
MICRO-SHAFT-POKING
A Novel Instrument for Mechanically Characterizing Soft Biomimetic Membrance
Kuo-Kang Liu, Mark Ahearne, Eleftherios Siamantouras and Ying Yang
Institute of Science Technology in Medicine, Keele University, Stoke-on-Trent, UK
Keywords: Instrumentation, hydrogel, mechanical characterization, microengineering.
Abstract: Characterizing viscoelastic properties of soft biomimetic membranes has become increasingly important due
to their biomedical applications such as tissue engineering/regenerative medicine and biosensors. This paper
presents a new micro-shaft-poking (MSP) technique which is free from the complication of substrate
backing, normally occurred as an intractable problem in the conventional indentation testing for soft
membranes. A tailored indention apparatus with a spherical indenter was constructed to achieve the force
resolution and displacement of 1μN and 1μm. The biomimetic membranes which were used for mechanical
testing were made of two types of hydrogel, alginate and agarose. The results showed that the elastic
modulus increased with gel concentration. A creep test has also been conducted to examine the time-
dependent behaviors of various hydrogel and a viscoelastic model has been correspondingly developed and
applied to describe the experimental results. Other potential applications of this new instrument to other
membranes, both artificial and biological, have been addressed in the paper.
1 INTRODUCTION
Recent advancements in biomimetic materials have
opened a new avenue for tissue regeneration/implant
and the construction of next generation biomedical
devices, such as developing engineered tissue and
designing cell-based biosensor. In many cases, their
viscoelastic/mechanical properties play an important
role in the performance and durability of these
membranes, and ultimately dictate whether the
applications are successful or not. A great need
therefore is required for the development of new
techniques for mechanically characterizing these
emerging biomimetic materials such as hydrogels
(Ratner et al., 1996). However, mechanical
characterization of these materials in a quantitative
manner is highly challenging due to their unique
mechanical characteristics, such as fragility and
viscoelasticity
Despite the intractability in experimentally
measuring the mechanical properties of soft
biological materials, recent progress in the advanced
instrument developments have made such
microscale characterization more feasible (Lu et al.,
2004). The common fundamental principle among
these advanced methods is to measure the material
deformation under an applied load. Among these,
bulge or blister testing, nanoindentation, and
microtensile testing are prevailingly used for
mechanical characterization of soft biomimetic
materials (Espinosa, et al., 2003; Liu et al., 2004).
Testing via bulging or nanoindentation alleviate
many of the problems such as mounting specimen
and providing sufficient resolution of measurements
(Scott et al., 2004). Moreover, the data measured by
using different testing techniques are often scattered.
Such variations have been recognized to be
attributed to the technological means employed by
each technique and the calibration. Also the different
requirements for conducting the experiments and
interpretation of results will also contribute to the
diecrepancy (Liu et al, 2004; Menciassi et al., 2001).
Therefore the mechanical properties of the soft
materials are difficult to be unequivocally
determined when the various techniques are
compared (Espinosa et al., 2003).
In this work, a new ultra-precise measuring
instrument, the MSP, for characterizing mechanical
properties of biomimetic materials are described.
Based on simultaneous force-displacement
measurements, the elastic modulus of soft
membranes can be determined. This instrument
provides a broad range of measurements to facilitate
large deformation analysis as well as time dependant
210
Liu K., Ahearne M., Siamantouras E. and Yang Y. (2008).
MICRO-SHAFT-POKING - A Novel Instrument for Mechanically Characterizing Soft Biomimetic Membrance.
In Proceedings of the First International Conference on Biomedical Electronics and Devices, pages 210-215
DOI: 10.5220/0001051402100215
Copyright
c
SciTePress
force measurements, with microscale resolution.
Firstly, the testing technique has been used to
characterize circular biomimetic hydrogel
membranes whose properties are of great interest for
biomedical applications and have not been
investigated well before.
