
THERMORESPONSIVE POLYMER-BASED MICRODEVICE FOR
NANO-LIQUID CHROMATOGRAPHY
Guillaume Paumier, Sovann Siv, Aur
´
elien Bancaud, Jan Sudor and Anne-Marie Gu
´
e
LAAS-CNRS, University of Toulouse, 7 avenue du Colonel Roche, Toulouse, France
Keywords:
PNIPAM, thermoresponsive polymer, nanoliquid chromatography, sample preparation.
Abstract:
We report here on the development of an integrated device for sample desalting and pre-concentration for
nanoLC / ESI-MS analysis combining poly-(N-isopropyl acrylamide) (PNIPAM) grafted microbeads and the
means to dynamically control their temperature. Thermoresponsive properties of PNIPAM induce switchable
hydrophobic/hydrophilic surfaces on which peptides can reversibly adsorb and desorb. The device is fabricated
on a glass or pyrex substrate with deposited Ti/Au electrodes serving as built-in resistive heating sources. Pre-
molded microfluidic channels and reservoirs made in PDMS are eventually assembled. Electrical and thermal
characterization together with multiphysics modeling have been performed. The SiO
2
surfaces of the channels
and silica beads used as carriers of the stationary phases have been end-grafted with PNIPAM and employed
to study the reversible adsorption and release kinetics of albumin-fluorescein conjugates by fluorescence video
microscopy. It is clearly shown albumin-fluorescein complexes adsorb on beads surfaces above the transition
temperature of PNIPAM (hydrophobic state), and are released when the temperature decreases (hydrophilic
state), yet not fully reversibly.
1 INTRODUCTION
The challenge of proteomics is to develop high-
throughput and integrated approaches to identify and
understand the structure, functions and interactions of
proteins. Nano-liquid chromatography (nanoLC) in
combination with electrospray ionization mass spec-
trometry (ESI-MS) detection has become a major ex-
perimental method owing to its high separation power
and sensitivity (Ishihama, 2005). In general, pro-
teins are fractionated, isolated and digested into pep-
tides to be analysed and identified by nanoLC / ESI-
MS. Miniaturization provides a number of advantages
such as low limit of detection, small volumes of an-
alyte required and reduced intermediate manipulation
steps (Gauthier and Grimm, 2006). Thus, many ef-
forts have been made to integrate on-chip separation
devices providing the column, connection capillaries
and MS coupling via a nanospray emitter (Hern
´
andez-
Borges et al., 2007). Though, due to MS high sensi-
tivity to salts, peptides need to be desalted and con-
centrated on C4 or C8 columns prior to their analy-
sis (Wilm and Mann, 1996). During this step, certain
hydrophobic peptides can be lost on the hydrocarbon
surfaces because they show a greater affinity to the
stationary phase as compared to the mobile one uti-
lized for desorption of the purified peptides (Peterson
et al., 2003).
We propose here a novel approach based on sta-
tionary phases prepared from poly(N-isopropyl acry-
lamide) (PNIPAM) that can reversibly adsorb and re-
lease peptides upon external activation in a purely
aqueous environment. We present the development
of an integrated device for sample desalting and pre-
concentration for nanoLC / ESI-MS analysis, com-
bining PNIPAM grafted surfaces and the means to
dynamically control their temperature by integrated
microheaters.
2 THEORY
PNIPAM is a stimuli-responsive polymer which un-
dergoes a reversible coil-to-globule transition at its
178
Paumier G., Siv S., Bancaud A., Sudor J. and Gué A. (2008).
THERMORESPONSIVE POLYMER-BASED MICRODEVICE FOR NANO-LIQUID CHROMATOGRAPHY.
In Proceedings of the First International Conference on Biomedical Electronics and Devices, pages 178-181
DOI: 10.5220/0001053101780181
Copyright
c
SciTePress
lower-critical solution temperature (LCST) around
32
◦
C. PNIPAM grafted surfaces can be switched from
a swollen, hydrophilic and non-fouling state to a col-
lapsed, hydrophobic and protein-adsorbing state us-
ing thermal actuation (Kanazawa et al., 1996; Huber
et al., 2002). Such surfaces have been previously re-
ported for spatio-temporal control of flows in fluidic
microsystems by our group (Sudor et al., 2006).
