
A NEW INSTRUMENTED BIOLOGICAL DEVICE DESIGNED TO
APPLY MECHANICAL SHOCKS TO BONE CELLS
Laurent Navarro
1
, Jean-Charles Pinoli
1
, Henri Besset
2
, Ren´e Guyonnet
2
Ecole Nationale Sup´erieure des Mines de Saint-Etienne
1
Centre Ing´enierie et Sant´e (CIS) and LPMG-UMR CNRS 5148
2
Sciences des Processus Industriels et Naturels (SPIN)
158 cours Fauriel, 42023 Saint- Etienne cedex 2, France
Laurence Vico, Alain Guignandon
Universit´e Jean Monnet de Saint Etienne, Laboratoire de Biologie du Tissu Osseux (LBTO) and INSERM U890
15 rue Ambroise Par´e, 42023 Saint-Etienne Cedex 2, France
Keywords:
Mechanical shocks, biomechanical device, energy, acceleration forces, Fourier analysis.
Abstract:
A new device called biomechanical stimulation device (BSD) has been recently developped and is under
patenting process. This BSD allows to apply shocks to a biomaterial disc, on which bone cells have been
seeded. To observe the real behaviour of the biomaterial under shock loading, the BSD is instrumented with
an impact hammer and an accelerometer. Force and acceleration signals are recorded, and signal analysis can
be performed, in particular Fourier analysis. The results obtained lead to a better understanding of the stimulus
that the cells can perceive at the top surface of the biomaterial disc. It appears that mechanical shocks applied
at 1 Hps (Hit per second) or 10 Hps generate a frequency content up to 35 kHz. The main further objective
will be to characterize the influence of mechanical shocks on bone cells proliferation.
1 INTRODUCTION
Bone cells activity deals with several medical stakes
like osteoporosis and osteogenesis imperfectae, but
also with biocompatibility in the case of bone pros-
thesis implantation. Bone cells activity is related to
mechanical stimulation. Actually, the right term for
”bone cells activity” is osteogenesis. Osteogenesis
consists in a balance between bone synthesis (ensured
by osteoblastic cells) and bone resorption (ensured by
osteoclastic cells) (Bilezikian JP, 2002). Osteoblasts
and osteoclasts do not act at the same time : it is a
continuous looped process. First, the osteoclasts de-
stroy the bone and make holes. Then, the osteoclasts
withdraw and the osteoblasts take their place to form
the bone. After bone creation, osteoblasts leave and
osteoclasts come again to destroy the bone.
H. M. Frost showed with his Mechanostat (Frost,
1987) that the osteogenic process is strongly influ-
enced by mechanical stimuli. Previous studies have
reported different kinds of mechanical stimuli that
are efficient for osteogenesis: ultrasounds, hydro-
static pressure, fluid shear stress, biaxial and uniaxial
stretch, bending, nanostimulation with atomic force
microscopy and acceleration forces (Tjandrawinata
et al., 1997; Kacena et al., 2003; Hatton et al., 2003).
The effect of acceleration forces on osteogenesis has
already been analysed, but the exact stimulation that
the cells could perceive remains not entirely known.
Recently, a new biomechanical stimulation device
(BSD) has been developed and patented. It has been
built in the Ecole Nationale Superieure des Mines de
Saint Etienne (ENSMSE). This device aims at apply-
ing shocks to cells withoutdirect contact (acceleration
forces) between the cells and a mechanical impactor.
Shocks are applied to a biomaterial disc on which
cells are seeded. This allows to use different biomate-
rials in order to test their biocompatibility. Adhesion
of the cells on the biomaterial depends on the physic-
ochemical properties of this biomaterial. This adhe-
sion is a very important factor for the cellular activity
(Anselme et al., 2000; Ignatius et al., 2005; Jayara-
man et al., 2004).
In order to better understand the mechanical strain
and vibrational content involved, a signal acquisition
and signal processing study have been performed on
the BSD. More precisely, the purpose of this study
was to analyse the vibrational content resulting from a
272
Navarro L., Pinoli J., Besset H., Guyonnet R., Vico L. and Guignandon A. (2008).
A NEW INSTRUMENTED BIOLOGICAL DEVICE DESIGNED TO APPLY MECHANICAL SHOCKS TO BONE CELLS.
In Proceedings of the First International Conference on Biomedical Electronics and Devices, pages 272-278
DOI: 10.5220/0001047002720278
Copyright
c
SciTePress
shock on a biomaterial. Biomaterial biocompatibility
can be tested with the BSD, and the size of the bio-
material enables imaging and force investigations by
using Atomic Force Microscopy (AFM) (with 10 mm
diameter and 2 mm thickness disc). The BSD will be
presented in this paper in section 2. Then, the exper-
imental setup allowing the signals to be recorded is
detailed in section 3. Next, in section 4, results of the
signal acquisition will be analyzed. Furthermore, a
synthesis on this study and a conclusion will be given.
