
SENSORIZED MICROCATHETER
Smart Microinstrumentation Adressing Fetal Surgery – First Results
A. Sieber
1, 2
, K. Houston
2
, A. Menciassi
2
, G. Nauer
3
and P. Dario
2
1
Profactor Research and Solutions GmbH, Seibersdorf, Austria
2
Scuola Superiore Sant’Anna,Pisa, Italy
3
ECHEM, Wiener Neustadt, Austria
Keywords: Fetal surgery, Endoscopy, Fetoscopy, Pulmonary Atresia, Bio-impedance, Catheter.
Abstract: Pulmonary Atresia is a malfunction that is diagnosed in about 1 out of 20.000 fetus. The authors propose a
novel surgical intervention where the fetal heart is accessed with an endoscopic intervention through the
umbilical cord. The key for this innovative procedure is a novel micro-catheter that is equipped with sensor
and actuators that allow active navigation inside the heart and also tissue characterisation. The present paper
presents the first prototype.
1 INTRODUCTION
1.1 Fetal Surgery
Birth defects occur in 1/28 of births and are the
leading cause of infant deaths. Costs for treatment
after birth are sometimes higher than surgery costs.
Surgical interventions on the fetus during pregnancy
allow a higher intra-uterine survival rate and an
improved postnatal outcome. Till now for a fetus
with diagnosed congenital malformation abortion,
continuation of the pregnancy until termination with
a Cesarean delivery, change in timing mode or place
of delivery were the only possibilities. Fetal surgery
may now provide a solution in these cases.
Starting from the two main American centres
(Harrison, M. R., 2003) that have been performing
fetal surgery for more than twenty years - University
of California, San Francisco Fetal Treatment Center
and Children’s Hospital of Philadelphia, Center for
Fetal Diagnosis and Treatment - nowadays about a
dozen worldwide centres provide prenatal surgical
intervention and many others carry on research and
experiments for specific fetal surgical applications.
(Raul A. Cortes and Diana L. Farmer, 2004)
Fetal surgery is still intended for a restricted
number of malformations that can not be
successfully or efficaciously treated after birth.
However, since 1981 many life-threatening fetal
pathologies have been treated through in-utero
surgical corrections, approaching prenatal
interventions as a valid alternative to neonatal
therapy or induced abortion.
At the moment open fetal surgery is the standard
procedure. It is similar to standard surgical
interventions, but causes a high level of stress for
both the fetus and the mother. An alternative can be
performing a key-hole surgical intervention on the
fetus with the help of endoscopic microtools. This
procedure is commonly known as fetoscopy and
allows an intervention on the fetus in its natural
environment causing less uterine trauma, less fetal
manipulation but preterm labor, damage to uterine
membranes and manipulation difficulties. (Sydorak,
R. M. , Albanese, C.T., 2003; Danzer, E., Sydorak,
R.M., Harrison M.R., Albanese C.T, 2003; Flake,
A.W., 2003 ; Berris M., Shoham M., 2006;
Papadopulos, N.A., Papadopoulos, M.A., Kovacs,
L., Zeihofer, H.F., Henke, J., Boettcher, P., Biemer,
E., 2005).
These procedures are performed through the use
of small trocars and a combination of
videofetoscopic and sonographic visualization.
Paediatric surgeons are trying to apply standard
minimal invasive instruments, designed by medical
engineers for different kind of surgery, to fetal
surgery applications. These instruments may be
suitable for some interventions, but are far too big
for interventions in an early development stage of
the fetus. Thus one of the main problems fetoscopy
is facing is the lack of suitable micro
instrumentation.
190
Sieber A., Houston K., Menciassi A., Nauer G. and Dario P. (2008).
SENSORIZED MICROCATHETER - Smart Microinstrumentation Adressing Fetal Surgery – First Results.
