ANIMAL STUDIES USING AN OXYGEN-TENSION SENSOR
FOR TISSUE VIABILITY MONITORING
Dafina Tanase
Electronic Instrumentation Laboratory, Delft University of Technology, Mekelweg 4, Delft, The Netherlands
Niels Komen
Erasmus Medical Centre, Rotterdam, The Netherlands
Arie Draaijer
TNO Quality of Life, Zeist, The Netherlands
Johan F. Lange, Gert-Jan Kleinrensink, Johannes Jeekel
Erasmus Medical Centre, Rotterdam, The Netherlands
Paddy J. French
Delft University of Technology, Delft, The Netherlands
Keywords: Oxygen-tension sensor, tissue viability, colon, optical method.
Abstract: Leakage at the site of an anastomosis is the main, yet unsolved reason for mortality in abdominal surgery.
Every year, a large number of patients die due to anastomotic leakage after surgery. An objective aid to
monitor the anastomotic site pre- and postoperatively and detect leakage at an early stage, is needed.
Therefore, a miniature, wireless measurement system to detect tissue viability during and after colon
surgery (continuously for 7 days) is being developed. The complete sensor chip should include an oxygen-
saturation sensor (sO
2
), an oxygen-tension sensor (pO
2
), a carbon-dioxide tension sensor (pCO
2
) and a
temperature sensor. The present work focuses on the use of the oxygen-tension and temperature sensors for
animal studies. Initial in-vivo measurements were carried out on the small and large intestines of male
wistar rats. The main goal was to measure the distribution of pO
2
on the colon around the anastomosis and
to determine the changes in pO
2
during repetitive ischemia-and-reperfusion experiments on the small
intestine. The paper presents the obtained measurement results.
1 INTRODUCTION
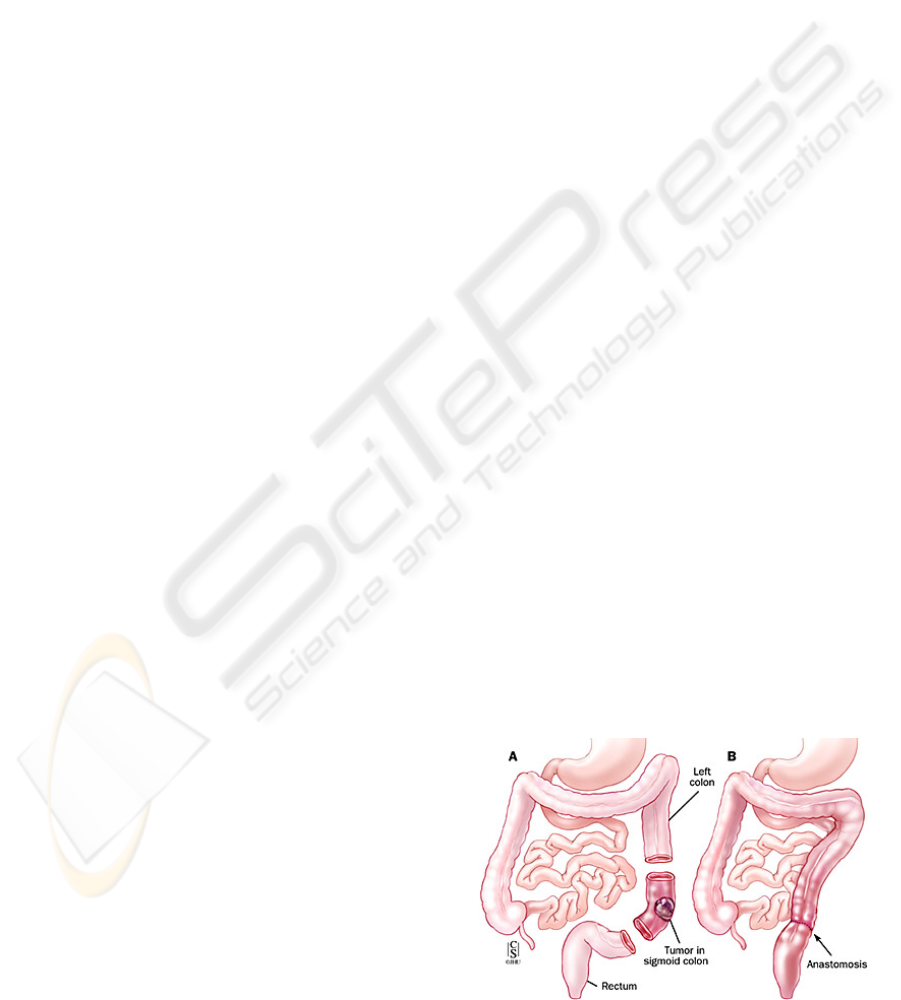
An anastomosis is the surgical connection of two
tubular segments to restore continuity (Figure 1).