2 EXPERIMENTAL SETUP
2.1 Instrumental Setup
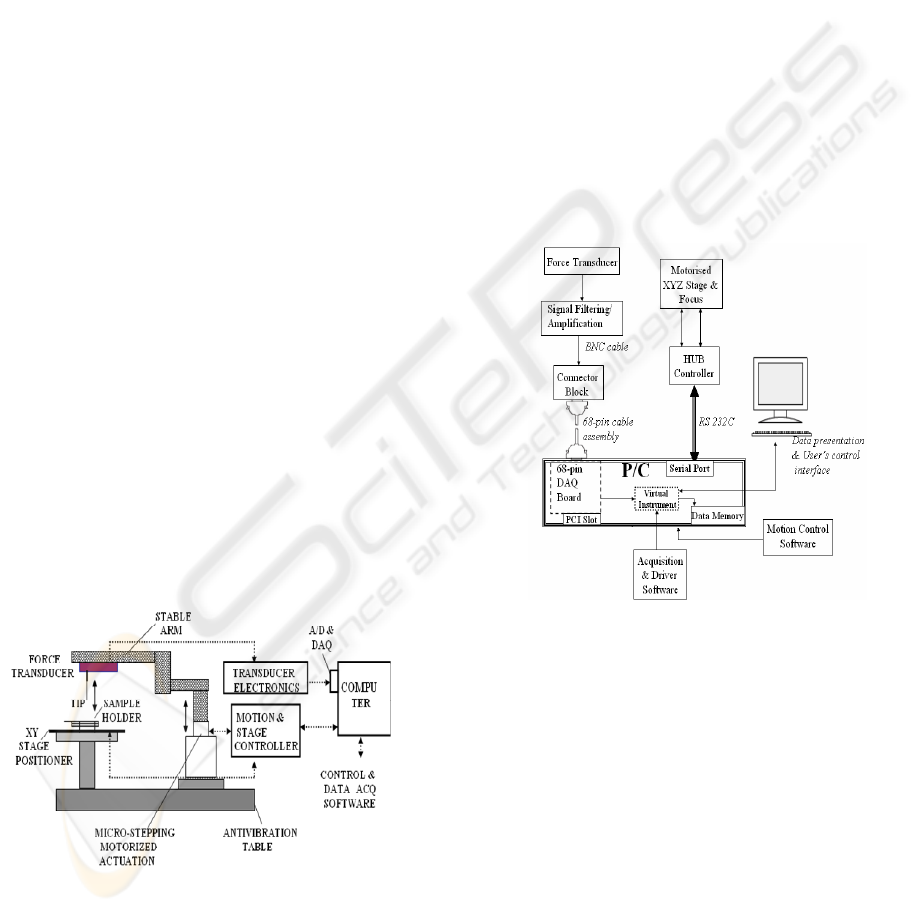
The system, schematically shown as Figure 1, was
newly developed for measuring force as a function
of displacement. The instrument is based around an
inverted optical microscope (Eclipse TE 2000S,
Nikon, USA), incorporated with a XYZ motorized
motion control interfaced with as external PC. The
microscope’s Z-axis motorized stage, which is
capable of 1μm step and a travelling distance up to
8.5mm, is used as a displacement actuator (ESP300,
Newport, Irvine, CA). Attached to Z-axis stage was
a specially designed solid arm, on which edge a
force transducer (404A, Aurora Scientific Inc.,
Canada), with 1μN force resolution and 100mN
maximum force capability, was mounted firmly. The
stability of the arm prevents from the “dead” weight
effect of the transducer’s head in the output signal
and avoids bending of the interior housing of the
transducer, which might offset the output. In
addition, it ensures precise loading in a vertical
position. In the final form the instrument had a force
and displacement resolution of 1μN and 1μm
respectively.
Figure 1: Schematic view of the instrumental set-up (not
to scale).
A fine, spherical tip is attached at the end of the
force transducer’s output tube for deforming the
sample. A handy sample holder facilitates mounting
of a thin, circular membrane between a set of
parallel plastic rings, without affecting the natural
properties of the material. The position of the
membrane for central alignment can be adjusted
precisely by tuning a two dimensional (X-Y)
translation stage (ASSY STAGE 25, Cell Robotics
Inc., USA), with a resolution higher than 2μm. The
force transducer signal is filtered and amplified by
using differential amplification (S 400A, Aurora
Scientific Inc., Canada). The amplified analogue
signal is transmitted through a connector block
(DAQ SCB-68, National Instruments, USA) into a
data acquisition (DAQ) board (PCI DAQ-6036E,
National Instruments, USA) for digitization and
further processing. The acquired data were displayed
and recorded by a tailored software design based on
the Labview platform (National Instruments, USA).
Figure 2 shows the interconnections among
instrumentation, data acquisition and control.
Figure 2: A block diagram of the system showing the
interconnections between instrumentation, data acquisition
and control interface.