The idea presented here is to use PNIPAM-
decorated beads as stationary phases to trap peptides
during desalting and pre-concentration steps prior to
the nano-LC / ESI-MS analysis. The reversible tran-
sition of PNIPAM surfaces upon temperature allows
controlled adsorption and release of peptides without
the change of quality of a solvent. To increase spe-
cific surface of interaction between PNIPAM and pep-
tides, PNIPAM is grafted on micrometric silica beads
injected into the channel. The channel height is re-
duced at its center to block the beads, while its width
is widened to preserve the constant surface area.
A resistive heating device is directly integrated
on the pyrex subtrate to control the temperature in-
side the channel. Microfluidic pre-molded PDMS
channels and reservoirs are eventually assembled to
the substrate to form the final fluidic microsystems
(Fig. 1).
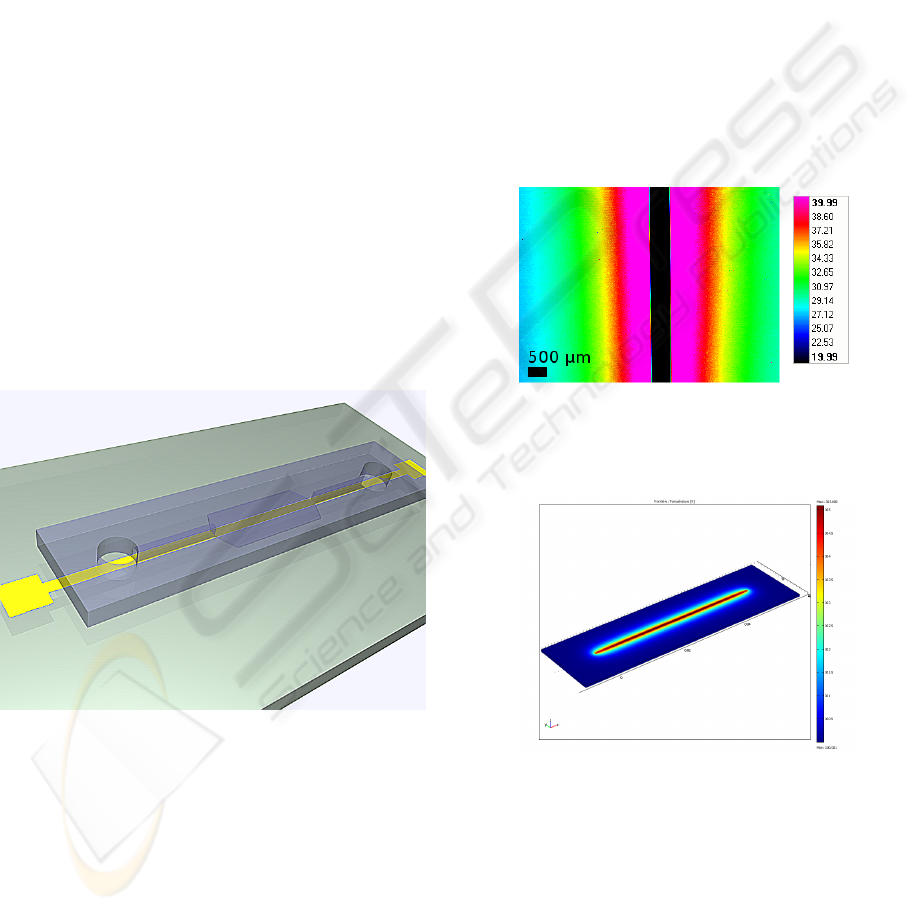
Figure 1: 3D view of the assembled prototype: pyrex sub-
strate, heating line and PDMS channel.