2 EXPERIMENTAL DEVICE
(BSD)
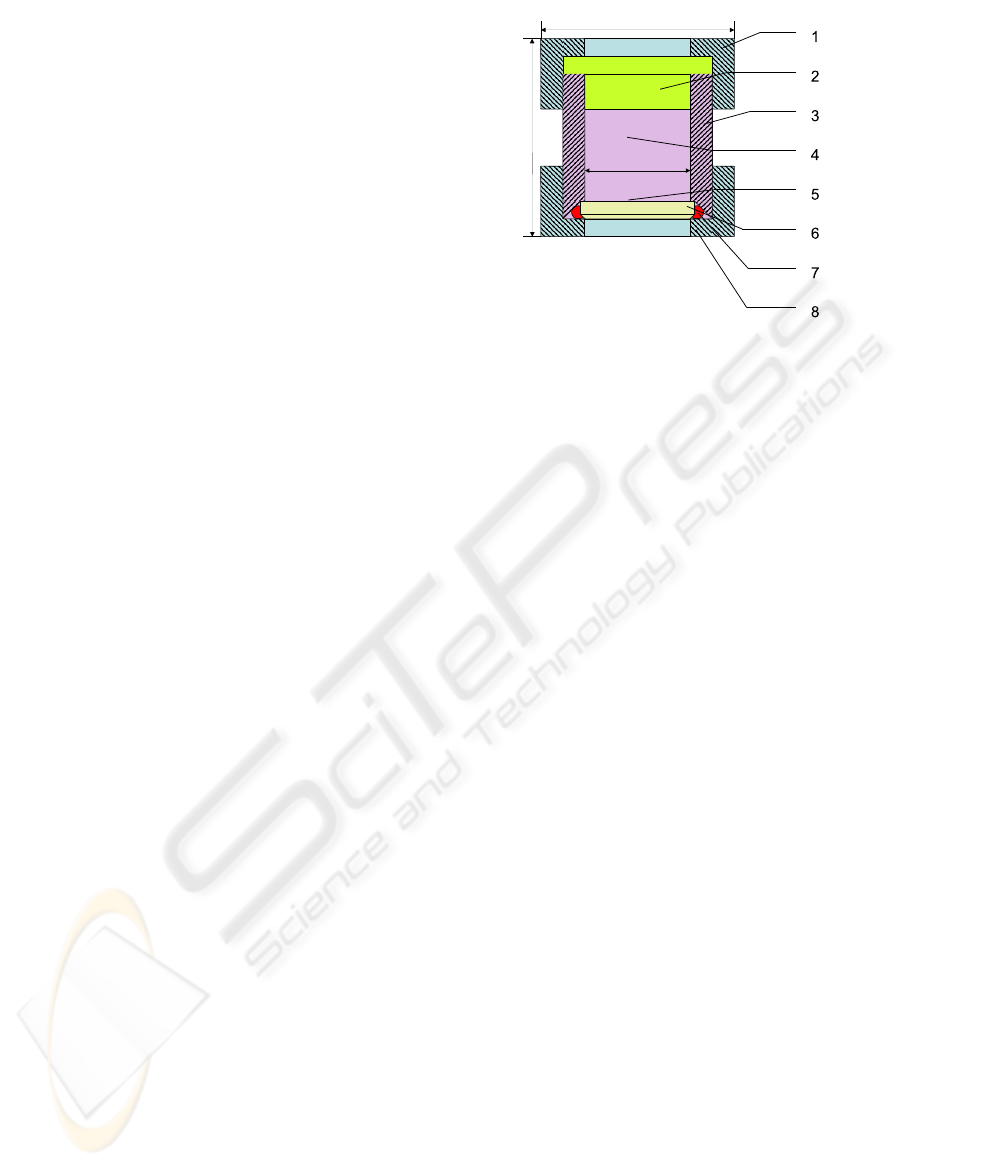
A schematic diagram of the BSD is shown in Fig. 1.
Four different biomaterial discs (Titanium (Ti6Al4V),
Hydroxy-apatite (HAP), cortical bone and trabecular
bone) have been used. The discs are normally sealed
into a culture chamber filled with culture medium,
and cells are seeded on the top of the disc. However,
all signal acquisitions have been performed without
medium and cells in order to only characterise the me-
chanical behaviour of the disc. This is a fundamental
step that is necessary to better understand what kind
of vibratory stimuli the cells can perceive at the top
of the biomaterial disc. During the mechanical stim-
ulations, a vertical actuator is activated with a small
and short stroke, high force solenoid, and a titanium
hammer head is adapted to the solenoid stalk.