2.2 Material Preparation
Two types of hydrogel, alginate and agarose, were
prepared. Alginate is a co-polymer consisting of β-
D-mannuronic (M block) and α-L-guluronic (G
block) acid. The ratios and lengths of these blocks
play an important role in the mechanical behaviours
of the alginate. A 2% (w/v) solution of sodium
alginate was formed by dissolving 2 g of Protanal
LF200 S (FMC BioPolymer, Norway) in 100 ml of
deionised water. The ratio of M block to G block in
this type of alginate has been found to be 0.23
(Drury et al., 2004). Different concentrations of the
MICRO-SHAFT-POKING - A Novel Instrument for Mechanically Characterizing Soft Biomimetic Membrance
211
alginate solution were formed by adjusting the ratio
of alginate powder to deionised water. When fully
dissolved, the solution was autoclaved for
sterilization. Autoclaving had the effect of
decreasing the viscosity of the solution. To fabricate
alginate hydrogels, rings made from filter paper
(Millipore, USA) were placed on the bottom of
small petri dish. These rings reduced the amount of
shrinkage of the hydrogel after crosslinking and
allowed the hydrogels to be lifted from the petri
dishes. 200 µl alginate solution was poured inside a
ring of inner diameter 11 mm. 5 ml of 0.5 M filtered
calcium chloride solution (CaCl
2
) was added over
the alginate. The application of CaCl
2
caused the
sodium in the alginate to be replaced by calcium,
which resulted in crosslinking and formation of a
hydrogel. Once applied, the CaCl
2
solution had to
cover the alginate quickly to prevent the hydrogel
from forming unevenly. After 10 minutes the CaCl
2
solution was removed and the hydrogel was washed
twice in phosphate buffered saline (PBS) (Sigma,
UK).
Agarose hydrogels were made using agarose
type 1 (Sigma, UK). A 2% (w/v) agarose solution
was produced by dissolving 0.2 g of agarose powder
in 10 ml PBS. For lower concentrations, less
agarose powder was required. The powder was
dissolved by heating the solution to over 60°C.
When fully dissolved, the solution was filtered to
remove any impurities. 200 µl of the solution was
applied to a petri dishes with circular filter paper
rings of inner diameters 11 mm. The hydrogels
were formed by cooling at room temperature. Once
the hydrogel had formed, water or PBS was added to
the petri dish to prevent the agarose from
