessel detection in
images of the retina can be categorized into two
groups. The tracking based approach and Template
or Model based approach. Tracking based
approaches work by first locating an initial point and
then exploiting local image properties to trace the
vasculature recursively. This technique may require
user intervention and appear to have proclivity for
termination near branch points. Model based
approaches apply explicit vessel models to extract
the vasculature.
Chaudhuri et al. (Chaudhuri et al. 1989)
introduced an algorithm based on directional two-
dimensional (2-D) matched filters to detect
piecewise linear segments of blood vessels. Twelve
different templates were used to search for vessel
segments along all possible directions. This method
is completely unsupervised and good for initial
estimation. However, the detected vessels may not
be continuous and small vessels get missed and
validity of the detected vessels is not checked.
Tolias and Panas (Tolias and Panas 1998)
presented a fuzzy vessel tracking algorithm for
retinal images based on fuzzy clustering. Salient
regions are initialized as starting point for vessel
tracking. Then Fuzzy C-means clustering algorithm
determines vessel and non-vessel region along a
vessel profile. Then trace the vessel based on
thresholding the membership values. This is also an
unsupervised technique and performed well on
detecting major vessels (98%). Nonetheless, this
algorithm still suffers from missing a large number
of minor vessels (detection rate only 23.53%).
Mendonca and Campilho (Mendonca and
Campilho 2006) proposed an algorithm based on
region growing process using vessel centreline as
seed point. Multi-scale morphological vessel
enhancement applying a modified top-hat transform
with variable size structuring elements is performed.
In order to obtain binary maps of the vessels a
binary morphological reconstruction is used. A set
of four directional differences of offset Gaussian
filters is used to detect vessel centreline. Vessel
filling by region growing process using as initial
seeds the pixel within the centrelines is used. The
growing is successively applied to the four scales
and, in each region growing step; the seed image is
the result of the previous aggregation. This
technique shows an improved detection rate with
accuracy of 94.7%.
Staal et al. (Staal et al. 2004) presented a ridge
based vessel segmentation algorithm in color retinal
images. The ridge is detected by applying Gaussian
scale space technique and grouped by applying
region growing algorithm. Therefore, the image is
grouped into patches or convex sets. Features of the
convex sets and the pixels belonging o the convex
sets are considered to construct vectors and
classified by the KNN (k-nearest neighbour)
classifier. Convex sets’ features are height, width
and their ratio, curvature, distance between first and
last points of a convex set, mean and standard
AN ADAPTIVE REGION GROWING SEGMENTATION FOR BLOOD VESSEL DETECTION FROM RETINAL
IMAGES
405

deviation of a green patch, etc. Pixel features are
value of red and green plane of the image at the
pixel location and their ratio, etc. This technique
also shows promising detection rate with maximum
accuracy of 0.944.
Wu et al. (Wu et al. 2006) introduced an adaptive
detection of blood vessels in the retinal images. At
first the blood vessel enhancement is performed by
adaptive histogram equalization technique. Then
vessels features are extracted using the standard
deviation of Gabor filter responses along different
orientations. Finally, the vessel is traced using
forward detection, backward verification and
bifurcation detection. The overall detection rate is
80.15% while small vessel pixel detection rate 42%
and small vessel detection rate 75%.
Jiang and Mojon (Jiang and Mojon 2003)
presented an adaptive local thresholding technique
by verification-based multithreshold probing to
detect blood vessels in the retinal images. At first,
the original retinal image is converted into binary
image through multiple thresholding by considering
curvilinear structure and width of the vessels. Then
Euclidian distance transformation from candidate
vessel point to background point is performed.
Following that the vessel candidate is pruned by
means of the distance map to only retain centreline
pixels (considering distance of two nearest
background pixel & angle from these two points) of
curvilinear bands. Finally, the curvilinear bands are
reconstructed from their centreline pixels. The
reconstructed curvilinear bands give the part of the
vessel network that is made visible by the particular
threshold. The overall detection rate reported is
86.5%. This technique needs further improvement in
vessel detection and background noise suppression.