The mechanical impacts are directly driven
through a current amplifier. Square type stimulation
signals are used according to the current supply mode
of the solenoid (DC current). Since the input signal
waveform is not sinuso¨ıdal, the signal frequencyis de-
fined in terms of hits per second (Hps) rather than in
Hz. This notation is used to avoid misunderstanding
between shocks application and resulting frequency
content. Some authors have shown that in vitro cul-
tured osteoblasts respond to mechanical strain at fre-
quency values between 1 Hz and 10 Hz (Lanyon,
1984; Neidlinger-Wilke et al., 1994; Kaspar et al.,
2000). The 1 Hz stimulation frequency corresponds
to the human locomotor behaviour. In this study, me-
chanical shocks are applied at these two stimulation
rates : 1 Hps and 10 Hps.
3 SIGNAL ACQUISITION
Signal acquisition is performed using two sensors
coupled on the BSD, as shown in Fig. 2.
An Integrated Circuit Piezoelectric (ICP
R
) ac-
Figure 1: Schematic diagram of the BSD culture chamber
used for in vitro experiments. Impact hammer is activated
by a solenoid, the head of the impact hammer strikes the
external surface of the biomaterial disc. Bone cells are cul-
tured on the surface of biomaterial and the culture cham-
ber is filled with medium. The impact hammer strikes the
biomaterial with predefined frequency and duration. The
different components of culture chamber and hammer head
represented in the figure : (1) Macrolon
R
top lid, (2)
teflon cork,(3) Macrolon
R
main part of culture chamber,
(4) culture medium, (5) cell culture, (6) biomaterial disc,
(7) Macrolon
R
bottom lid, (8) seal.
celerometer (model 352C23, PCB Piezotronics, Inc.,
NY, USA) is fixed on the discs top surfaces by direct
adhesive mounting and an Impulse Force Test Ham-
mer (model 086D80, PCB Piezotronics, Inc., NY,
USA) is adapted to the solenoid stalk and used as me-
chanical impactor. These sensors are connected to a
signal conditioner (model 442B104, PCB Piezotron-
ics) and acquired signals are recorded in a PC by
means of an acquisition card (NI DAQ Pad-6015, Na-
tional Instruments) with a sampling rate of 100 kHz
using a program written with LabVIEW
R
software.
A functional diagram of sensors monitoring, and main
sensors characteristics are given in Fig. 3, 4 respec-
tively. Accelerometer and Impulse force Test Ham-
mer signals are recorded simultaneously during the
impact. Acceleration and Force signals samples are
interlaced to ensure synchronized acquisition for fu-
ture joint analysis.
4 RESULTS
4.1 Signal Analysis
The experimental BSD produces two different types
of signal : force signals from the instrumented impact
hammer and acceleration signal from the accelerom-
eter. Ten discs of each biomaterial have been pro-
A NEW INSTRUMENTED BIOLOGICAL DEVICE DESIGNED TO APPLY MECHANICAL SHOCKS TO BONE
CELLS
273
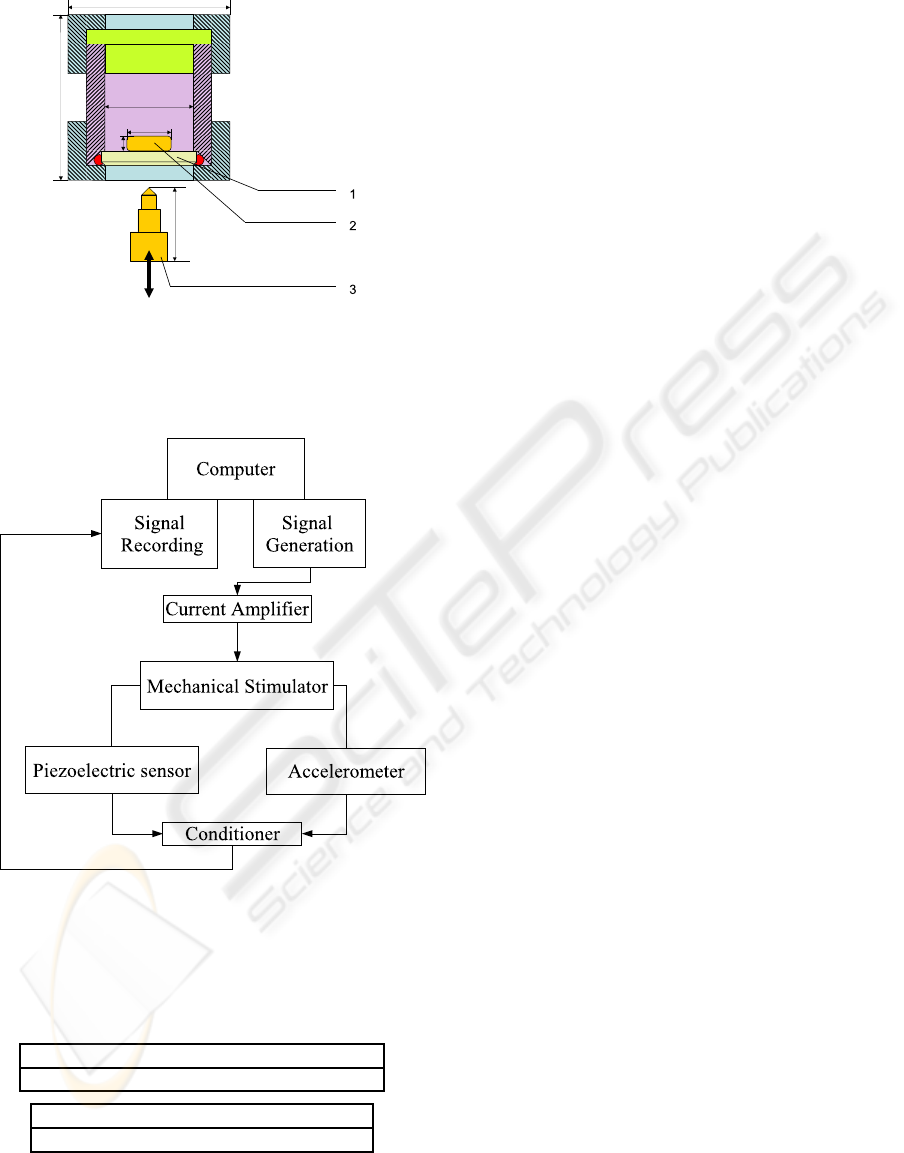
Figure 2: Schematic diagram of the culture chamber used
for signal acquisition in air : (1) Biomaterial disc, (2) ICP
R
.