dehydrating.
3 THEORETICAL ANALYSES
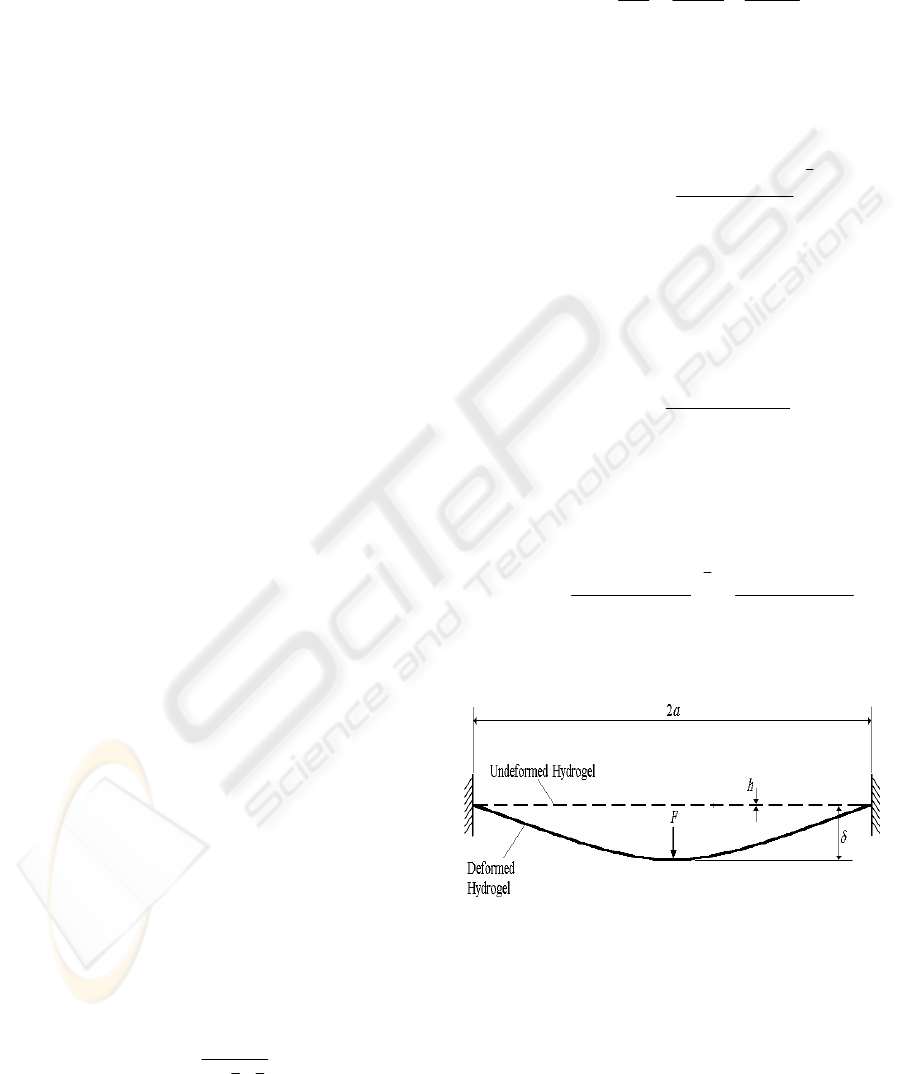
The Young’s modulus was calculated from the
indentation data using a previously described
theoretical model (Scott et al., 2004). For a
hydrogel material suspended around its outer edge
(Figure 3), the total displacement that the indenter is
lowered (δ) is equal to the sum of the depth of
penetration into the hydrogel (δ
1
) and the vertical
deformation displacement of the hydrogel (δ
2
). δ
1
can be calculated using the Hertz model (Johnson,
1985);
2
3
1
2
1
4
3
*
δ
R
F
E =
(1)
where F is the force applied to the hydrogel by the
indenter, R is the radius of the indenter tip and E* is
the elastic modulus and can be derived from the
equation;
H
H
I
I
EEE
22
11
*
1
νν
−
+
−
=
(2)
where E
I
and E
H
are the moduli of the indenter and
hydrogel respectively and ν
I
and ν
H
are their
Poisson’s ratios. Since the modulus of the indenter
is much larger than the hydrogel, the term (1 - ν
I
)/E
I
was neglected and equation (1) can be rewritten as;
3
1
2
222
1
16
)1(9
⎟
⎟
⎠
⎞
⎜
⎜
⎝
⎛
−
=
RE
F
ν
δ
(3)
where E and
ν
are the Young’s modulus and
Poisson’s ratio of the hydrogel materials. The
vertical deformation displacement of the hydrogel
was calculated using plate-bending theory
(Timoshenko & Woinowsky-Krieger, 1959) from
the equation;
3
22
2
π4
)1(3
Eh
Fa
ν
δ
−
=
(4)
where h is the thickness and a is the radius of the
hydrogel inside the sample holder. By adding
equations (3) and (4), the total displacement of the
indenter on the bending-governing deformation was
determined from the equation;
3
22
3
1
2
222
π4
)1(3
16
)1(9
Eh
Fa
RE
F
νν
δ
−
+
⎟
⎟
⎠
⎞
⎜
⎜
⎝
⎛
−
=
(5)
The Young’s modulus was calculated from equation
(5) using Matlab software (MathWorks, USA).
Figure 3: Schematic representations of a membrane
deformed by micro-shaft poking (MSP) indentation.
Two theoretical models were used to examine the
relaxation behaviour of the hydrogels during
indentation. The 3-parameter standard linear model
and 5-parameter Maxwell-Weichert model were
both used to describe the viscoelastic relaxation
response under a constant strain (Figure 4).
BIODEVICES 2008 - International Conference on Biomedical Electronics and Devices
212
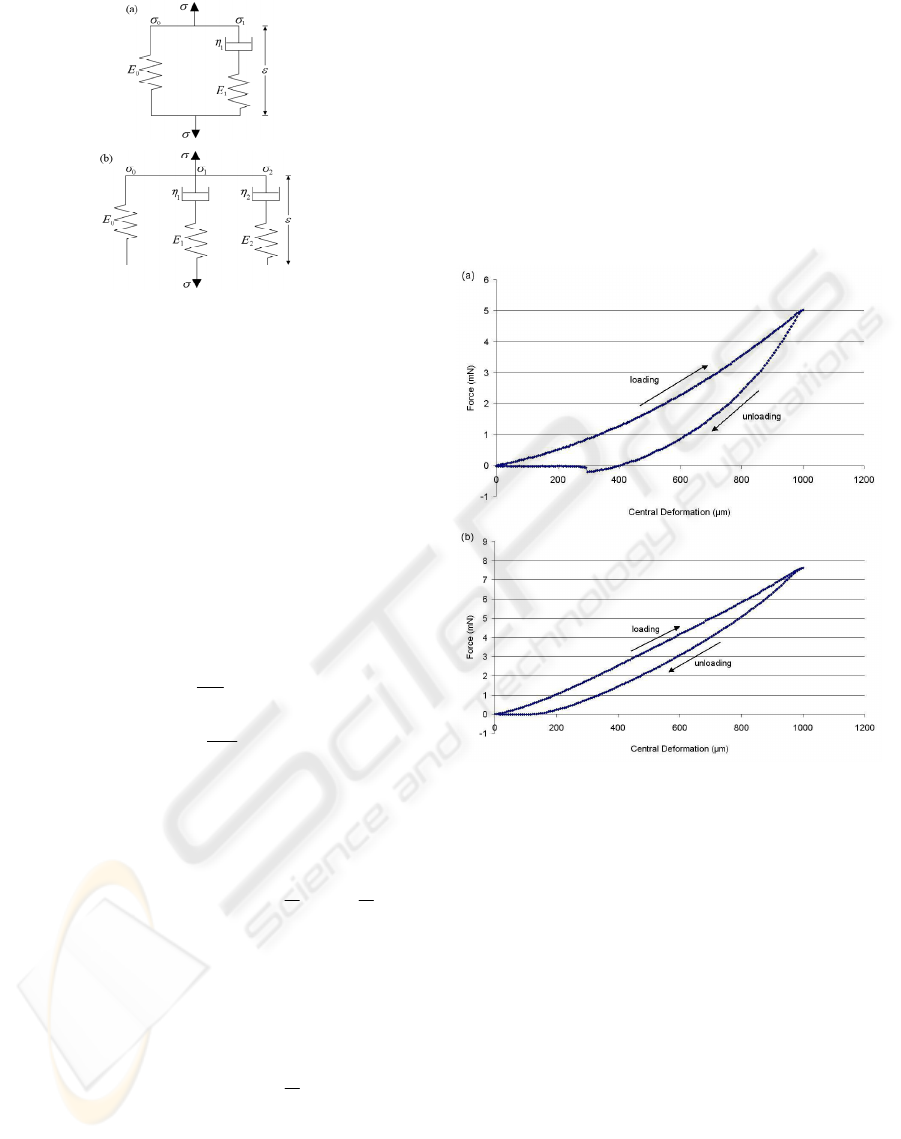
Figure 4: Schematic representation of the (a) 3-parameter
standard linear model and (b) 5-parameter Maxwell-