3 EXPERIMENTAL
3.1 Heating Device
Our heating device was made with lines fabricated
on a silicon, glass or pyrex substrate with deposited
Ti/Au (1000 / 8000
˚
A) electrodes serving as built-
in resistive heating sources. Lines of 100 µm and
500 µm width (respectively 32.5 Ω and 6.75 Ω on
pyrex) were realized and characterized. Infrared
imaging showed the heated zone was localized around
the heater (Fig. 2). Suitable temperatures were ob-
tained for acceptable voltages: 51
◦
C for 4 V (500 µm
wide) and 7 V (100 µm wide), given that LCST
of PNIPAM is around 32
◦
C. For 500 µm-wide lines
around 4 V, we obtained a homogeneous heated zone
more than 1.5 mm wide (microfluidic colons are
50 µm wide). Response time in heating is very short
(< 1s); cooling happens in seconds.
Multiphysics modeling using Comsol was also
performed. First modeling results fit relatively well
with experimental data (Fig. 3), however, a slight re-
finement of the model is still necessary. Work is also
underway to develop more complex heating devices
allowing more precise control of heated zones (Pau-
mier et al., 2007).
Figure 2: Infrared thermal imaging of the Ti/Au electrode
(
◦
C). The resistor appears black because of the infrared re-
flection on gold.
Figure 3: Multiphysics modeling of the heating process us-
ing Comsol.
3.2 Surface Chemistry
A 500 nm SiO
2
layer was deposited by plasma-
enhanced chemical vapor deposition (PECVD) on
the substrate and the electrodes to provide an elec-
tric insulator, and to allow homogeneous PNIPAM
grafting. The SiO
2
surfaces of the channels and sil-
ica beads (used as carriers of the stationary phases)
THERMORESPONSIVE POLYMER-BASED MICRODEVICE FOR NANO-LIQUID CHROMATOGRAPHY
179
were end-grafted with PNIPAM according to litera-
ture (Hjert
´
en, 1985), through an intermediate silane
layer (3-trimethoxysilyl propylmethacrylate). Sur-
faces were characterized with dynamic contact an-
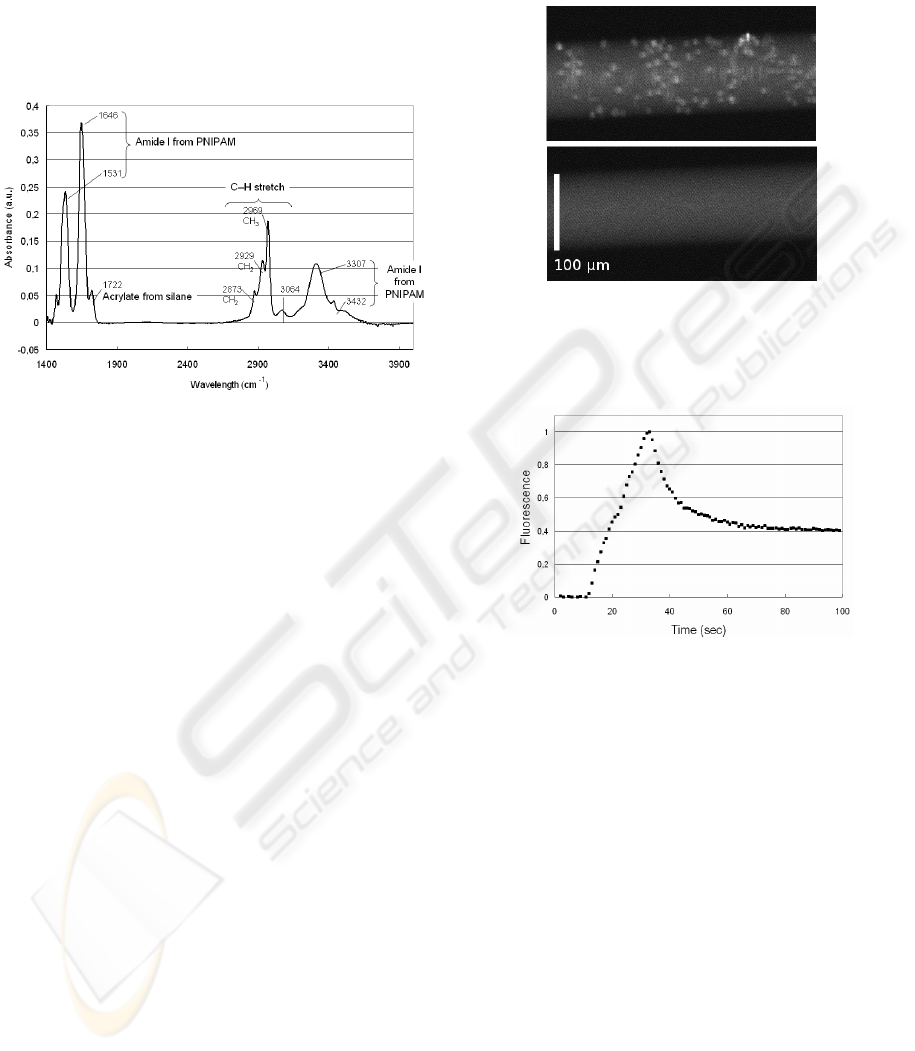
gle measurements and multireflection infra-red spec-
troscopy. The latter showed specific peaks identifying
chemical groups from PNIPAM (Fig. 4).