accelerometer, (3) ICP
R
Impulse Force Test Hammer.
Figure 3: BSD is driven by amplified voltage current sig-
nals from the computer. During mechanical shocks, signals
of ICP
R
. accelerometer and ICP
R
impulse hammer con-
nected to BSD are simulteanously transmitted to the com-
puter.
Accelerometer : 5 mV/g ( 15%) sensitivity
50 kHz frequency range
Impact hammer : 22.5 mV/N sensitivity
12 kHz Frequency Range ( 5%)
Figure 4: Sensitivity and frequency range given by the man-
ufacturer for the two sensors.
cessed. Standard mean deviation have been calcu-
lated for each acceleration signals: Ti6Al4V, 783±31
g; HAP, 1197±87 g; Cortical bone, 1215±21 g; Tra-
becular bone, 225±24 g; and for each force signals:
Ti6Al4V, 34±0.6 N; HAP, 28±0.2 N; Cortical bone,
23±0.2 N; Trabecular bone, 8±0.6 N. These values
exhibit that Trabecular bone is softer than the other
biomaterials.
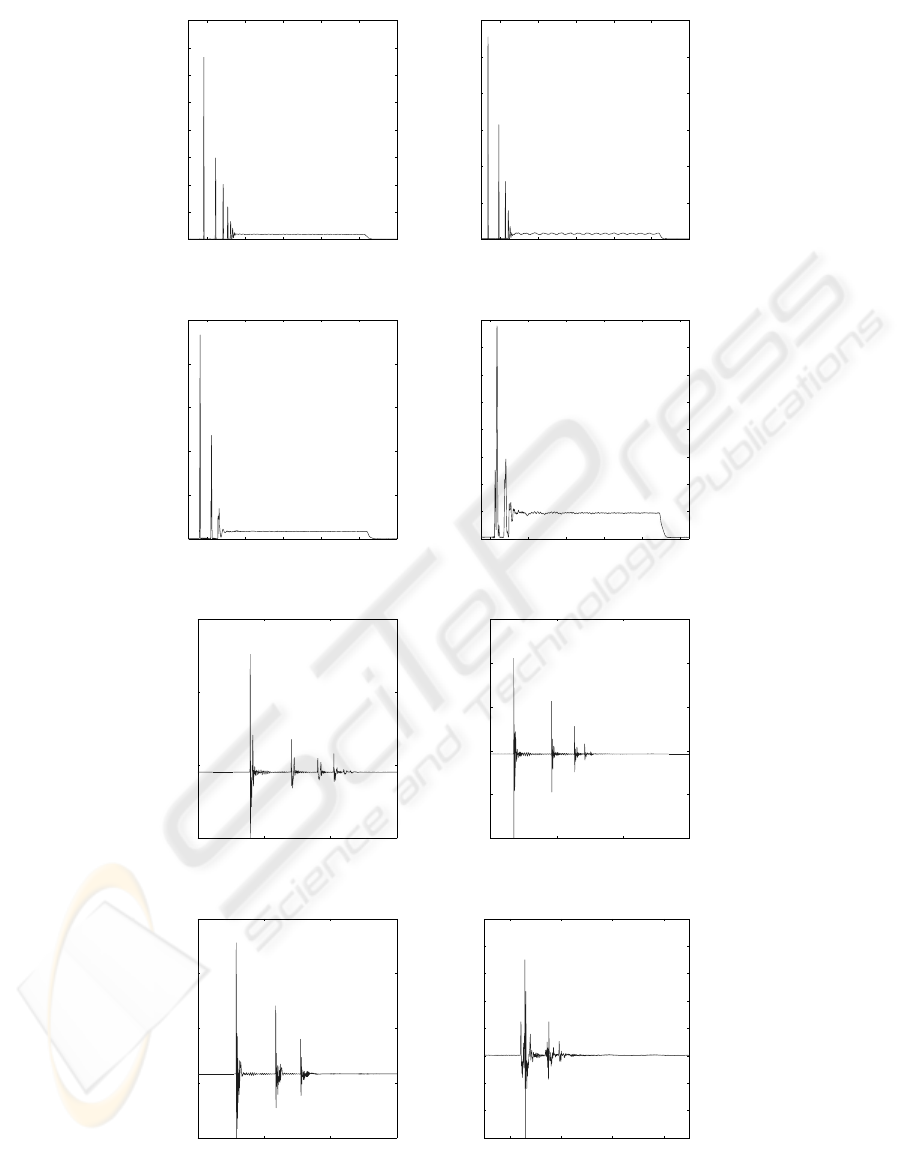