Leakage of a colorectal anastomosis is a
complication in which intestinal content leaks into
the abdominal cavity due to a “defect” in the
anastomosis. This defect can be caused by a reduced
oxygen supply and it can lead to cell death and
necrosis of the anastomosis. As a result, leakage can
occur and as a consequence, peritonitis may develop
and can lead further to sepsis, multiple-organ failure
Figure 1: A colon anastomosis.
50
Tanase D., Komen N., Draaijer A., F. Lange J., Kleinrensink G., Jeekel J. and J. French P. (2008).
ANIMAL STUDIES USING AN OXYGEN-TENSION SENSOR FOR TISSUE VIABILITY MONITORING.
In Proceedings of the First International Conference on Biomedical Electronics and Devices, pages 50-55
DOI: 10.5220/0001052300500055
Copyright
c
SciTePress
and ultimately death. Therefore, anastomotic leakage
of a colorectal anastomosis is considered a
potentially lethal complication.
The reported incidence varies between 10 % and
13 % (Kanellos, 2004; Guenaga, 2003; Peeters,
2005), with a mortality rate that can be as high as
32 % (Choi, 2006). To date, no peroperative
methods to avoid or predict anastomotic leakage, or
any validated, objective parameters for detection of
anastomotic leakage in an early postoperative phase,
exist. Current diagnostic methods include
observation of clinical signs and symptoms (fever
and pain), while confirmation is obtained by
imaging. These methods are faced with several
disadvantages. When anastomotic leakage has
progressed to a state of clinical manifestation, the
patient is already ill and treatment needs to be
initiated. Imaging modalities, more specifically
abdominal CT-scans and/with contrast enemas, are
normally used to confirm a clinical diagnosis of
anastomotic leakage, meaning the patient is already
ill (Eckmann, 2004).
At present, clinically relevant anastomotic
leakage is usually diagnosed approximately 6 to 8