Zana and Klein (Zana and Klein 2001) presented
a vessel segmentation algorithm using mathematical
morphology and curvature evaluation. At first the
vessels are highlighted using their morphological
properties (sum of top hats reduces small bright
noise and improve the contrast of all linear part).
After that the cross curvature is evaluated using the
Laplacian operator. Then the alternating filter is
used to produce the final result. The technique is not
sensitive to sudden changes in the global gray level.
However, results in missing pixels of the dilated line
because of surrounding texture.
Hoover et al. (Hoover et al. 2000) proposed an
algorithm for locating blood vessels in retinal
images by piece-wise threshold probing of a
Matched Filter Response (MFR). At first, the
original image is filtered by MFR. Then the filtered
image is thresholded and thinned. Finally, use the
probing technique while the probe examines the
image in pieces (initial threshold is the MFR image
value at the starting pixel, then regions grow using a
conditional paint-fill technique), testing a number of
region based properties (e.g., segment length). If the
probe decides a piece is vessel (if the resulting
region belong to a minimum number of threshold
pixels but less than maximum or connects two
previously probed pieces, then the region is labelled
as vessel), then the constituent pixels are
simultaneously segmented and classified. The
overall detection rate is 90% true positive and 4%
false positive. This technique has limitations on
detecting background or non vessel removal.
3 PROPOSED METHOD
As we mentioned earlier that the automated retinal
segmentation is complicated by the fact that the
local contrast of vessels is unstable, the width of
retinal vessels can vary from very large to very small
especially unhealthy ocular fundus images. We
present a method for the segmentation of blood
vessels in retinal images based on the partial
derivative of intensity image, which gives
information about its topology and also overcome
the problem of image intensity variation. We use
STARE (Hoover 2002) retinal imaging dataset. Our
proposed method performed much better in
detecting both major and minor vessels.
The procedure is as follows: at first we enhance
the contrast of the original retinal image by applying
Adaptive Histogram Equalization method then we
apply the first order directional derivative operator,
normalize it and convert the original image into
gradient image. Each vessel will show up as parallel
edges, which will be segmented by applying
