
MICROWAVE DIELECTRIC SPECTROSCOPY
OF LOW-VOLUME FRACTION HUMAN CANCER CELLS
EMBEDDED IN COLLAGEN GELS
Experimental Feasibility Study with an Open-ended Coaxial Probe
Stéphane Egot-Lemaire*, Pierre-Olivier Bagnaninchi*, Jacek Pijanka*
Josep Sulé-Suso** and Serguei Semenov*
Institute for Science and Technology in Medicine,*Keele University and **University Hospital of North Staffordshire
Guy Hilton Research Center, Thornburrow drive, Stoke-on-Trent, U.K.
Keywords: Cancer cells, SK-MES cell line, Collagen gels, Volume fraction, Dielectric spectroscopy, Complex
permittivity, Open-ended coaxial probe, Microwaves.
Abstract: This paper addresses and demonstrates the feasibility for microwave dielectric spectroscopy to detect small
volume fractions of SK-MES lung cancer cells embedded in collagen gels with an open-ended coaxial
probe. Measurements were performed on the frequency range 200 MHz – 2 GHz. For all the cell volume
fractions tested (1.4%-4.4%), a significant difference in complex permittivity was observed between
composite gels (containing cells) compared to gels alone. Statistically significant changes were especially
found in the real part of the permittivity, which decreased consistently when the volume fraction increased.
1 INTRODUCTION
The dielectric properties of biological tissues and
cells have been widely investigated for many
decades (Foster, 1996; Gabriel 1996). They are
characterized by the so-called complex permittivity,
expressing the polarization response of a material in
the presence of a time-varying applied electric field.
Measuring its complex response as a function of the
field frequency is referred to as dielectric
spectroscopy (DS).
The difference in the dielectric properties
between various biological tissues is exploited in
electromagnetic tomography techniques, such as
biomedical microwave imaging, a promising
imaging modality (Tofighi, 2001; Semenov, 2003;
Fear, 2005). One of its particular interests is the
ablility to detect tumours (Hagness, 1998; Bulyshev,
2001; Shao, 2005; Bindu, 2006). Indeed malignant
tissues have mainly been found to have significantly
different dielectric properties than the corresponding
normal tissues regarding both the real and the
imaginary parts of the complex permittivity
(Chaudary, 1984; Surowiec, 1988; Smith, 1986;
Joines, 1994; Sha, 2002). In this regard, DS studies
related to cancer have mostly dealt with bulk tissues.
In these kinds of studies bulk tissues are either
investigated in vitro or in vivo which have both
disadvantages. In vitro studies have mostly
investigated non-living tissues. Moreover it is
difficult to deal with in vivo human tissues mainly
because surgery is needed, and it does not
necessarily give information at cell level.
On the contrary, working on cell culture samples
is a good alternative way to get a better biophysical
knowledge of living cells. In this regard, a number
of DS experiments have been carried out on various
types of cell suspensions. The most commonly
investigated cell suspensions, whatever the volume
fraction (ranging from a few percent to roughly
70%), have logically been blood samples (Lisin,
1996; Chelidze, 2002; Bordi 2002; Jaspard, 2003;
Treo, 2005) and yeast suspensions (Claycomb,
2002). A few of them have especially focused on
measuring the dielectric properties of white blood
cancer cells in suspension which were shown to be
also different from those of normal cells (Polevaya,
1999; Ermolina, 2001). Furthermore, in vitro cell
culture samples embedded in microporous scaffolds
have also been successfully investigated for tissue
engineering purposes (Bagnaninchi, 2003 and 2004).
156
Egot-Lemaire S., Bagnaninchi P., Pijanka J., Sulé-Suso J. and Semenov S. (2008).
MICROWAVE DIELECTRIC SPECTROSCOPY OF LOW-VOLUME FRACTION HUMAN CANCER CELLS EMBEDDED IN COLLAGEN GELS -
Experimental Feasibility Study with an Open-ended Coaxial Probe.
In Proceedings of the First International Conference on Biomedical Electronics and Devices, pages 156-161
DOI: 10.5220/0001055401560161
Copyright
c
SciTePress

The method was proved to be a good way to monitor
cell growth and differentiation in scaffolds used in
tissue engineering. The aforementioned studies
dealing with cell culture samples were shown to be
able to retrieve cell signature. This term refers to the
dielectric properties of the main cell compartments,
such as membrane, cytoplasm but also nucleus.