The first step of the signal analysis consists in a
zoom on the force and acceleration signals (Fig. 5) of
the four materials : Ti6Al4V, Hydroxy-apatite(HAP),
cortical bone and trabecular bone. This analysis en-
ables to show important characteristics that can not be
seen easily. For example, several rebounds between
the impactor and the disc can be seen for only one
hit. The delay between two rebounds is shortening
as their amplitude decreases. A flat part can also be
seen at the end of all the signals : it corresponds to the
sustain time of the solenoid due to the square driving
signal.
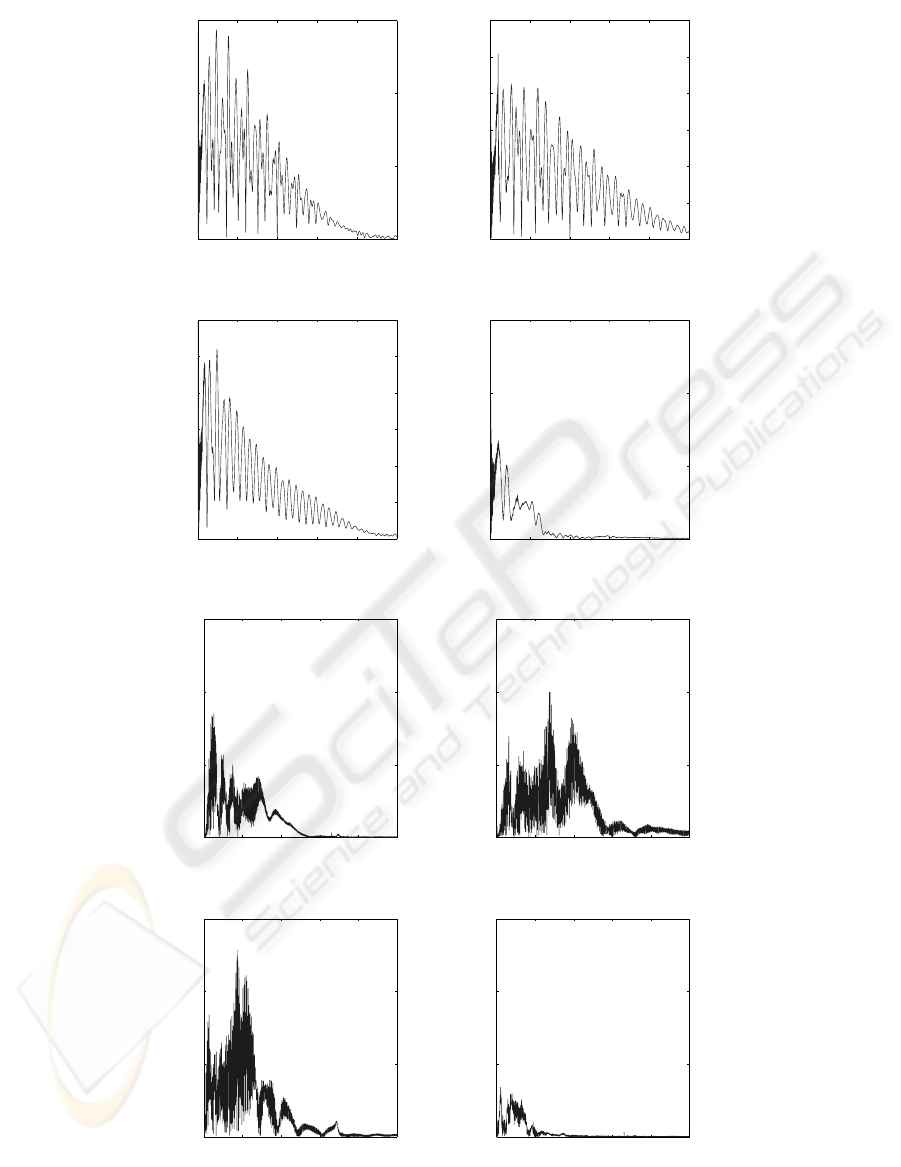
The second step of the signal analysis is the calcu-
lation of the Fourier Transform (FT) and the represen-
tation of the magnitude (Flandrin, 1993). The Fourier
Transform (FT) X( f) of a signal x(t) is expressed as :
FT
x
( f) =
Z
+∞
−∞
x(t)e
−i2π ft
dt
where t denotes the time and f the frequency.