Adaptive region growing algorithm. Since the
contrast of the vessels is unstable it is not viable to
apply a threshold value to segment the edges of a
vessel. Due to the curvilinear structure of the vessels
the gradient direction is also changeable. So, we
apply Adaptive value of gradient magnitude with
region growing process to segment the edges.
Parallel edges are selected considering the gradient
direction of each pixel belonging to the parallel
regions. We can, therefore, segment the vessels and
remove the background noise and other objects.
Finally, we map the vessel pixels from the original
retinal image based on the segmented gradient image
to show the detected vessels.
VISAPP 2007 - International Conference on Computer Vision Theory and Applications
406
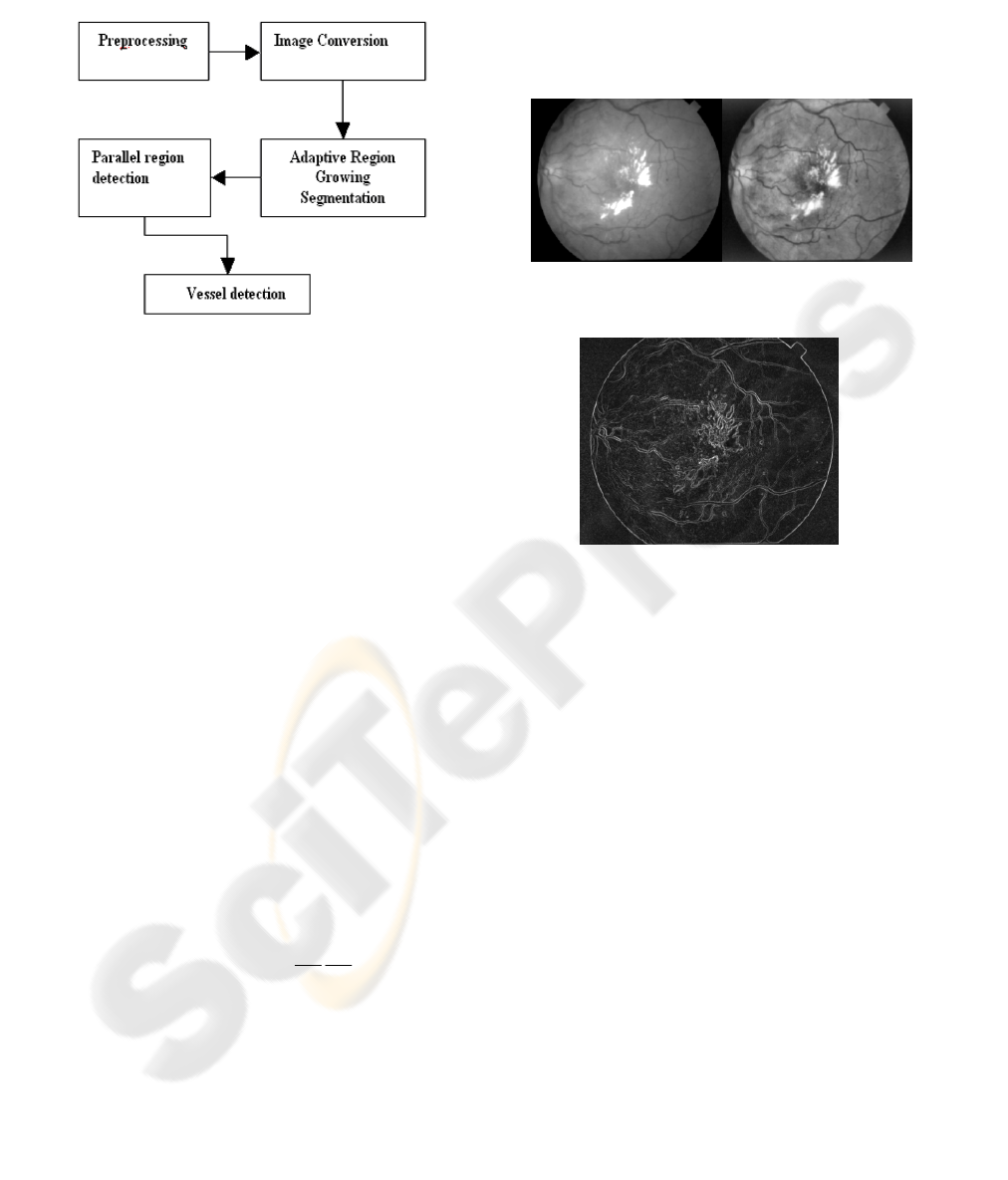
Figure 1 displays the overall technique of our
proposed method. We describe each step in detail in
the following subsections.
Figure 1: The vessel segmentation model.
3.1 Preprocessing of Retinal Image
In the preprocessing step the aim is to enhance the
contrast of the original retinal image. The Adaptive
Histogram Equalization method is implemented,
using MATLAB, to enhance the contrast of the
image intensity by transforming the values using
contrast-limited adaptive histogram equalization. It
operates on small regions in the image, called tiles,
rather than on the entire image. Each tile contrast is
enhanced, so that the histogram of the output region
approximately matches the histogram specified by
the 'Distribution' parameter. The neighboring tiles
are then combined using bilinear interpolation to
eliminate artificially induced boundaries.
3.2 Image Conversion
The enhanced retinal image is converted into
gradient image using first order partial differential
operator. The gradient of an image f(x,y) at location
(x,y) is defined as the two dimensional vector
(Gonzalez and Wintz 1987)
G [f(x,y)]= [G
x
G
y
] =
⎥
⎦
⎤
⎢
⎣
⎡
∂
∂
∂
∂
y
f
x
f
(1)
It is well known from vector analysis that the
vector G points in the direction of maximum rate of
change of f at location (x,y). For edge detection, we
are interested in the magnitude of the vector,
generally referred to simply as the gradient and
denoted G [f(x,y)] and commonly takes the value of
G [f(x,y)]
≈ |G
x
| +
|G
y
|. (2)
The direction of the gradient vector is calculated
as follows. Letting
),( yx
α
represent the direction
angle of G at location (x,y),
),( yx
α
=tan
-1
(G
y
/ G
x
) (3)
where the angle is measured with respect to the x-
axis.