In Proceedings of the First International Conference on Biomedical Electronics and Devices, pages 190-195
DOI: 10.5220/0001047301900195
Copyright
c
SciTePress
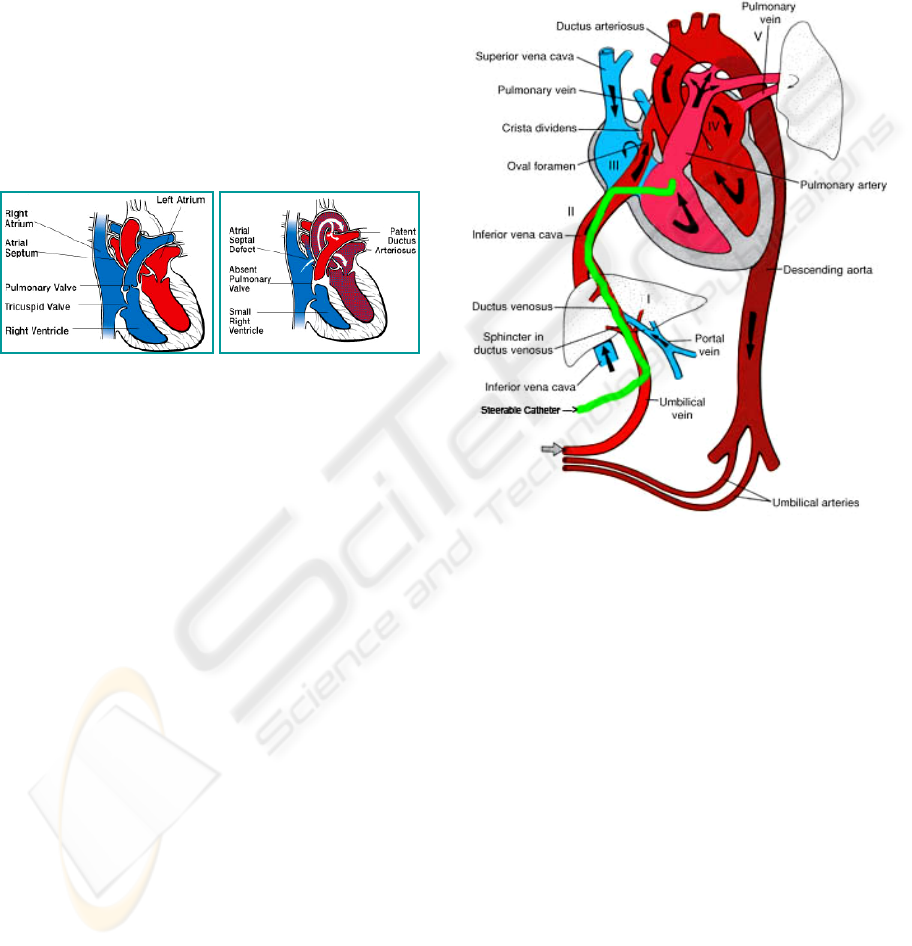
1.2 Pulmonary Atresia
During pregnancy the necessary oxygen is not
supplied through the fetal lungs but by the placenta.
The Foramen Ovale is an opening between the right
and the left atrium that allows blood to pass by the
pulmonary tract. After birth this opening is usually
closed. Pulmonary Atresia (Daubeney, P.E.F.,
Wang, D., Delany, D.J., Keeton, B.R., Anderson,
R.H., Slavik, Z., Flather, M., Webber, S.A., K.,
2005; Litovsky, S., Choy, M., Park, J., Parrish, M.,
Waters, B., Nagashima, M., Praagh, R.V. & Praag,
S.V., 2000) is a malfunction that may appear during
pregnancy: it is an incorrectly developed pulmonary
valve.that is, instead of a valve there is just a
membrane (compare Figure 1A and Figure 1B).
Figure 1A: Healthy heart 1B: Heart with absent pulmonary
valve (http://www.americanheart.org/).