days after surgery (Kanellos, 2004; Alves 1999).
Some studies report an even longer interval
(12 days) between operation and diagnosis of
anastomotic leakage (Hymann, 2007). The long
intervals between the construction of the
anastomosis and the diagnosis of anastomotic
leakage are detrimental for the prognosis, increasing
mortality rates (Macarthur, 1998).
Therefore, a biomarker reflecting the viability of
the anastomosis, could be a fast and objective
diagnostic tool in addition to current methods,
allowing diagnosis of anastomotic leakage before its
clinical presentation.
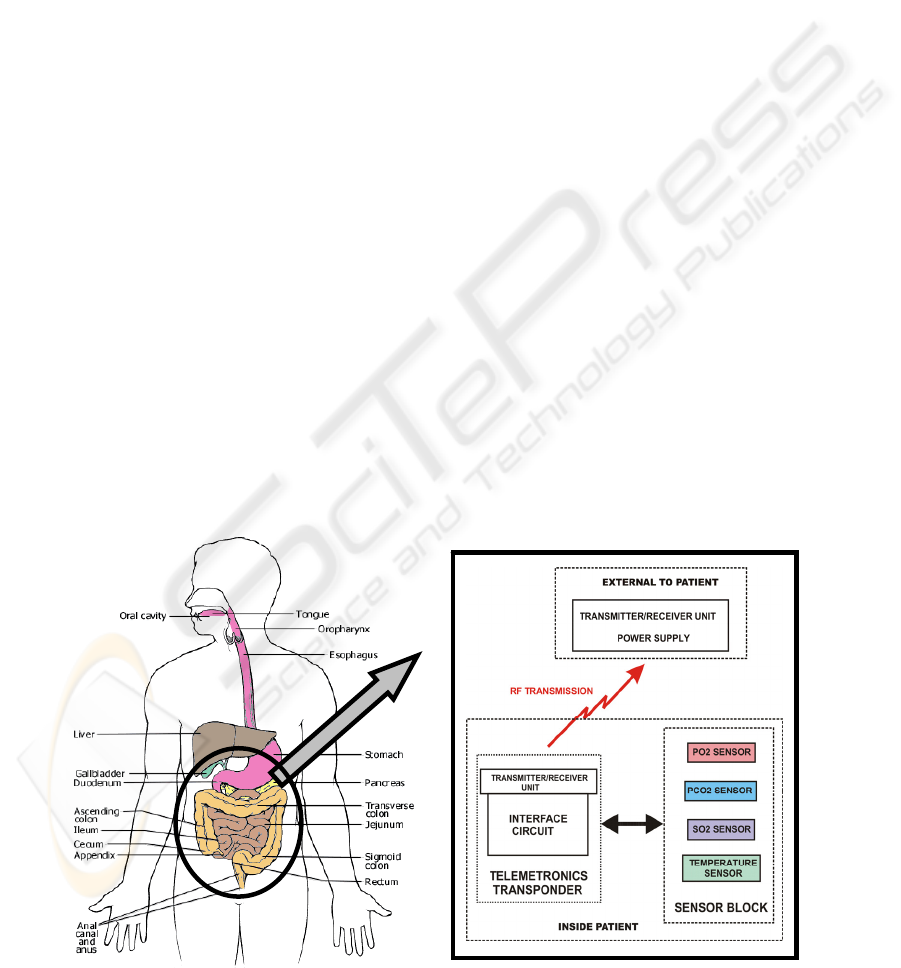
In this respect, the main goal of this research is
to develop a miniature, wireless sensor system to
monitor tissue viability pre- and postoperative,
continuously for 7 days. The complete sensor chip
should include an oxygen-saturation sensor (sO
2
), an
oxygen-tension sensor (pO
2
), a carbon-dioxide
tension sensor (pCO
2
) and a temperature sensor
(Figure 2).
The present work focuses on the use of the
oxygen-tension and temperature sensors for animal
studies.
2 MEASUREMENT SETUP
The measurement setup for the animal studies is
shown in Figure 3. It consists of the pO
2
and
temperature sensor block and a notebook for reading
and processing the data from the sensors. The
investigations were performed in the Erasmus
Medical Centre in Rotterdam, using male wistar rats,
12 weeks old. They were prepared for surgery by
shaving their abdomen and disinfecting it with 70%
alcohol.
.
Figure 2: Schematic of the complete sensor system for tissue viability monitoring.
ANIMAL STUDIES USING AN OXYGEN-TENSION SENSOR FOR TISSUE VIABILITY MONITORING
51
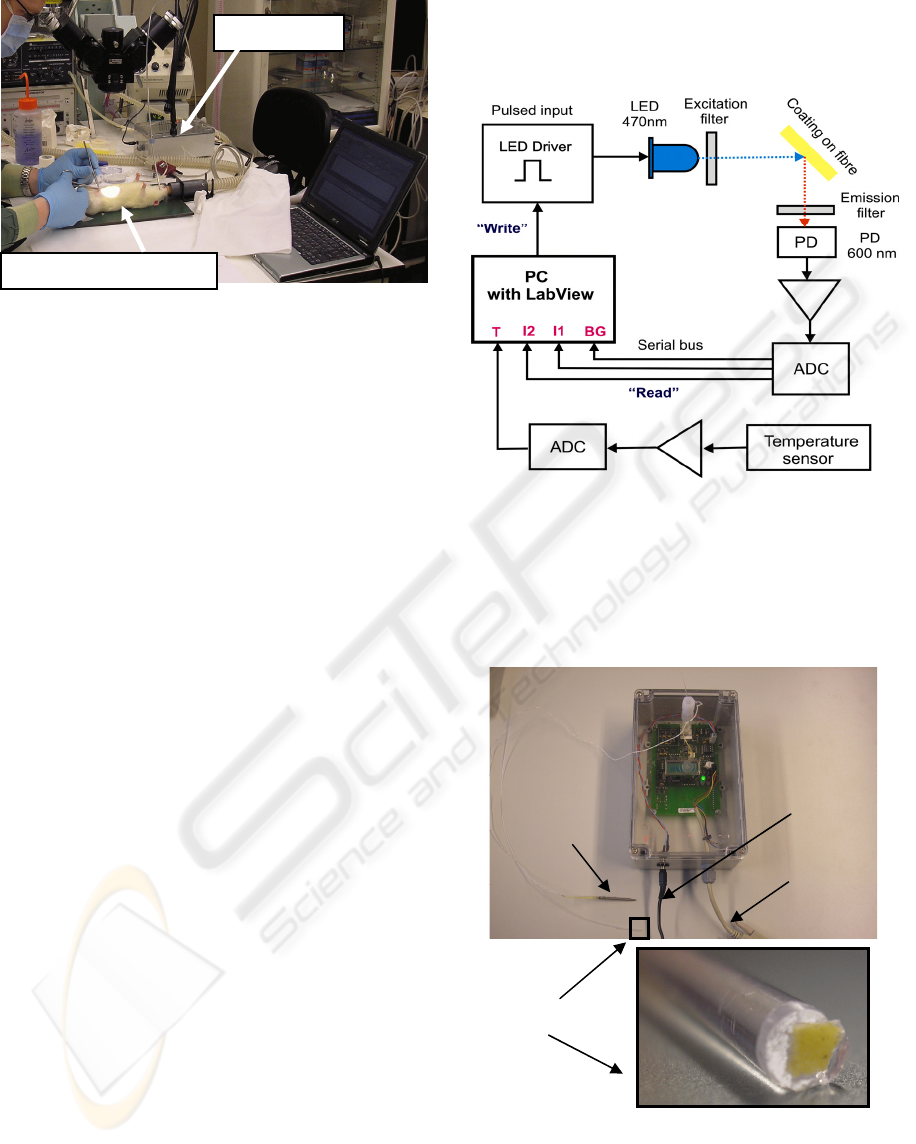
Figure 3: Measurement setup at Erasmus Medical Centre
in Rotterdam comprising the sensor block and the
notebook for data processing.
