
DEVELOPMENT OF AN AMPEROMETRIC SENSOR FOR
POTASSIUM IONS
Marcos F. S. Teixeira, Alex S. Lima, Patricia Monteiro Seraphim
Dep. of Physical, Chemistry and Biology , Faculty of Science and Technology, University of State of Sao Paulo (UNESP)
Rua Roberto Simonsen, 305 - Presidente Prudente – SP - Brazil
Nerilso Bocchi
Department of Chemistry, Federal University of São Carlos
Via Washington Luiz, Km 235 - São Carlos – SP - Brazil
Keywords: Sensor for potassium ions, hollandite, voltammetry.
Abstract: Hollandite-type manganese oxides are nanofibrous crystals with sub-nanometer open tunnels that provide a
unique property for sensing applications. Sensor based on hollandite-type manganese oxide was investigated
for amperometric detection of potassium. With an operating potential of +0.63 V versus SCE, potassium
ions produce oxidation currents at the sensor, which can be exploited for quantitative determinations. The
amperometric signals are linearly proportional to potassium ions concentration in the range 2.7 × 10
−4
to 9.1
×10
−4
mol l
−1
with a correlation coefficient of 0.9990. The construction and renewal are simple and
inexpensive.
1 INTRODUCTION
Determination of potassium contents of serum,
urine, and foods is very important in clinical and
medical fields, since the potassium contents are
related to renal diseases. These diseases restrict
patients to a diet containing a large amount of
potassium. From the potassium determination,
medical information concerning physical conditions
of the patient can be obtained. In the case of
hypopotassemia, alkalosis, cirrhosis of liver, diuretic
drugs, etc. are suspected. On the other hand, when
potassium concentration in human serum becomes
higher than 9 mmol L
−1
, heart often stops (Harrison
et al., 1966). Hence, accurate, easy and rapid sensing
of potassium ions is very important.
The development of chemical sensors for non-
electroactive ions based with modified electrodes
has been based in the participation of non-
electroactive cations in redox reactions of metal
hexacyanoferrates (Karyakin, 2001). Other
compound with ability of accommodate non-
electroactive cations and promote the electroactivity
in function of the insertion cation is the manganese
oxide. Manganese oxides represent a large class of
materials that have layered and tunneled structures
consisting of edge-shared MnO
6
octahedral units.
They have attracted considerable interest due to
broad potential applications in heterogeneous
catalysis, chemical sensing, toxic wastewater
treatment, and rechargeable battery technology. In
our laboratories, we are also interested in developing
highly sensitive and selective methods for the
determination of non-electroactive using electrodes
modified with different allotropic forms of
manganese oxide (Teixeira et al., 2004 and Teixeira
et al., 2004).
In this paper, we propose a new sensor to
determine potassium ions.
2 EXPERIMENTAL
2.1 Apparatus
All voltammetric measurements were carried out in
a 30ml thermostated glass cell at 25
◦ C, containing
three electrodes: carbon-paste electrode as a working
electrode, saturated calomel as reference electrode
(SCE), and platinum wire as an auxiliary electrode.
During the measurements, the aqueous solution
(TRIS buffer solutions) in the cell was not stirred
198
F. S. Teixeira M., S. Lima A., Monteiro Seraphim P. and Bocchi N. (2008).
DEVELOPMENT OF AN AMPEROMETRIC SENSOR FOR POTASSIUM IONS.
In Proceedings of the First International Conference on Biomedical Electronics and Devices, pages 198-201
DOI: 10.5220/0001054001980201
Copyright
c
SciTePress
and deaerated. Voltammetric measurements were
performed with a micro-Autolab Type III controlled
by an appropriated software.
2.2 Reagents and Solutions
All solutions were prepared using a Millipore Milli-
Q water. All chemicals were analytical reagent grade
and were used without further purification. The
supporting electrolyte used for most of the
experiments was a 0.1 mol l
-1
Tris buffer solution
(pH = 8.30). A 0.01 mol l
-1
potassium ions solution
was prepared daily by dissolving potassium chloride
(Merck) in 100 ml of such Tris buffer solution.
Graphite powder (1–2 μm particle size from
Aldrich) and mineral oil (Aldrich) of high purity
were used for the preparation of the sensor.
2.3 Preparation of Hollandite-Type
MnO
2
Hollandite-type manganese(IV) oxide was prepared
using a reflux method according to literature (Ching
et al. 1997). For conversion to hollandite-type
manganese oxide, a KMn
2
O
4
sample was treated in
an aqueous diluted sulfuric acid solution kept under
constant stirring during 120 min. When the pH of
this mixture was stabilized at a particular value, the
solution was decanted and the remaining solid
material washed by decantation with deionized
water, filtered and dried in air at 90
ºC.