No blood supply to the lungs is possible in this
case which usually causes the death of newborns
when oxygen supply by the placenta is not given
anymore. Furthermore anatomic obstruction to the
right or left ventricular outflow tract may cause
ventricular dysfunction, can divert fetal blood flow
in the uterus and result in cardiac chamber
hyperplasia. Thus severe aortic or pulmonary
stenosis can result in a hypoplastic left or right
ventricle with an inability for the ventricular
chambers to support the systemic or pulmonary
circulation. Theoretically early relief of the fetal
aortic or pulmonary stenosis may prevent such
occurrence and might preserve the right or left
ventricular function. In the case of pulmonary
atresia this can be achieved by a punctuation of the
pulmonary membrane to enable a pulmonary blood
flow and a further correct development of the valve.
(Tworetzky, W., Wilkins-Haug, L., RW. Jennings,
2004; Kohl, T., Witteler, R., Strämper, D.,
Gogarten, W., Asfour, B., Reckers, J., Merschhoff,
G., Marcus, A.E., Weyand, M., Van Aken, H.,
Vogt, J., Scheld, H.H., 2003)
Pulmonary atresia can be diagnosed in the 12-
14th week of gestation. The surgical intervention
should be performed as soon as possible. In the 14th
week the fetus size is about 9-14cm and has a
weight in the range of 60 - 200g. In this
development stage the pulmonary membrane has a
diameter of approximately 1mm.
Pulmonary atresia occurs in about one out of
every 20,000 live births. An early surgical
intervention is the only alternative to abortion and
could allow normal development of the pulmonary
valve and the right ventricle.
Figure 2: Fetoscopic approach to access the fetal right
ventricular through the umbilical cord
2 METHODS
2.1 Fetoscopic Approach
We propose a minimally invasive surgical
procedure in the case of pulmonary atresia which
includes the following steps:
(1) Externalisation of the uterus where the fetus
remains in its own environment
(2) Accessing the right ventricle with a flexible
and steerable microcatheter through the
umbilical cord (need for a microcatheter
(outer diameter <1mm), steering mechanism
(3 degrees of freedom), the catheter needs to
be highly flexible, position feedback systems
need to be available to track the catheters tip)
(Figure 2)
SENSORIZED MICROCATHETER - Smart Microinstrumentation Adressing Fetal Surgery – First Results
191

(3) guiding the catheters tip in front of the
pulmonary membrane (need for a steerable
catheter)
(4) recognition of the tissue in front of the
catheter: Due to the small dimensions of the
pulmonary tissue and the surrounding tissue it
is very difficult to distinguish between those
just by vision on an ultrasound picture. Tissue
characterisation and recognition sensors may
then be the solution for a reliable tissue
distinction.
(5) once the pulmonary membrane is detected the
perforation takes place (need for a cutting
tool)
It is clearly visible that the development of
suitable microinstrumentation is the key to this novel
surgical technique. To proof the feasibility of the
approach, we designed a first prototype for such a
smart catheter that is equipped with tissue
characterisation sensors and a steering mechanism.
2.2 Steering Mechanism
To be able to reach the right ventricle through the
umbilical cord (figure 2), the catheter needs to be
equipped with steering capabilities (Ascari, L.,
Stefanini, C., Menciassi, A., Sahoo, S., Rabischong,
P., Dario, P., 2003). The multi-lumen catheter
consists of a very flexible ending and a less flexible
part. In the walls of the catheter 4 thin diameter
lumen are integrated, each one for one steering wire.
Pulling on these 4 wires and releasing at the same
time the wire which is on the opposite side in the
catheter will primarily result in a movement of the
flexible end part of the catheter. Two
microcontroller driven servo drives are used to pull
and release the wires. This microcontroller is then
connected to a personal computer, which, equipped
with a haptic force feedback joystick, allows a
precise control of the catheter. A third degree of
freedom is realized by either manually or servo
supported driving the catheter forward and
backwards.