Afterwards, the animal undergoing surgery was
placed on a hot plate and anesthetised throughout the
intervention by administering a mixture of
isoflurane, oxygen and air (the fraction of inspired
oxygen, FiO
2
=66 %). Access to the internal
anatomical structures of the animals was gained by
laparotomy (surgical incision into the abdominal
wall) with an incision length of 4 cm. After opening
the abdomen and exposing the ascending colon, the
oxygen-tension and temperature sensors were fixed
together and placed at pre-defined locations along
the ascending colon, laterally (with respect to the
peritoneal membrane) and antimesenterial (opposed
to the peritoneal membrane).
The sensors were fabricated at TNO Quality of
Life, The Netherlands (Draaijer, 1999) and they
have been tested in a previous study (Tanase, 2007).
The block diagram of the sensors is shown in
Figure 4. The pO
2
sensor consists of a coating at the
tip of an optical fibre (3 mm diameter) and works on
the principle of dynamic quenching by oxygen of
fluorescent particles immobilized in a gas permeable
polymer. In our case, the fluorescent particle is
ruthenium, which enters an excited state caused by
the LED excitation with a wavelength of 470 nm.
The excited state of ruthenium is deactivated by the
collision process with oxygen, the particles emitting
light with a wavelength of 600 nm. The emitted
signals are detected by a photodiode (PD) and
converted to a digital signal using an on-board
analogue-to-digital (ADC) converter. The oxygen
concentration is determined by measuring the
fluorescence lifetime. In addition to the pO
2
sensor,
the sensor block contains the temperature sensor
(NTC type, Farnell), whose output is also converted
via an ADC. The total sequence of data sent to the
computer (via a serial connection RS 232) is BG, I1,
I2 and T (the background, the two intensities at
successive times and the temperature). From these
data, the software (LabView, National Instruments)
computes the oxygen tension and indicates the
temperature.
Figure 4: Schematic of the sensors with the LED driver
circuit, the read-out of the photodiode and of the
temperature sensor.
Figure 5 presents a photograph of the sensor block
with the two sensors and a magnified view of the
fibre tip with the oxygen-sensitive coating.
Figure 5: Photograph of the sensor block with the two
sensors and a magnified view of the fibre tip showing the
oxygen sensitive coating.
Power supply
RS 232
Temperature
sensor
Coating on fibre
pO
2
senso
r
Sensor block
Rat undergoing surgery
BIODEVICES 2008 - International Conference on Biomedical Electronics and Devices
52

3 MEASUREMENT RESULTS
Initial tests were performed by placing the sensors
on the colon after the construction of the
anastomosis. Figure 6 shows the sensors at a
distance of 1 cm away from the anastomosis, while
Figure 7 presents the table and graph with the
measurement results, for different sensor locations
around the anastomosis. The sensors were placed
radial (lateral and antimesenterial) and longitudinal,
at ten different locations on both sides of the
anastomosis, as indicated in Figure 7.
Figure 6: Photograph showing the sensors on the colon,
1 cm away from the anastomosis.
The lowest oxygen-tension values are obtained
on the anastomosis (part 3 and part 8 in Figure 7).