The FT has the property of expressing a signal in
a frequency space.
The magnitude of the Fourier transform gives the
global frequency content of a signal (Fig. 5). Usually,
only the positive frequencies of the Fourier Transform
magnitude are shown in Fig. 6, since they possess a
physical meaning. In addition, the Shannon principle
is taken into account to avoid aliasing effect. This
principle is respected by the BSD signal acquisition
process.
The frequency spectrums of the signals are multi-
modal and different for the four discs. The Ti6Al4V
force signal frequencyspectrum (Fig. 6a) shows that a
slightly higher amplitude occurs for the Ti6Al4V than
for the other materials. It is interesting to notice that
HAP and cortical bone force frequency spectrums are
regular (Fig. 6b,c), it might be due to the fact that they
have almost the same chemical composition. Never-
theless, the cortical bone force frequency spectrum’s
pattern is smooth, whereas the HAP force frequency
spectrum pattern is sharper. The frequency spectrum
of the trabecular bone is very low, up to 1500 Hz, be-
cause the trabecular bone is softer than the cortical
bone. The acceleration frequency spectrums are not
regular and present several peaks, but relevant peaks
can not be observed on these spectrums. However, it
BIODEVICES 2008 - International Conference on Biomedical Electronics and Devices
274
0.52 0.54 0.56 0.58 0.6 0.62
0
5
10
15
20
25
30
35
40
Ti6Al4V
Time (s)
Force (N)
0.52 0.54 0.56 0.58 0.6 0.62
0
5
10
15
20
25
30
HAP
Time (s)
Force (N)
(a) (b)
0.52 0.54 0.56 0.58 0.6 0.62
0
5
10
15
20
25
Cortical bone
Time (s)
Force (N)
0.48 0.5 0.52 0.54 0.56 0.58
0
1
2
3
4
5
6
7
8
Trabecular bone
Time (s)
Force (N)
(c) (d)
0.51 0.52 0.53 0.54
0
500
1000
1500
Ti6Al4V
Time (s)
Acceleration (g)
0.51 0.52 0.53 0.54
0
500
1000
1500
2000
2500
HAP
Time (s)
Acceleration (g)
(e) (f)
0.51 0.52 0.53 0.54
0
500
1000
1500
2000
Cortical bone
Time (s)
Acceleration (g)
0.48 0.49 0.5 0.51
0
50
100
150
200
250
300
350
400
Trabecular bone
Time (s)
Acceleration (g)
(g) (h)
Figure 5: Force and Acceleration signals, recorded at 100 kHz sampling frequency with 12 bit amplitude resolution. (a) :
Ti6Al4V acceleration signal. (b) : HAP acceleration signal. (c) : Cortical Bone acceleration signal. (d) : Trabecular bone
acceleration signal. (e) : Ti6Al4V force signal. (f) : HAP force signal. (g) : Cortical Bone force signal. (h) : Trabecular bone
force signal.
A NEW INSTRUMENTED BIOLOGICAL DEVICE DESIGNED TO APPLY MECHANICAL SHOCKS TO BONE
CELLS
275
1000 2000 3000 4000 5000
0
500
1000
1500
Ti6Al4V
Frequency (Hz)
Amplitude
1000 2000 3000 4000 5000
0
200
400
600
800
1000
1200
HAP
Frequency (Hz)
Amplitude
(a) (b)
1000 2000 3000 4000 5000
0
200
400
600
800
1000
1200
Cortical bone
Frequency (Hz)
Amplitude
1000 2000 3000 4000 5000
0
500
1000
1500
Trabecular bone
Frequency (Hz)
Amplitude
(c) (d)
1 2 3 4 5
x 10
4
0
5000
10000
15000
Ti6Al4V
Frequency (Hz)
Amplitude
1 2 3 4 5
x 10
4
0
5000
10000
15000
HAP
Frequency (Hz)
Amplitude
(e) (f)
1 2 3 4 5
x 10
4
0
5000
10000
15000
Cortical bone
Frequency (Hz)
Amplitude
1 2 3 4 5
x 10
4
0
5000
10000
15000
Trabecular bone
Frequency (Hz)
Amplitude
(g) (h)
Figure 6: Fourier transform (FT) spectrums of the acquired force and acceleration signals. (a) : magnitude of Ti6Al4V force
FT. (b) : magnitude of HAP force FT. (c) : magnitude of cortical bone force FT. (d) : magnitude of trabecular bone force
FT. (e) : magnitude of Ti6Al4V acceleration FT. (f) : magnitude of HAP acceleration FT. (g) : magnitude of cortical bone
acceleration FT. (h) : magnitude of trabecular bone acceleration FT.