2.3 Electrical Impedance Sensor
Bio-impedance spectroscopy allows tissue
classification and identification by recording and
analyzing the electrical impedance at different
frequencies (Rigaud, B., Hamzaoui, L., Chauveau,
N., Martinez, E., Morucci, J., 1994; Cao, H.,
Tungjitkusolmun, S., Choy, Y. B., Tsai, J. Z.,
Vorperian, V. R., Webster, J. G., 2002). From the
electrical point of view cell membranes appear as
capacitors. In comparison to low frequency electrical
current where the current path is leading mainly
through extra cellular fluid, high frequency electrical
current is able to penetrate the cells. Different tissues
can be distinguished by comparison of their
characteristic impedance over frequency and phase
over frequency plots. Principle Component Analysis
can then be used to classify a tissue by a recorded
data set.
For impedance spectroscopy two or four
electrodes configuration are state of the art. Four
electrodes impedance measurement allows higher
accuracy, as two electrodes are used to drive in the
electrical current and the other two, which are
normally arranged in between the first two ones, are
used the small sensing electrode.
It must be kept in mind that for tissue
classification it is not necessary to record accurate
impedance data from the electrical point of view. It
is just important that the training data sets are
recorded with the same electrode configuration to
give comparable recordings.
2.4 Spectrophotometric Sensor
To enable a reliable tissue distinction a second
sensor system based on a different principle is
required. Tissues can be distinguished by their
colour, so pulmonary valve tissue appears rather
white in comparison to the surrounding more red
endocardium. Integration of two optical fibres is the
basis for the recording of optical reflectance
spectrum in front of the catheters tip. One fibre is
used for guiding the necessary light from a white
LED to the point of interest. Reflected light is then
received with a second fibre leading to an optical
spectrophotometer working in the visible range.
Unfortunately in normal condition the heart is filled
with blood. Haemoglobin is a strong light absorber,
where furthermore the wavelength dependent light
absorption also is dependent on the oxygenation
status of haemoglobin. Measuring tissue
characteristics with a spectrophotometric method is
therefore not possible in the presence of whole
blood.
2.5 Washing System
To solve the above described problem we integrated
another lumen in the catheter to provide washing
solution with a small amount of physiological saline
solution blood in front of the catheters tip can be
washed away. Thus blood in the measuring zone is
substituted with the washing solution, which enables
BIODEVICES 2008 - International Conference on Biomedical Electronics and Devices
192

P
1
2
3
4
5 7
6
8
9
10
1: Tip
2: flexible and steerable part of the catheter
3: not steerable but flexible part of the catheter
4: connector/distributor
5: pressure sensor
6: op tical fibers
7: impedance electrode(s)
8: tube
9: precision pump
10: reservoir with physiological solution
11: controller for the precision pump
12: light source and spectrophotometer
13: electrical impedance spectrometer and rf generator
14: Master computer
11
12
13
14
Figure 3: System setup.
spectrophotometric reflectance measurement
(Sieber, A., 2007). In figure 3 the principle setup
including the peristaltic pump for the physiological
saline solution is shown.
2.6 Tissue Fixation
Impedance and photometric spectrum recording
requires mechanically stable conditions – the tissue
in front of the catheter should not move relatively to
the catheter, which is difficult to realize in a moving
environment like a beating heart. To solve this
problem the washing system described above has a
second functionality: After the blood in front of the
catheter is substituted with a small amount of
physiological saline solution (Figure 4, 1-3), the
washing solution pump can be driven backwards,
thus sucking in washing solution and creating
suction in front of the catheter. Tissue in front of the
catheter is sucked to the tip and a reliable electrical
and mechanical connection is established (Sieber, A.,
2007), and electrical impedance and optical
spectroscopy are performed (Figure 4, 4-5). The
maximum suction pressure used in this setup is -50
mbar.
Figure 4: tissue characterisation scheme.