2.4 Sensor Construction
Sensors with hollandite-type MnO
2
were prepared
by carefully mixing the dispersed graphite powder
with manganese oxide
at varying ratio. Exactly 1 g
of this mixture was subsequently added to 0.200 g of
mineral oil (20% m/m) and mixed in a 50 ml beaker
containing 20 ml of hexane. The final paste was
obtained with the solvent evaporation. The carbon-
paste electrode was finally obtained packing the
paste into a plastic tube (1 ml insulin plastic syringe)
and arranged with a copper wire serving as an
external electric contact.
3 RESULTS AND DISCUSSION
3.1 Electrochemical Behavior
First, the voltammetric behavior of the CPEM with
hollandite-type manganese oxide in Tris buffer
solution (pH 8.3) containing 5.0 x 10
-4
mol L
-1
potassium ions was investigated. The cyclic
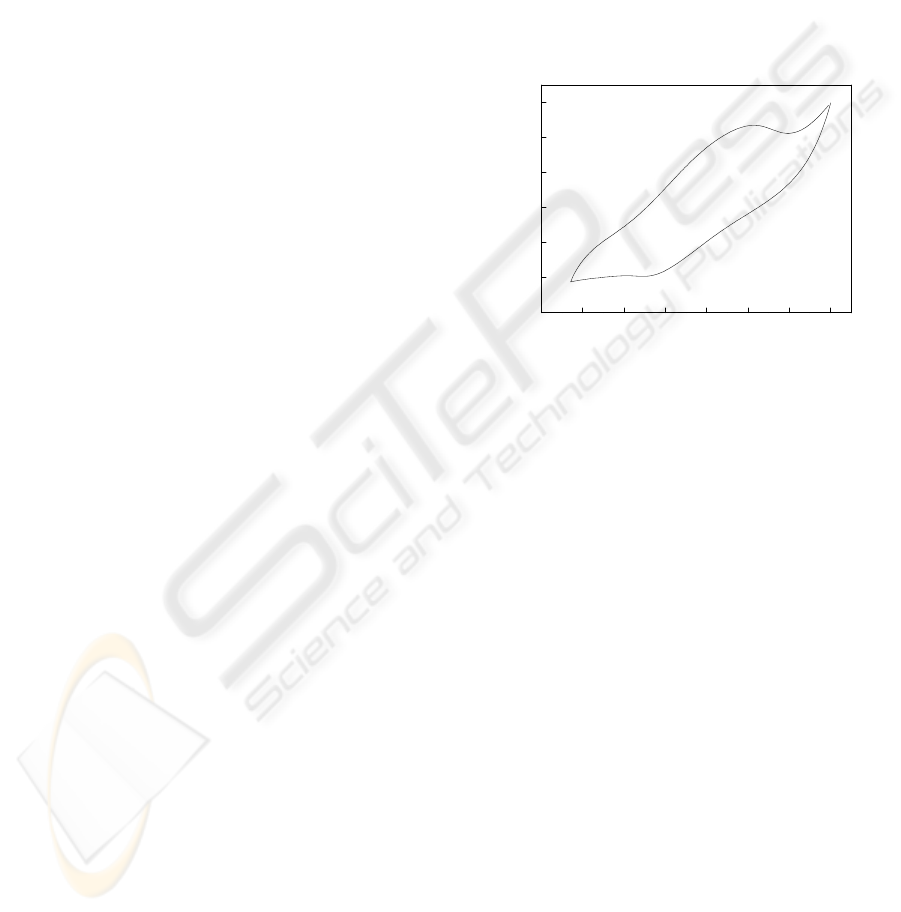
voltammograms obtained with the sensor (see Fig.
1) presented one anodic peak (peak I = 0.63 V vs.
SCE) and another cathodic peak (peak II = 0.08 vs.
SCE). This electrochemical activity is due to the
extraction topotactic process of the potassium ions
from the hollandite structure, which occurs in two
steps to the electrochemical insertion/extraction
processes of the potassium ions (Feng et al., 1995):
4
4
2
3
OMnMnK
+
−
+
xxx
(s)
→ 2.MnO
2(s)
+ x K
+
(aq)
+ x e
-
(1)
2.MnO
2(s)
+ x K
+
(aq)
+ x e
-
→
4
4
2
3
OMnMnK
+
−
+
xxx
(s)
(2)
-0.2 0.0 0.2 0.4 0.6 0.8 1.0
-200
-100
0
100
200
300
peak II
peak I
I / μA
E (V) vs. ECS
Figure 1: Cyclic voltammogram of the sensor in 5.0 x 10
-4
mol L
−1
of potassium ions, at a scan rate of 50 mV s
−1
between − 0.15 and 1.00 V versus SCE.