This is an expected effect - due to local cell death,
tissue oxygenation at the site of the anastomosis is
reduced. The farther from the anastomosis we
measure, the better the oxygenation, and the higher
the oxygen-tension values. The spikes on the graph
are artefacts visible only at the moments when the
sensors are moved from one tissue location to
another, because then, for a short period of time, the
fibre is in air. The temperature changes
corresponding to tissue and air are visible on the
temperature graph.
Another series of measurements were performed
with the sensors on the small intestine (Figure 8). In
this case, the blood supply to the central part of the
small intestine was obstructed by two strings that
were fastened for ischemia and released for
reperfusion. The measurement results during the
ischemia-reperfusion experiment are shown in
Figure 9.
At the beginning of the test, the sensors were
placed on the small intestine and by fastening the
strings, the intestine was made ischemic (part 1).
The values readily decreased to 4 mmHg, indicating
total ischemia. Once the strings were released, an
overshoot was noted, showing a maximum at
202 mmHg. Two other cycles were repeated to test
the correctness of the measurement. Also in this
case, the results of the tests met our expectations.
In addition to these measurements, other tests
were performed by changing the levels of inspired
oxygen (33.4 %, 42.8 %, 66.7 % and 91 %). We
noted that the local pO
2
changed accordingly to the
inspired oxygen. For an even better characterisation,
a new series of tests is currently performed, during
which the animals are intubated. In this way, the
inspired oxygen can be accurately controlled, while
the rats are being continuously monitored.
4 CONCLUSIONS
The paper has presented the initial measurements
and results with an optical oxygen-tension sensor
and a temperature sensor. The performed tests have
shown that the principle of optical sensing is suitable
for tissue measurements.
The first series of measurements has shown a
significant decrease (approximately 40 mmHg) in
pO
2
on the anastomosis as compared to the other
measurement sites on the colon. It was also shown
that on two points (lateral and antimesentery) of the
anastomosis, the values for the pO
2
were
approximately the same.
The ischemia and reperfusion experiments have
shown that the sensor system reacted as expected to
the local changes on the small intestine. When the
intestine was made ischemic, the pO
2
decreased and
when the obstruction was removed, the pO
2
increased significantly, with an overshoot.
This cycle was repeated three times to test the
correctness and repeatability of the measurement.
Sensors
Anastomosis
ANIMAL STUDIES USING AN OXYGEN-TENSION SENSOR FOR TISSUE VIABILITY MONITORING
53
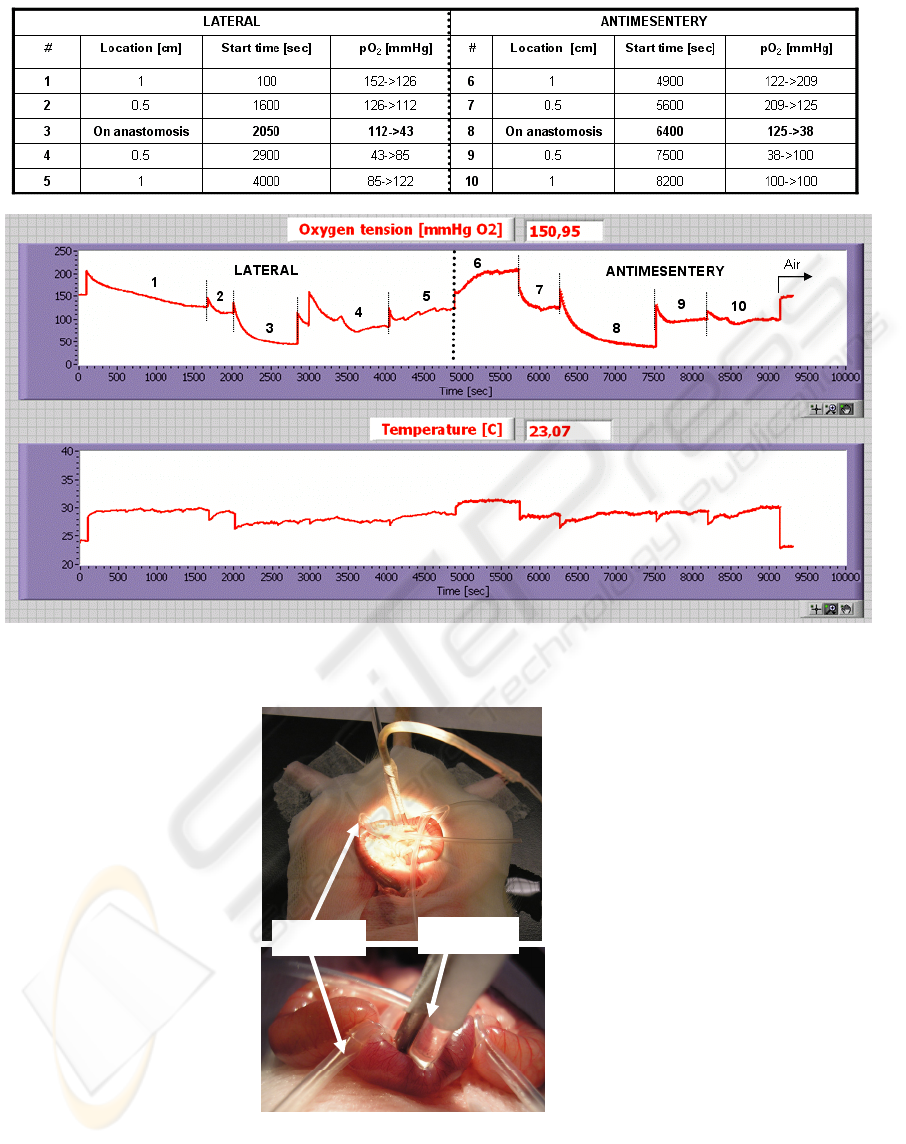