Figure 4: Multiple internal reflection infrared spectrum of
surfaces grafted with PNIPAM.
4 RESULTS AND DISCUSSIONS
4.1 Controlled Adsorption/Release of
Proteins
To prove feasibility of using beads decorated with
PNIPAM to adsorb/desorb proteins, we made our first
experiments in capillaries. We used silica beads (5 µm
in diameter) end-tethered with PNIPAM chains in
fused silica capillaries (100/385 µm inner/outer di-
ameter). The inner surface of the capillary was end-
grafted with polyacrylamide (PAM), which is not sen-
sitive to temperature changes in the studied range.
Bovine serum albumine (BSA) - fluoresceine conju-
gate (1 mg/ml) dissolved in sodium phosphate buffer
(pH 7) was injected into the capillary. When tem-
perature was increased to about 40
◦
C (above LCST),
PNIPAM chains on beads became hydrophobic; they
trapped and concentrated BSA-fluorescein conjugates
on beads, making them fluorescent, as shown in
Fig. 5. By decreasing the temperature below the
LCST, BSA-fluorescein conjugates were released into
the solution.
The kinetics of reversible adsorption and release
was also studied. The graph on Fig. 6 shows the
adsorption and release of albumin-fluorescein conju-
gates on beads. We observed the release of proteins
was not fully reversible. A proposed explanation is
the low grafting density of PNIPAM chains and con-
sequent protein adsorption on the non-modified sur-
faces of silica beads.
Figure 5: Albumine-fluorescein conjugates adsorbed on 5-
µm beads (top, T > LCST ) and then released (bottom, T <
LCST ). Background fluorescence is due to complexes in
solution.
Figure 6: Adsorption/release kinetics of albumin-
fluorescein conjugates from glass beads functionalized
with PNIPAM (normalized units).
4.2 Beads in Microchannels
Then, we went a step further and injected silica beads
inside our PDMS microchannel. Silica beads were
not functionalized with PNIPAM at this point. The
microchannel was made of three parts. At the center,
the channel was 1250 µm wide, 1500 µm long and
4 µm high. Side channels were 50 µm wide, 3000 µm
long and 100 µm high each. This geometry allows to
trap beads where section changes.