Weichert model.
For a 5-parameter Maxwell-Weichert model, which
consists of a single spring and two Maxwell
elements in parallel, the total stress, σ(t), equals to
the sum of the stresses applied to the spring and the
Maxwell elements;
210
)(
σ
σ
σ
σ
+
+=t (6)
where σ
0
is the stress applied to the spring and σ
1
and
σ
2
are the stresses applied to each Maxwell element
and whose values can be described as;
00
E
ε
σ
=
(7)
1
1
11
η
εσ
tE
eE
−
=
(8)
2
2
22
η
εσ
tE
eE
−
= (9)
where η refers to the dashpot viscosity. By
substituting equations (7), (8) and (9) into equation
(6), the stress relaxation function g(t), which equates
to σ(t)/σ(0), can be described as;
21
210
)(
ττ
tt
eAeAAtg
−−
++=
(10)
where A
0
, A
1
and A
2
represents the strain dependent
amplitudes, and τ
1
(=
η
1
/E
1
) & τ
2
(=
η
2
/E
2
) represent
strain dependent time constants.
For the standard linear model, the stress relaxation
function is written as;
1
10
)(
τ
t
eAAtg
−
+=
(11)
The values for A and τ were determined using non-
linear regression analysis for both relaxation models.
4 RESULTS AND DISCUSSIONS
4.1 Young’s Moduli of Hydrogels
A typical force-displacement curve for a 2% w/v
alginate and 2% w/v agarose hydrogel indented to
1000µm are shown (Figure 5 (a) & (b)). It can be
seen that the loading and unloading curves did not
match. This type of behaviour, referred as
hysteresis, is common in viscoelastic materials and
is the result of energy dissipation during loading.
Figure 5: The MSP-measured loading-unloading curves
of a hydrogel membrane with (a) 2% alginate & (b) 2%
agarose.
The loading-unloading curves appear less linear for
alginate hydrogel than agarose hydrogels and there
was a more significant difference between the
loading and unloading curves for alginate. This
suggests that alginate has non-linear viscoelastic
characteristics while agarose has more linear elastic
characteristics. This type of mechanical behaviour
agrees with the findings from previous publications
(Bonn et al., 1998; Zhang et al., 2005). It was also
found for some of the alginate hydrogels that the
indentation force became negative for a short period
during unloading. This can be explained by
adhesion forces between the indenter and the
hydrogel causing the indenter to adhere onto the
hydrogel while unloading (Gupta et al., 2007).