Figure 2: Original retinal green channel image (left) and
its Adaptive Histogram Equalized Image (right).
Figure 3: Gradient image of the Adaptive histogram
equalized of Figure 2.
3.3 Adaptive Region Growing
Technique
The edges of vessels are segmented using region
growing procedure that groups pixels or sub regions
into larger regions based on gradient magnitude. As
the gradient magnitude is not constant for the whole
vessel we need to consider an adaptive gradient
value that gradually increases or decreases to append
the pixel to a region. We call it an adaptive
procedure, as the difference of neighbouring pixels
intensity value is always adapted for the region
growing process.
The region growing process starts with
appending the pixels that pass certain threshold
value (Gonzalez, Woods et al. 2004). For region
growing we find the intensity difference between a
pixel belonging to a region and its neighbouring
potential region growing pixels. The pixel is
considered for appending in that region if the
difference is less than a threshold value. The
threshold value is calculated by considering the
maximum differential gradient magnitude for any
neighbouring pixels with equal (approximately)
AN ADAPTIVE REGION GROWING SEGMENTATION FOR BLOOD VESSEL DETECTION FROM RETINAL
IMAGES
407
gradient direction. Region growing should stop
when no more pixels satisfy the criteria for inclusion
in that region. In the region growing process each
region is labelled with a unique number. For that
purpose we construct a cell array with region
number and its pixel position. The image is scanned
in a row-wise manner until its end, and each pixel
that satisfies our criteria is taken into account for
growing a region with its 8-neighborhood
connectivity.
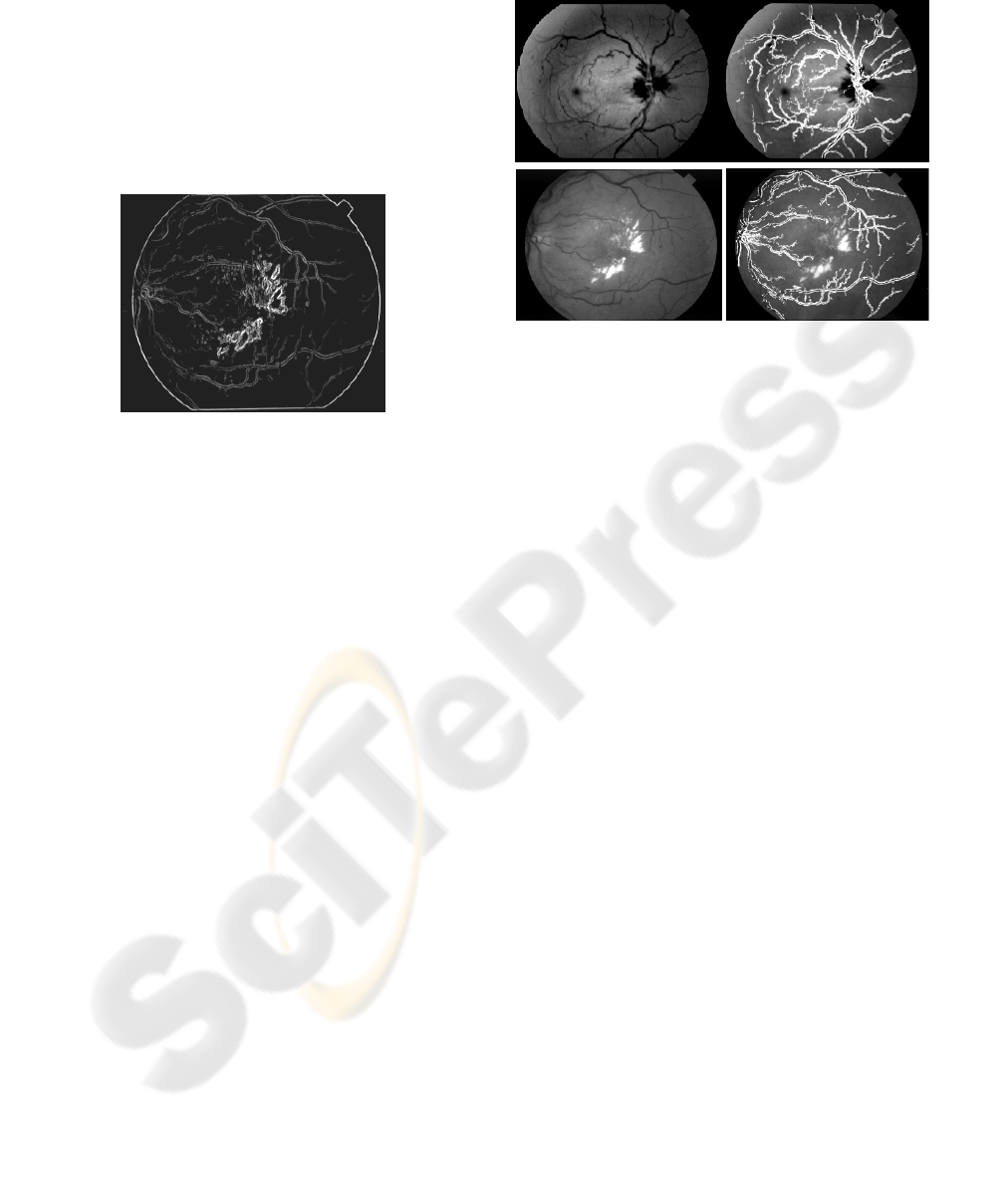
Figure 4: Output image after applying adaptive region
growing with minimum pixel number.
3.4 Parallel Region Detection
We calculate the parallel edges (regions) by
considering pixel orientation belonging to each
region. At first, we pick the region number and
belonging pixel coordinates from the constructed
cell array. Then we grouped the region/regions
parallel to each region, which is calculated by
mapping the pixels gradient direction. For each