The enhanced response to potassium ions occurs
because the cathodic polarization of the sensor with
hollanditel-type manganese oxide is quite enough to
reduction the manganese in the solid. Consequently,
the potassium ions from the adjacent solution are
able to diffuse though the hollandite structure to
maintain the electroneutrality principle.
In the absence of potassium ions, no voltammetric
response was observed for the sensor, confirming
that the response of the sensor is a function of the
insertion reaction of potassium ions in the hollandite
structure as mentioned previously (see Eq. 3):
2.MnO
2(s)
+ x K
+
(aq)
+ (x/2) H
2
O
(l)
→
4
4
2
3
OMnMnK
+
−
+
xxx
(s)
+ x H
+
(aq)
+ (x/4) O
2(g)
(0 < x < 1)
(3)
where is vacant site (tunnel) of the manganese
oxide.
The apparent electrochemical rate constant k
e and
the electron-transfer coefficient α
anodic
were calculated
for the sensor according to the method described by
Larivon (Larivon, 1979). It has been shown by
Laviron that for a surface redox couple, α
anodic
and ke
can be determined from the variation of Epa with
scan rate. Figure 2 presents the plot of E
pa (V)
DEVELOPMENT OF AN AMPEROMETRIC SENSOR FOR POTASSIUM IONS
199
versus log ν (V s
−1
) of the sensor in Tris buffer
solution (pH 8.3) containing 5.0 x 10
-4
mol L
-1
potassium ions. For large enough values of scan rate
the E
p − log ν plots gave one straight line with
slopes of 2.303RT/(1− α
anodic
)nF for the anodic
branch, where R is the gas constant, T the absolute
temperature, F the Faraday constant and n number of
electrons involved in the redox couple. Considering
that the number of electrons involved in the redox
process is 1, the calculated value for the coefficient
α
anodic
was 0.83. These results suggest the redox
process tends towards an irreversible system. The
apparent electrochemical rate constant can then be
determined applying the equation k
e =
2.303α
anodic
nFν
o
/RT, in which the value of scan rate
(ν
o
) is determined by extrapolation of the linear
branch at higher scan rates and its intersection with
the constant peak potential, represented by the peak
of the voltammogram at the lower scan rate. The
observed value was k
e = 32.2 s
−1
.
-2.4 -2.2 -2.0 -1.8 -1.6 -1.4 -1.2 -1.0
0.4
0.5
0.6
0.7
0.8
E
pa
/ V vs. ECS
log v (V/s)
Figure 2: Dependence of E
pa
with log(ν) for the sensor in
Tris buffer solution (pH 8.3) containing 5.0 x 10
-4
mol L
-1
potassium ions.
The effect of the carbon paste composition in the
amperometric response of the sensor was evaluated
in Tris buffer solution (pH 8.3) containing 5.0 x 10
-4
mol L
-1
potassium ions. The anodic peak current
increased with the amount of manganese oxide in
the paste up to 25% (
m
/
m
). The anodic peak current
decreased significantly when more than 25% is used
in the electrode preparation. This probably occurs
due to a decrease in the conductive area at the sensor
surface. According to these results a sensor
composition of 25% (
m
/
m
) modified hollandite-type
manganese oxide, 55% (
m
/
m
) graphite and 20% (
m
/
m
)
mineral oil was used in further studies.