Several loading and unloading cycles were
recorded for individual hydrogels. It was noticeable
MICRO-SHAFT-POKING - A Novel Instrument for Mechanically Characterizing Soft Biomimetic Membrance
213
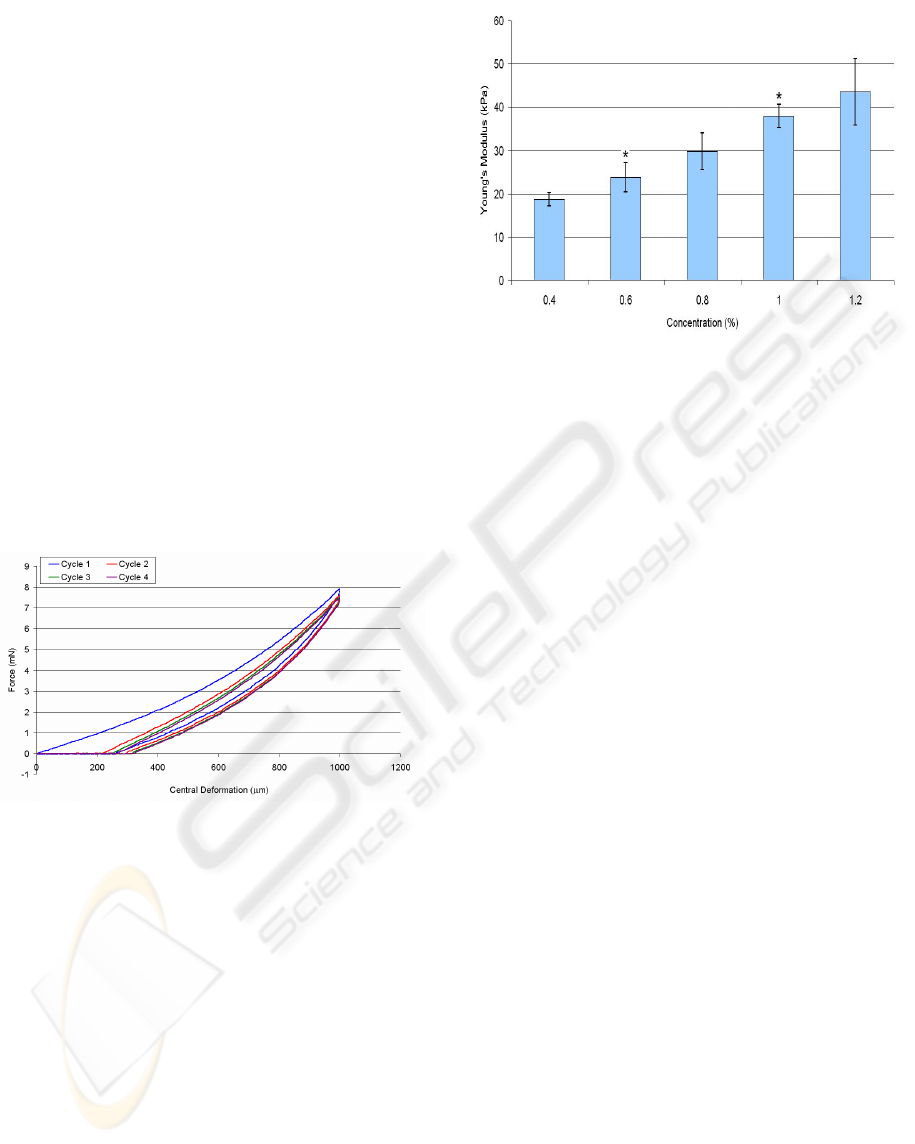
that different indentation cycles on the same
hydrogel did not match although later indentation
cycles appeared to match much more closely than
earlier cycles. It can be seen (Figure 6) that for a 2%
alginate hydrogel, after the first indentation cycle an
increase in force was only detected after the indenter
has been lowered by over 200 μm. This would
suggest that plastic deformation of the alginate
hydrogels had occurred in addition to elastic and
viscoelastic deformation. The plastic deformation,
in addition to the viscous properties of the hydrogel,
prevented the hydrogel from fully returning to its
original pre-indentation shape. There was also a
small decrease in the amount of force required to
indent the hydrogel with each cycle. This decrease
in force was reduced with each cycle until reaching
there was no functional force decrease was
detectable. This phenomenon is common in
biological materials (Fung, 1993) and is the
combination of fibre reorganization and fluid
movement within the tissue. The loading-unloading
cycles for agarose appeared to match more closely
than for alginate.
Figure 6: The MSP-measured loading-unloading cycles for
a 2% alginate hydrogel.
The Young’s moduli of agarose hydrogels of
different concentrations indented up to 1000 μm are
displayed (Figure 7). Agarose was preferred to
alginate for calculating the Young’s modulus since it
had a more linear elastic response to indentation.
The Young’s modulus of alginate hydrogels varies
in literature with a range between 1 kPa and 100 kPa
(Awad et al., 2004; Drury et al., 2004). A non-linear
loading curve would result in the values for Young’s
modulus becoming dependent on the indentation
depth. It can be seen that there was an almost linear
increase in Young’s modulus with agarose
concentration. Simple regression analysis was used
to confirm the linearity of Young’s modulus with
agarose concentration between 0.4−1.2% with a
coefficient of determination (R²) equal to 0.9935.