region every pixel is searched from its potential
parallel region and once a maximum number of
pixels match with the other region we consider it as
parallel to that region. We consider all regions and
once a region is considered we assigned a flag value
to that region so that it will not be considered again.
In this way we can only filter the vessels from the
region and discard all other regions, which are
background noise or other objects like haemorrhage,
macula, etc in the retinal image.
3.5 Vessel Detection
We mapped the original retinal image with the
pixels from the segmented gradient image to show
the vessels. We can also find the centreline of each
vessel (parallel region pixels) edges and then expand
it with setting the stopping criteria of facing edge
pixels to determine the total pixels of each vessel.
For simplicity, we only produce the images with
mapping the original retinal images. Figure 5 below
displays two examples of images from the dataset
and their output.
Figure 5: Original retinal image (left) and detected vessels
(right).
4 RESULTS AND DISCUSSION
Figure 5 shows two example images and the output
after blood vessel segmentation using the proposed
method. The first retinal image is almost a normal
eye and we observe that 99% vessels are detected
properly. However, the second retinal image suffers
from noise and the detection rate falls to
approximately 90%. This fall in detection rate is due
to the fact that if any part of the retinal image suffers
from noise like block (eg. haemorrhage) then the
whole area is removed as we consider only the
parallel regions. Therefore, it is possible to miss
vessels from that particular part of the retinal image.
Table 1 displays observations of vessel detection for
five different images.
5 CONCLUSION AND FUTURE
WORK
In this paper we proposed a novel approach for
adaptive region growing technique and applied to
retinal gradient images. The results obtained are
promising. Currently, we are working on to produce
the retinal output binary image by expanding the
vessel centreline, which can be used as a vessel
width and crossover measurement.
VISAPP 2007 - International Conference on Computer Vision Theory and Applications
408

Table 1: Vessel detection accuracy.