3 RESULTS
3.1 Catheter Prototype
To prove the feasibility of the concept a catheter
was fabricated with the following specifications
(Figure 5 and 6):
diameter: 3,5 mm
steerable tip, 2 DOF, servo actuated
four electrodes for electrical impedance
spectroscopy and radio frequency cutting
two 500 μm optical fibers for optical
reflectance spectroscopy
housing micromachined from PEEK with 5
axis Kern CNC milling centre
integrated washing / suction channel
Figure 5: Catheter tip design (A) and first prototype (B).
Figure 6: Catheter prototype (A) and control with a
joystick (B).
A joystick is deployed for the control of the bending
of the catheter tip (Figure 6B).
SENSORIZED MICROCATHETER - Smart Microinstrumentation Adressing Fetal Surgery – First Results
193
3.2 System Setup
Figure 3 shows the principle setup. The catheter
consists of the PEEK tip (1) with the integrated
electrodes, the washing channel and the optical
fibers, the steerable part (2) and the passive flexible
part (3). It is connected to a distributor (4) where a
pressure sensor (5) is mounted. Another port is
connected to the pump providing the washing
solution from a reservoir (10). The pump is
connected to a microcontroller ATMEL Atmega 32
(11). This microcontroller also serves as a controller
for two servos driving the Bowden cables of the
catheter needed for active bending of the catheter
tips (not displayed). The optical part (12) consists of
a light source (white LED) and a microspectrometer
[Microparts]. The electrodes are connected to a
programmable precision LCR meter [TEGAM]. All
the components 11, 12 and 13 are then connected to
a PC. The software is written under National
Instruments LabView.
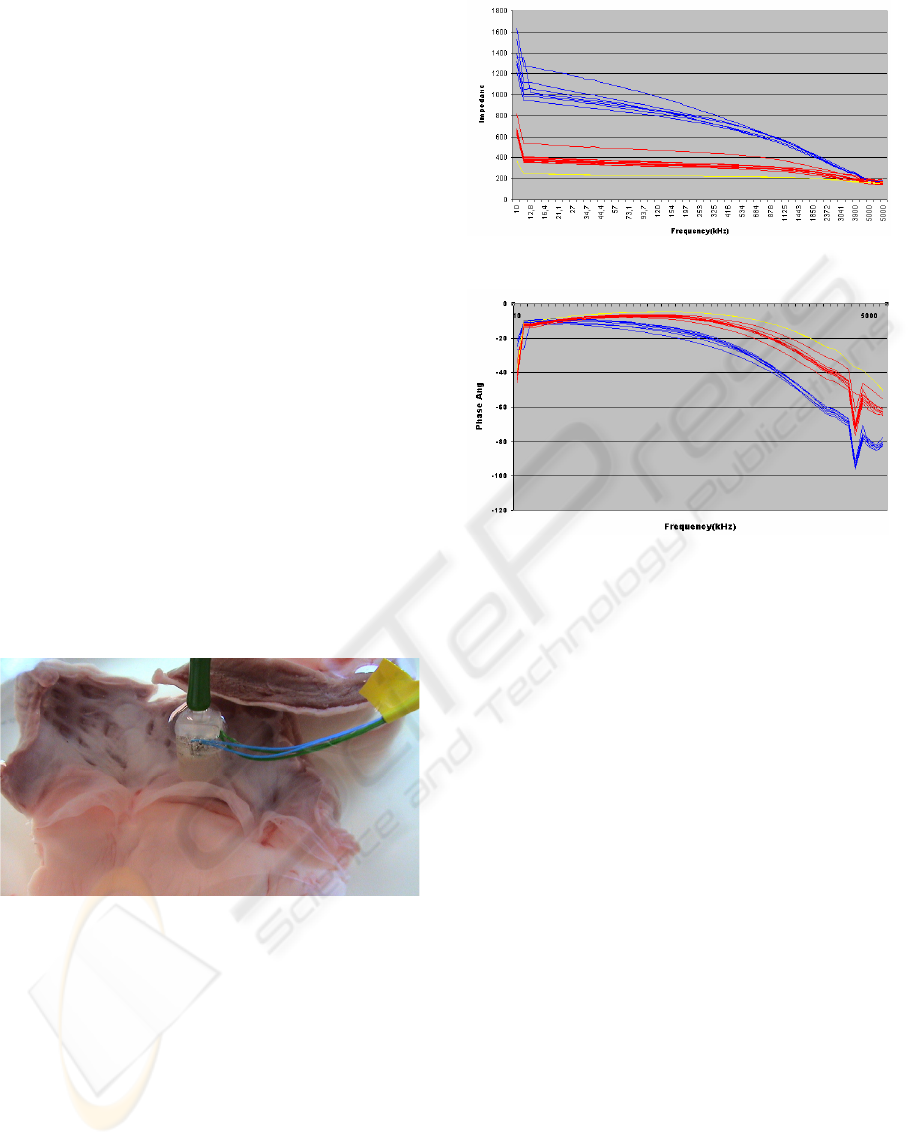
3.3 Tissue Distinction
Several electrical impedance spectra of in total 10
dissected bovine pulmonary valves and surrounding
endocardium were recorded (in the presence of
saline solution).