Figure 7: Young’s modulus of agarose hydrogels
measured by the MSP indentation (± standard deviation,
n=4). *represents a significant difference with a 95%
confidence over the previous concentration determined
using ANOVA-tukey test.
The standard deviation bars show a high degree of
repeatability in measuring different agarose hydrogel
with the same concentration. Interestingly when the
same hydrogel was measured several times, the
standard deviation was further reduced i.e. for 0.5 %
agarose hydrogel indented to 1000 µm four times, E
= 20.9 ± 0.7 kPa. The values obtained for 1%
agarose by the MSP method appear resemble those
found by Bonn et al. (1998) using 3-point bending
and Nyland & Maughan (2000) using atomic force
microscopy.
4.2 Stress Relaxation of Hydrogels
Normalized force relaxation data was collected for
both agarose and alginate hydrogels. The hydrogels
were indented to a central displacement depth of
1000µm, which was maintained for 45 minutes. For
times longer than 45 minutes, dehydration of the
hydrogel would affect the measurement readings.
Both agarose and alginate appeared to exhibit
relaxation behaviour consistent with viscoelastic
materials (Figure 8 (a) & (b)). The amount of force
required to maintain the indentation displacement
was reduced over time. The normalized force
initially decreased quickly but then slowed until
reaching a plateau. Alginate appeared to
demonstrate a greater relaxation response than
agarose, which suggests it is more viscoelastic than
agarose, which has more elastic characteristics.
Nonlinear regression analysis, performed
using XLStat (Addinsoft, USA), was used to
determine the ability of the 3-parameter standard
BIODEVICES 2008 - International Conference on Biomedical Electronics and Devices
214
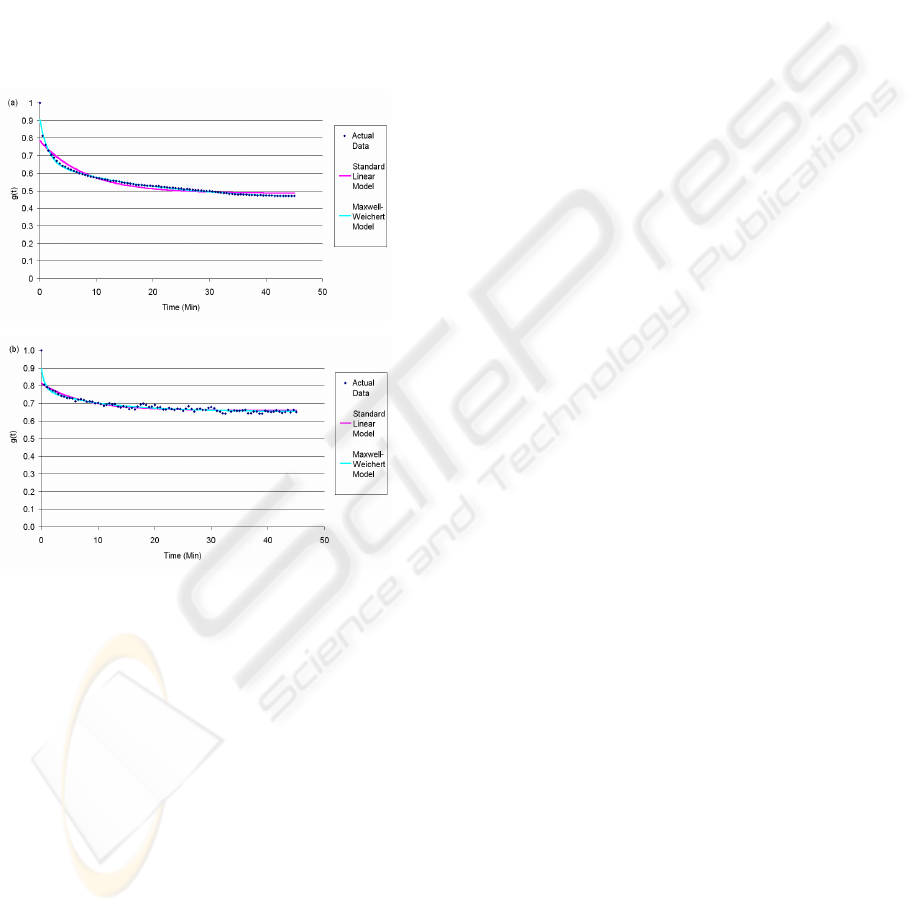
linear model and 5-parameter Maxwell-Weichert
model to describe the hydrogels’ relaxation
responses to the deformation applied by MSP. Both
models appear to show a high degree of correlation
between the actual data and the theoretical model
data (Figure 8). The values for coefficients of
determination (R²) for agarose and alginate
hydrogels were found to be greater than 0.9 using
the standard linear model and greater than 0.95 for
the Maxwell-Weichert model. This suggests that the
5-parameter Maxwell-Weichert model is capable of
providing a more accurate representation of the
relaxation response than the standard linear
model.