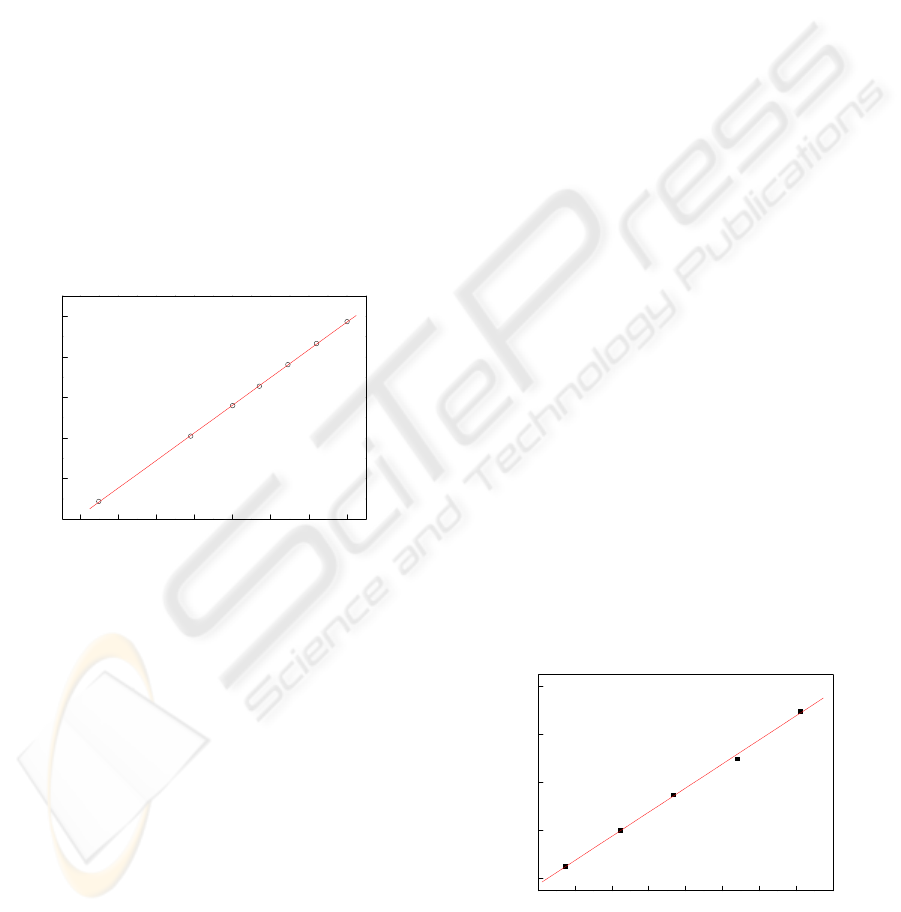
3.2 Analytical Curve and Repeatability
After optimizing the best operating conditions for
the sensor (scan rate of 20 mV s
−1
), cyclic
voltammetries were carried out in Tris buffer
solution containing different potassium ion
concentrations in order to obtain the analytical curve
for such electrode. This curve, illustrated in the
Figure 3, was constructed using the anodic peak
currents resulting a linear relationship with the
potassium ion concentrations from 2.7 × 10
−4
to 9.1
×10
−4
mol l
−1
(I
pa
(μA) = 2.07 + 200.8 [K
+
] (mol
L
−1
); r = 0.9990) with a detection limit of 1.5 × 10
−4
mol L
−1
potassium ions. The precision of the method
was also tested by analyzing five replicates
containing 5.0 x/10
−4
mol L
−1
potassium ions. For
each voltammogram, the surface of the sensor was
renewed. The variation coefficient was 2.0 %.
4 CONCLUSIONS
As shown above, a sensor based on the hollandite-
type manganese oxide exhibits an obvious response
to potassium ions. It is necessary to investigate the
interaction mechanism between potassium ion and
the hollandite-type manganese oxide. The presence
of Mn
3+
in hollandite may increase its activity for
the reaction. During the amperometric detection,
potassium ions diffuse through the hollandite
structure to produce Mn
+3
, which can be
electrochemically reoxidized to Mn
+4
. The oxidative
current is directly related to the concentration of
potassium ions. In view of its sensitivity, stability,
low working potential and simplicity and low cost of
construction, the sensor based on the hollandite-type
manganese oxides exhibits prospects for future
biosensor work.
0.3 0.4 0.5 0.6 0.7 0.8 0.9
2.12
2.16
2.20
2.24
2.28
I
pa
/ μA
[potassium ions] / mmol L
-1
Figure 3: The curve analytical obtained from the anodic
currents using sensor based hollandite-type manganese
oxide.
BIODEVICES 2008 - International Conference on Biomedical Electronics and Devices
200

ACKNOWLEDGEMENTS
The research support from FAPESP under contract
no. 05/01296-4 and also grant by CNPq (no.
372010/2006-7) to A.S.L. are gratefully
acknowledged (SJT).
REFERENCES
Ching, S., Roark, J.L., Duan, N., Suib, S.L., 1997. Chem.
Mater. 9, 750.
Feng, Q., Kanoh, H., Miyai, Y., Ooi, K., 1995. Chem.
Mater. 7, 148.
Harrison, T.R., Adams, R.D., Bennett, I.L., Resnik, W.H.,
Thorn, G.W., Wintrobe M.M., (Eds.), 1966. Principles
of Internal Medicine, 5
th
edn., McGraw-Hill, New
York.
Larivon, E., 1979. J. Electroanal. Chem. 101, 19.
Karyakin, A.A., 2001. Electroanalysis 13, 813.
Teixeira, M.F.S., Cavalheiro, E.T.G., Bergamini, M.F.,
Moraes, F.C., Bocchi, N., 2004. Electroanalysis 16,
633.
Teixeira, M.F.S., Bergamini, M.F., Bocchi, N., 2004.
Talanta 62, 603.
DEVELOPMENT OF AN AMPEROMETRIC SENSOR FOR POTASSIUM IONS
201