Figure 7: The numeric results and the graphical representation of the tests performed on the colon, to determine the
distribution of the oxygen radially and longitudinally with respect to the anastomosis, at different locations.
Figure 8: An overall view and a close-up of the small intestine showing the strings (used to obstruct the blood flow) and the
sensors.
Strings
Sensors
BIODEVICES 2008 - International Conference on Biomedical Electronics and Devices
54

Figure 9: The graphical representation of the ischemia-reperfusion experiment on the small intestine.
Although not presented in this paper, the first
steps towards an integrated sensor system have been
taken. The sensor-system design is currently
underway and issues regarding device sterilisation
and packaging are already taken into account.
A new study is currently planned for more
detailed investigations, considering also the aspects
of biocompatibility.
REFERENCES
Alves A., Panis Y., Pocard M., Regimbeau J.M., Valleur
P. (1999). Management of anastomotic leakage after
nondiverted large bowel resection. J Am Coll Surg,
189(6):554-559
Choi H-K., Lau W-L., Ho J.W.C., (2006). Leakage after
resection and intraperitoneal anastomosis for
colorectal malignancy: analysis of risk factors, Dis.
Colon Rectum, 49:1719-1725
Draaijer A., Konig J.W., Gans O., Jetten J., Douwma A.C.
(1999). A novel optical method to determine oxygen
in beer bottles. EMC Congress, France
Eckmann C., Kujath P., Schiedeck T.H., Shekarriz H.,
Bruch H.P. (2004). Anastomotic leakage following
low anterior resection: results of a standardized
diagnostic and therapeutic approach. Int J Colorectal
Dis, 19(2):128-133
Guenaga K.F., Matos D., Castro A.A., Atallah A.N.,
Wille-Jorgensen P. (2003). Mechanical bowel
preparation for elective colorectal surgery. Cochrane
Database Syst Rev 2:CD001544
Hyman N., Manchester T.L., Osler T., Burns B., Cataldo
P.A. (2007). Anastomotic leaks after intestinal
anastomosis: it’s later than you think. Ann Surg,
245(2):254-258
Kanellos I., Vasiliadis K., Angelopoulos S. et al. (2004).
Anastomotic leakage following anterior resection for
rectal cancer. Tech Coloproctol, 8 Suppl 1:s79-81
Macarthur D.C., Nixon S.J., Aitken R.J.(1998). Avoidable
deaths still occur after large bowel surgery. British J
Surg, 85(1):80-83
Peeters K.C., Tollenaar R.A., Marijnen C.A. et al. (2005).
Risk factors for anastomotic failure after total
mesorectal excision of rectal cancer. British Journal of
Surgery, 92(2):211-216
Tanase D., Komen N., Draaijer A., Kleinrensink G.J.,
Jeekel J., Lange J.F., French P.J. (2007), Oxygen-
tension measurements – the first step towards
prevention and early detection of anastomotic leakage,
To be published in Proceedings of the IEEE Sensors
2007 Conference, Atlanta, USA
ANIMAL STUDIES USING AN OXYGEN-TENSION SENSOR FOR TISSUE VIABILITY MONITORING
55