Figure 8: Actual and theoretical model normalised force
data for (a) a 2% alginate and (b) a 1% agarose hydrogel at
a constant indentation of 1000µm.
5 CONCLUSIONS
The MSP method has been applied to examine the
mechanical and viscoelastic characteristics of
various biomimetic materials, i.e., agarose and
alginate hydrogel membranes and their results have
been demonstrated to be satisfactory. Incorporated
with simple analyses, the new instrument has been
shown to be capable of determining quantitatively
viscoelastic and mechanical properties based on
experimental data of loading/unloading and stress
relaxation curves. The instrument has potentials for
testing other soft biological materials, such as
human and animal skins.
REFERENCES
Awad H. A., Quinn Wickham M., Leddy H. A., Gimble, J.
M., Guilak F. 2004, ‘Chondrogenic differentiation of
adipose-derived adult stem cells in agarose, alginate
and gelatin scaffolds’, Biomaterials, Vol. 25, no. 16,
pp. 3211-3222
Bonn D., Kellay H., Prochnow, M. Ben-Djemiaa K.,
Meunier J. 1998, ‘Delayed fracture of an
inhomogeneous soft solid’, Science, Vol. 280, no.
5361, pp. 265-267
Drury, J. L., Dennis, R. G. & Mooney, D. J. 2004, ‘The
tensile properties of alginate hydrogels’, Biomaterials,
Vol. 25, no. 16, pp. 3187-3199
Espinosa H. D., Prorok B. C., Fischer M. 2003, ‘A
methodology for determining mechanical properties of
freestanding thin films and MEMS materials’, J. of the
Mechanics and Physics of Solids, Vol. 51, no. 1, pp.
47-67
Fung, Y.C. 1993, Biomechanics: mechanical properties of
living tissues, Springer-Verlag, 2
nd
edition, New York.
Gupta, S., Carrillo, F., Li, C., Pruitt, L., Puttlitz, C. 2007,
‘Adhesive forces significantly affect elastic modulus
determination of soft polymeric materials in
nanoindentation’, Materials Letters, Vol. 61, no. 2, pp
448-451
Johnson, K.L. 1985, Contact Mechanics, Cambridge
University Press, Cambridge
Liu K. K., Khoo, H. S., Tseng, F. G. 2004, ‘In situ
mechanical characterization of square microfabricated
elastomeric membranes using an improved
microindentation’, Review of Scientific Instruments,
Vol. 75, no. 2, pp. 524-531
Lu, S., Dikin, D. A., Zhang, S., Fisher, F. T. 2004,
‘Realization of nanoscale resolution with a
micromachined thermally actuated stage’, Review of
Scientific Instruments, Vol. 75, no. 6, pp. 2154-2162
Menciassi, A., Scalari, G., Eisinberg, A., Anticoli, C.,
Francabandiera, P., Carrozza, M. C., Dario, P. 2001,
‘An instrumented probe for mechanical
characterization of soft tissues’, Biomedical
Microdevices, Vol. 3, no. 2, pp. 149-156
Nyland, L. R., Maughan, D. W. 2000, ‘Morphology and
transverse stiffness of drosophila myofibrils measured
by atomic force microscopy’, Biophysical Journal,
Vol. 78, no. 3, pp 1490-1497
Ratner, B. D., Hoffman, A. S., Schoen, F. J., Lemons J. E.
1996, ‘An Introduction to Materials in Medicine’,
Academic Press, New York
Scott, O. N., Begley, M. R., Komaragiri, U., Mackin T. J.
2004, ‘Indentation of freestanding circular elastomer
films using spherical indenters’, Acta Materialia, Vol.
52, no. 16, pp. 4877-4885
Timoshenko S. P.,Woinowshy-Kreiger S. 1959, Theory of
plates and shells, McGraw-Hill, Singapore, 2
nd
Edition
Zhang, J., Daubert, C. R., Foegeding, E. A. 2005,
‘Fracture analysis of alginate gels’, Journal of Food
Science E: Food Engineering and Physical Properties,
Vol. 70, no. 5, pp. 425-431
MICRO-SHAFT-POKING - A Novel Instrument for Mechanically Characterizing Soft Biomimetic Membrance
215