Several sizes of beads and central height of the
middle section of the channel were tested and charac-
terized through fluorescence microscopy. When these
sizes were too close, beads managed to slip inside the
central part, due to PDMS ductility: Young’s modu-
lus of PDMS depends on the mixing ratio of elastomer
and curing agent but it remains about 10
5
Pa (Armani
et al., 1999). We observed this phenomenon with
BIODEVICES 2008 - International Conference on Biomedical Electronics and Devices
180

5 µm beads and 4 µm central height. To prevent this,
we injected a limited amount of 10 µm beads prior
to the 5 µm beads, that were prevented from entering
the central part because of the bigger beads. Exper-
iments with PDMS devices and beads modified with
PNIPAM are currently underway.
Figure 7: Top: 3D close-up on the channel zone where
beads are blocked. Bottom: Top-view of fluorescent 10 µm
and 5 µm beads blocked at the entry of the central part.
5 CONCLUSIONS
Thermoresponsive properties of PNIPAM upon tem-
perature are well known and have been demonstrated
as switchable surfaces for protein adsorption. We
demonstrated in this work the possibility to inte-
grate such switchable surfaces into fluidic microsys-
tems dedicated to sample preparation for nanoLC /
ESI-MS. We have developed essential components
and know-how about heating sources, reversible pro-
tein adsorption and release, and injection of beads in
PDMS microchannels. We are now demonstrating the
feasibility of the microsystems for desalting and pre-
concentration of various peptide samples.
REFERENCES
Armani, D., Liu, C., and Aluru, N. (1999). Re-configurable
fluid circuits by PDMS elastomer micromachining. In
Proc. IEEE MEMS ’99.
Gauthier, G. and Grimm, R. (2006). Miniaturization: Chip-
based liquid chromatography and proteomics. Drug
Discov. Today Techn., 3(1):59–66.
Hern
´
andez-Borges, J., Aturki, Z., Rocco, A., and Fanali, S.
(2007). Recent applications in nanoliquid chromatog-
raphy. J. Sep. Sci., 30(11):1589–1610.
Hjert
´
en, S. (1985). High-performance electrophoresis:
Elimination of electroendosmosis and solute adsorp-
tion. J. Chromatogr. A, 347:191–198.
Huber, D., Manginell, R., Samara, M., Kim, B.-I., and
Bunker, B. (2002). Programmed adsorption and re-
lease of proteins in a microfluidic device. Science,
301:352–354.
Ishihama, Y. (2005). Proteomic LC–MS systems using
nanoscale liquid chromatography with tandem mass
spectrometry. J. Chromatogr. A, 1067(1-2):73–83.
Kanazawa, H., Yamamoto, K., Matsushima, Y., Takai,
N., Kikuchi, A., Sakurai, Y., and Okano, T.
(1996). Temperature-responsive chromatography
using poly(n-isopropylacrylamide)-modified silica.
Anal. Chem., 68(1):100–105.
Paumier, G., Sudor, J., Coll
´
e, E., Marty, B., Bancaud, A.,
Camps, T., and Gu
´
e, A.-M. (2007). Electrokinetic
mixers based on stimuli-responding surfaces. In Proc.
11th Int. Conf. on Miniaturized Systems for Chemistry
and Life Sciences (µTAS’2007).
Peterson, D., Rohr, T., Svec, F., and Fr
´
echet, J. (2003).
Dual-function microanalytical device by in situ pho-
tolithographic grafting of porous polymer monolith:
integrating solid-phase extraction and enzymatic di-
gestion for peptide mass mapping. Anal. Chem.,
75(20):5328–5335.
Sudor, J., Paumier, G., Gu
´
e, A.-M., Vinet, F., Est
`
eve, A.,
and Djafari-Rouhani, M. (2006). Spatio-temporal tun-
ing of stimuli-responding surfaces for dynamic con-
trol of electroosmotic flows. In Proc. 10th Int. Conf.
on Miniaturized Systems for Chemistry and Life Sci-
ences (µTAS’2006).
Wilm, M. and Mann, M. (1996). Analytical properties of the
nanoelectrospray ion source. Anal. Chem., 68(1):1–8.
THERMORESPONSIVE POLYMER-BASED MICRODEVICE FOR NANO-LIQUID CHROMATOGRAPHY
181