Image
Total
Number of
vessels
Number of
detected
vessels
Accuracy
Image 1 93 92 98.92
Image 2 74 70 94.59
Image 3 85 79 92.94
Image 4 81 76 93.82
Image 5 75 71 94.66
REFERENCES
Chanwimaluang, T. and G. Fan (2003). "An efficient
blood vessel detection algorithm for retinal images
using local entropy thresholding." Proceedings of the
2003 International Symposium on Circuits and
Systems (ISCAS '03) 5: 21-24.
Chaudhuri, S., S. Chatterjee, N. Katz, M. Nelson and M.
Goldbaum (1989). "Detection of Blood Vessels in
Retinal Images Using Two-Dimensional Matched
Filter." IEEE Transactions on Medical Imaging 8(3):
263-269.
Gonzalez, R. C. and P. Wintz (1987). Digital Image
Processing, Second Edition. Addison-Wesley
Publishing Company, Inc.
Gonzalez, R. C., R. E. Woods, S. L. Eddins (2004).
Digital Image Processing Using MATLAB, Prentice
Hall.
Hoover, A. (2002). STARE-project
http://www.ces.clemson.edu/~ahoover/stare (last
accessed on 21November, 2006).
Hoover, A., V. Kouznetsova and M. Goldbaum (2000).
"Locating Blood Vessels in retinal Images by Piece-
wise Threshold Probing of a Matched Filter
Response." IEEE Transactions on Medical Imaging
19(3): 203-210.
Jiang, X. and D. Mojon (2001). "Blood Vessel Detection
in retinal Images by Shape-Based Multi-threshold
Probing." Lecture Notes in Computer Science 2191:
38-44.
Jiang, X. and D. Mojon (2003). "Adaptive local
thresholding by verification-based multithreshold
probing with application to vessel detection in retinal
images." IEEE Transactions on Pattern Analysis and
Machine Intelligence, 25(1): 131-137.
Li, Q., J. You, L. Zhang and D Zhang (2006). "A New
Approach to Automated Retinal Vessel Segmentation
Using Multiscale Analysis." Proceedings of
International Conference of Pattern recognition
(ICPR06): 1-4.
Martinez-Perez, M. E., A. D. Hughes, A. V. Stanton, S. A.
Thom, A. A. Bharath and K. H. Parker (1999).
"Segmentation of retinal blood vessels based on the
second directional derivative and region growing."
Proceedings of the International Conference on Image
Processing (ICIP 99) 2: 173 - 176.
Mendonca, A. M. and A. Campilho (2006). "Segmentation
of retinal blood vessels by combining the detection of
centerlines and morphological reconstruction." IEEE
Transactions on Medical Imaging 25(9): 1200 - 1213.
Staal, J., M. D. Abramoff, M. Niemeijer, M. A. Viergever
and B. V. Ginneken (2004). "Ridge-Based Vessel
Segmentation in Color Images of the Retina." IEEE
Transactions on Medical Imaging 23(4): 501-509.
Tolias, Y. A. and S. M. Panas (1998). "A fuzzy vessel
tracking algorithm for retinal images based on fuzzy
clustering." IEEE Transactions on Biomedical
Engineering 17(2): 263-273.
Wu, D., M. Zhang and J-C Liu (2006). "On the Adaptive
Detetcion of Blood Vessels in retinal Images." IEEE
Transactions on Biomedical Engineering 53(2): 341-
343.
Zana, F. and J. C. Klein (1999). "A multimodal
registration algorithm of eye fundus images using
vessels detection and Hough transform." IEEE
Transactions on Biomedical Engineering 18: 419-428.
Zana, F. and J. C. Klein (2001). "Segmentation of vessel-
like patterns using mathematical morphology and
curvature evaluation." IEEE Transactions on Image
Processing 10(7): 1010-1019.
AN ADAPTIVE REGION GROWING SEGMENTATION FOR BLOOD VESSEL DETECTION FROM RETINAL
IMAGES
409