BIODEVICES 2008 - International Conference on Biomedical Electronics and Devices
276

is noticeable that the cortical acceleration frequency
spectrum is higher than the others.
4.2 Energy
From the acceleration signal recorded, the kinetic en-
ergy of the Ti6Al4V disc/accelerometer unit during
the mechanical shock is computed with the classical
formula
E
k
=
1
2
m
Z
v(t)
2
dt.
(with E
k
: kinetic energy, m: mass , v: speed, given for
each disc-accelerometer unit.)
The speeds of the different disc-accelerometer
units are computed by integration of the acceleration
signals, which occurs in the data processing sequence
after application of 30 Hz Butterworth high-pass fil-
ter to remove continuous component. The frequency
response curve of the Butterworth filter is practically
flat in the passband. However, the use of this kind
of filter introduces a non linear frequency dephasing.
In this study, only the frequency content is expected
and not the signal phase, thus the application of But-
terworth filter is well adapted. The kinetic energy is
calculated per surface unit: Ti6Al4V, 4.8±0.1 pJ/µm
2
for 1 Hps and 50.2±1.1 pJ/µm
2
for 10 Hps; HAP,
2.0±0.04 pJ/µm
2
for 1 Hps and 20.6±0.6 pJ/µm
2
for
10 Hps; Cortical bone, 8.5±0.2 pJ/µm
2
for 1 Hps
and 65.1±3.6 pJ/µm
2
for 10 Hps; Trabecular bone,
0.9±0.2 pJ/µm
2
for 1 Hps and 8.8±3.2 pJ/µm
2
for 10
Hps. Notice that values of kinetic energy at 10 Hps
are 10 times higher than those at 1 Hps.
5 SYNTHESIS
This new BSD was initially developed to simulate the
effects of mechanical impacts on bone cells cultured
on biomaterials to compare them with the effects of
impacts during walking or running activities. A pre-
vious study on Ground Reaction Forces (GRF) (Gi-
akas G, 2001) has shown that frequency content dur-
ing impact phase of running is limited to 100 Hz, ac-
celeration magnitude is ∼10 g. During shocks ap-
plied with the BSD, a frequency range comprised be-
tween 100 Hz and 4 kHz, an acceleration magnitude
of 783,1± 32,5 g at 1 Hps and an acceleration time of
∼1 ms have been found on Ti6Al4V for example. So
it is quite difficult to directly put in relation the results
obtained during this kind of mechanical impact with
those obtained by GRF studies.
In this study, recording conditions for acceleration
and force signals were slightly different from condi-
tions used in case of in vitro experiments, particularly
concerning the sealing of the culture chamber.
The disc should be excited by a frequency higher
than his resonnant frequency to enter in resonnance.
To verify this assumption, a numerical simulation was
perfomed and the first resonant frequency mode of
the Ti6Al4V disc was found to be at 144 kHz, which
is much higher than the frequencies observed on the
force signal Fourier transform. Consequently, the disc
can not enter in resonance and induces some self-
frequencies. The results found during signal record-
ing can be extrapolated to in vitro conditions.
When mechanical shocks were applied to the
discs, whatever the signal stimulation frequency was
(1 Hps and 10 Hps), one could observe that the shape
and the mean maximal value of the force and accel-
eration signals were identical. Analysis of accelera-
tion signals leads to an estimation of the kinetic en-
ergy of the tested disc during one impact. It has been
found that the amount of kinetic energy at 10 Hps is
10 times greater than that calculated at 1 Hps. Consid-
ering these results, in the case of mechanical shock,
it appears that it is more interesting to analyse more
accurately the frequency content of force signals, to
compare kinetic energy derived from accelerations
signals, and to focus on the force perceived by cells
when they are subjected to acceleration phase. By ap-
plying the fundamental law of dynamic, the acceler-
ation force during impact has been calculated by es-
timating cellular mass (1 picogramm): a force of ∼1
nN per cell has been found. This acceleration force
is comparable to forces for which biological events
have been previously observed. Consequently, it can
be expected that this new kind of mechanical stimula-
tion would have biological effects on bone cells.
A critical analysis can be done concerning the
reproducibility of the acceleration and force peaks’
measurements. The values and their errors are for
each material: Ti6Al4V, 783±31 g; HAP, 1197±87 g;
Cortical bone, 1215±21 g; Trabecular bone, 225±24
g; and for each force signals: Ti6Al4V, 34±0.6 N;
HAP, 28±0.2 N; Cortical bone, 23±0.2 N; Trabecu-
lar bone, 8±0.6 N. The error is less important in the
case of hard materials (cortical bone acceleration er-
ror is < 2% for example). However, a 10% error oc-
curs on the measurements of acceleration and force
peaks on the trabecular bone. This can be due to the
non-heterogeneity of the material and probably to the
non-flatness of the surface. In fact, the guidance of
the hammer is not perfect so the head of the hammer
does not hit in the same place every time. This is an
improvement to ensure to the BSD for future work.