Figure 7: test setup: recording impedance spectra of
pulmonary valve tissue.
Therefore a second slightly larger (5mm
diameter) catheter tip was fabricated from peek
again using the Kern CNC milling centre. During
the recording the negative suction pressure was kept
constant at -25 mbar.
The impedance spectra were recorded from
10kHz to 5 Mhz with a induced signal of 1V peak to
peak. Pulmonary valve and endocardium tissue can
be clearly distinguished by electrical impedance
spectroscopy (see plots shown in Figure 8 and 9).
Figure 8: Impedance vs frequency endocardium: blue;
pulmonary valve: red; saline solution: yellow.
Figure 9: Phase vs frequency: endocardium: blue;
pulmonary valve: red; saline solution: yellow. The
negative peak at approximately 4 Mhz seems to be a result
of resonance.
4 CONCLUSIONS
Pulmonary atresia is a malfunction that occurs in
approximately 1 out of 20000 fetus. It can be
diagnosed in the 15
th
week of pregnancy. A feasible
approach to correct the malfunction is described,
but it requires sophisticated instrumentation.
The fabrication of the first prototype is a major step
towards the final catheter, which will be the key for
a successful early treatment of pulmonary atresia
thus offering an affected fetus a prospect to a future
without handicaps.
5 FUTURE WORK
Next steps will be catheter insertion tests of the
prototype on the animal model, enlargement of the
impedance spectra database and in parallel the
design of the miniaturized version. We envisage the
substitution of the bowden wires (the actuation
wires for bending of the catheters tip) by smart
actuators such as Ion Polymer Metal Composites –
which will enable a reduction of the overall
BIODEVICES 2008 - International Conference on Biomedical Electronics and Devices
194

diameter of the catheter to 0,8 mm, or SMA
actuators (Mineta, T., Mitsui, T., Watanabe, Y.,
Kobayashi, S., Haga, Y., Esashi, M., 2002) .
Additionally coils will be integrated, in order to
give a position feedback (Salomon, O., Kosa, G.,
Shoham, M., Stefanini, C., Ascari, L., Dario, P.,
Zaaroor, M., 2006; Aurora NDI).
ACKNOWLEDGEMENTS
The work described in this paper was supported by
the Austrian Research Centers GmbH, by the
Fondazione Cassa di Risparmio di Pisa in the
framework of the ”microSURF” project for the
development of innovative tools and techniques in
fetal surgery, and by the ASSEMIC project, a Marie
Curie Research & Training Network (MRTN-CT-
2003-504826).
REFERENCES
Harrison, M. R., 2003, Fetal Surgery: Trials, Tribulations,
and Turf, Journal of Pediatrics Surgery, 2003, Vol. 38,
pp. 275-282.
Cortes, R.A., Farmer, D. L., 2004, Recent advances in fetal
surgery. Seminars in Perinatology, 28(3):199–211
Sydorak, R. M., Albanese, C.T., 2003, Minimal access
techniques for fetal surgery, World J Surg, Vol. 27,
pp. 95–102.
Danzer, E., Sydorak, R.M.; Harrison M.R.; Albanese C.T,
2003, Minimal assecc fetal surgery, European Journal
of Obstetrics & Gynecology and Reproductive
Biology 1008 (2003) 3-13
Flake, A.W., 2003, Surgery in the human fetus: the future.
J Physiol (Lond), 547(1):45–51
Berris M., Shoham M., 2006, Febotics – a marriage of
fetal surgery and robotics, Computer Aided Surgery;
11(4): 175–180
Daubeney, P.E.F.; Wang, D.; Delany, D.J.; Keeton, B.R.;
Anderson, R.H.; Slavik, Z.; Flather, M.; Webber, S.A.;
K., 2005, Pulmonary atresia with intact ventricular
septum: predictors of early and medium-term outcome
in a population-based study, J Thorac Cardiovasc Surg
130(4), 1071.
Litovsky, S.; Choy, M.; Park, J.; Parrish, M.; Waters, B.;
Nagashima, M.; Praagh, R.V. & Praag, S.V., 2000,
Absent pulmonary valve with tricuspid atresia or
severe tricuspid stenosis: report of three cases and
review of the literature, Pediatr Dev Pathol 3(4), 353--
366.
Tworetzky, W., Wilkins-Haug, L., RW. Jennings, 2004,
Balloon Dilation of Severe Aortic Stenosis in the
Fetus: Potential for Prevention of Hypoplastic Left
Heart Syndrome: Candidate Selection, Technique, and
Results of Successful Intervention. CIRCULATION
2004,110: 2125-2131
Kohl, T., Witteler, R., Strämper, D., Gogarten, W.,
Asfour, B., Reckers, J., Merschhoff, G., Marcus, A.E.,
Weyand, M., Van Aken, H., Vogt, J., Scheld, H.H.,
2003, Operative techniques and strategies for
minimally invasive fetoscopic fetal cardiac
interventions in sheep, JOURNAL OF DIGITAL
IMMAGING , DECEMBER; 16, 3:
Ascari, L., Stefanini, C., Menciassi, A., Sahoo, S.,
Rabischong, P., Dario, P., 2003, A new active
microendoscope for exploring the sub-arachnoid
space in the spinal cord, in Proceedings of the 2003
International Conference on Robotics and Automation
(ICRA), 2003.
Rigaud, B., Hamzaoui, L., Chauveau, N., Martinez, E.,
Morucci, J., 1994, Tissue characterization and
modeling by electrical bioimpedance spectrometry,
Proceedings of the 16th Annual International
Conference of the IEEE Engineering in Medicine and
Biology Society, 1994, Vol. 2, pp. 866 - 867.
Cao, H., Tungjitkusolmun, S., Choy, Y. B., Tsai, J. Z.,
Vorperian, V. R., Webster, J. G., 2002, Using
Electrical Impedance to Predict Catheter-Endocardial
Contact During RF Cardiac Ablation, IEEE Trans.
Biomed. Eng., 2002, Vol. 49, pp. 247-253
Sieber, A., 2007, Patent: Catheter Tip, BI783F/BI/fpd,
filed on May 30th, 2007
Mineta, T., Mitsui, T., Watanabe, Y., Kobayashi, S., Haga,
Y., Esashi, M., 2002, An active guide wire with shape
memory alloy bending actuator fabricated by room
temperature process, Sensors and Actuators, A:
Physical, 2002, Vol. 97-98, pp. 632–637.
Salomon, O., Kosa, G., Shoham, M., Stefanini, C., Ascari,
L., Dario, P., Zaaroor, M., 2006, Enanching
endoscopic image perception using a magnetic
localization system, Computer Assisted Surgery
AURORA, NDI, http://www.ndigital.com/aurora.php
SENSORIZED MICROCATHETER - Smart Microinstrumentation Adressing Fetal Surgery – First Results
195