An other improvement has been already done, it con-
sists in a screw that allows to ajust the stroke of the
A NEW INSTRUMENTED BIOLOGICAL DEVICE DESIGNED TO APPLY MECHANICAL SHOCKS TO BONE
CELLS
277

hammer. This leads to the possibility of controlling
the peak value and it reduces drastically the errors be-
tween different discs (The error for one disc is about
10 times smaller than that between discs).
6 CONCLUSIONS
A new device designed to apply mechanical shocks
to bone cells cultured on biomaterials has been devel-
oped. It allows to measure and compute shock pa-
rameters during impact : value and frequency content
of force impact, acceleration and kinetic energy for
each disc. When signals’ characteristics during im-
pact at different stimulation frequencies (1 Hps and 10
Hps) are compared, similar characteristics are found
for force signals but acceleration signals and kinetic
energy are different. Moreover, the computed value
of the acceleration force should lead to the observa-
tion of cellular responses. In conclusion, this new me-
chanical stimulator could be used for in vitro studies
to better understand bone cells mechanotransduction
during impacts.
Further studies, particularly concerning the bio-
logical effects of mechanical shocks on bone cells,
will be presented in future papers. Studies concern-
ing other signal analysis tools like time-frequency or
time-scale representations will also be held, since it
seems interesting to know if the BSD is a new way
to characterize biomaterials. Other biomaterial will
be tested, and Atomic Force Microscopy (AFM) will
be used in order to observe the bone cells behaviour
before and after mechanical shocks loading.
REFERENCES
Anselme, K., Linez, P., Bigerelle, M., Le Maguer, D.,
Le Maguer, A., Hardouin, P., Hildebrand, H., Iost, A.,
and Leroy, J. (2000). The relative influence of the
topography and chemistry of tial6v4 surfaceson os-
teoblastic cell behaviour. Biomaterials, 21(15):1567–
77.
Bilezikian JP, Raisz LG, R. G. (2002). Principles of Bone
Biology-Second Edition. ACADEMIC PRESS.
Flandrin, P. (1993). Temps-fr´equence. Herm`es.
Frost, H. (1987). Bone ”mass” and the ”mechanostat”: a
proposal. The Anatomical record, 219(1):1–9.
Giakas G, Baltzopoulos V, D. P. D. J. D. S. (2001). Com-
parison of gait patterns between healthy and scoliotic
patients using time and frequency domain analysis of
ground reaction forces. Spine, 21(19):2235–42.
Hatton, J., Pooran, M., Li, C., Luzzio, C., and Hughes-
Fulford, M. (2003). A short pulse of mechanical force
induces gene expression and growth inmc3t3-e1 os-
teoblasts via an erk 1/2 pathway. J Bone Miner Res.,
18(1):58–66.
Ignatius, A., Blessing, H., Liedert, A., Schmidt, C.,
Neidlinger-Wilke, C., Kaspar, D., Friemert, B., and
Claes, L. (2005). Tissue engineering of bone: effects
of mechanical strain on osteoblasticcells in type i col-
lagen matrices. Biomaterials, 26(3):311–8.
Jayaraman, M., Meyer, U., Buhner, M., Joos, U., and Wies-
mann, H. (2004). Influence of titanium surfaces on
attachment of osteoblast-like cells invitro. Biomateri-
als, 25(4):625–31.
Kacena, M., Todd, P., and Landis, W. (2003). Osteoblasts
subjected to spaceflight and simulated space shut-
tle launchconditions. In Vitro Cell Dev Biol Anim.,
39(10):454–9.
Kaspar, D., Seidl, W., Neidlinger-Wilke, C., Ignatius, A.,
and Claes, L. (2000). Dynamic cell stretching in-
creases human osteoblast proliferation and cicpsyn-
thesis but decreases osteocalcin synthesis and alkaline
phosphataseactivity. J Biomech., 33(1):45–51.
Lanyon, L. (1984). Functional strain as a determinant for
bone remodeling. Calcif Tissue Int., 36(Suppl 1):S56–
61.
Neidlinger-Wilke, C., Wilke, H., and Claes, L. (1994).
Cyclic stretching of human osteoblasts affects prolif-
eration and metabolism:a new experimental method
and its application. J Orthop Res., 12(1):70–8.
Tjandrawinata, R., Vincent, V., and Hughes-Fulford, M.
(1997). Vibrational force alters mrna expression in
osteoblasts. FASEB J., 11(6):493–7.
BIODEVICES 2008 - International Conference on Biomedical Electronics and Devices
278