These so-called cell signatures were shown to be
distinct for different cell lines or types. This
property allows for cell separation of cancer cells
from normal cells by dielectrophoresis (Gascoygne,
1997). In these different studies, measurements have
covered different parts of the frequency spectrum,
extending from the extremely low frequencies to the
lower part of the microwave range depending on the
measurement method used.
The motivations of the present study are mainly
threefold. The first aim would be to get a better
biophysical understanding of the differences in
dielectric properties between epithelial lung cancer
cells and the corresponding normal ones. Secondly,
DS could also be used as a method to analyse the
response and effectivnesses of various anti-cancer
treatments such as chemotherapy drugs on in vitro
cell culture samples. Some authors have already
used DS in the radio and microwave frequency
range to do so (Santini, 1991 and 1995; Hübner
2005; Duncan, 2006). Thirdly, this study is a
preliminary step to investigate the capability of DS
to be used on intraoperative tissue biopsies for on-
line assessment of tissue resection effectiveness.
To address these issues, our approach is an
adjunct approach of cell suspensions and allows to
have an in vitro realistic biological 3D model for
living lung epithelial cells. As a relatively dense
matrix, a collagen gel is a good model for
investigation. Collagen is the major component of
the natural extracellular matrix (Pietrucha, 2005) and
has already been used in other biomedical studies
involving similar lung cancer cell culture samples
(Yang, 2004). We used low volume fractions of cells
in order to have an in vitro system that could detect
small number of cells so this could have the clinical
application of detecting tumours when they are still
small enough to undergo radical treatment.
This paper deals with the preliminary
investigation of the dielectric properties of lung
cancer cell samples embedded in collagen gels in the
lower part of the microwave range (UHF). The cells
in question belong to a human epithelial lung cancer
cell line, namely SK-MES cell line. This paper
especially addresses the feasibility of distinguishing
low-volume fraction of these cells from the matrix in
which they are embedded in this part of the
frequency spectrum.
The first section describes in details the materials
and experimental set-up used and the second section
gives and discusses some obtained results.
2 MATERIALS AND METHODS
We mainly conducted 6 different experiments, on
6 different cell volume fractions: 1.4%, 1.9%, 2.7%,
3.2%, 3.8%, 4.4% corresponding respectively to
about 4, 5.3, 7.4, 9, 10.6, 12.3 million cells per gel.
They were counted with the aid of a grid-counting
chamber (Hycor Kova Glasstic). The volume
fraction was estimated by supposing the cells are
spherical. Their diameter (mean: 18 μm ± standard
deviation: 2.2 μm) was measured on a slide with a
light microscope with a computerized ruler.
For each of the 6 experiments, two sets of
7 collagen gels were prepared and measured. The
first set did not include any cells and the second set
included the same volume fraction of SK-MES cells
for a given experiment.
2.1 SK-MES Cell Culture
SK-MES cells (ECACC, UK) were cultured in
175 cm
2
-cell culture flasks and incubated at 37°C
and 5% CO
2
. Each culture vessel contained
complete culture medium composed of high-glucose
Dulbecco’s Modified Eagle’s Medium supplemented
by 10% volume of foetal calf serum and other
standard components according to the provider’s
instructions and previous studies (Yang, 2004).
2.2 Collagen Gels Preparation
Collagen type I gels were prepared according to the
supplier’s (BD Biosciences) instructions. To ensure
the viability of SK-MES cells reported in (Yang,
2004), the concentration of collagen was 1.5 mg/mL.
Cells were added and mixed with the gel at a
temperature of 4°C when collagen is in liquid form.
Gels were allowed to set by incubation at 37°C for 3
hours.
Each gel was prepared in a cylinder well and had
a diameter of 19 mm and a height of 3 mm. These
dimensions are justified in the next section.
Therefore each gel had a volume of 0.85 mL.
MICROWAVE DIELECTRIC SPECTROSCOPY OF LOW-VOLUME FRACTION HUMAN CANCER CELLS
EMBEDDED IN COLLAGEN GELS - Experimental Feasibility Study with an Open-ended Coaxial Probe
157
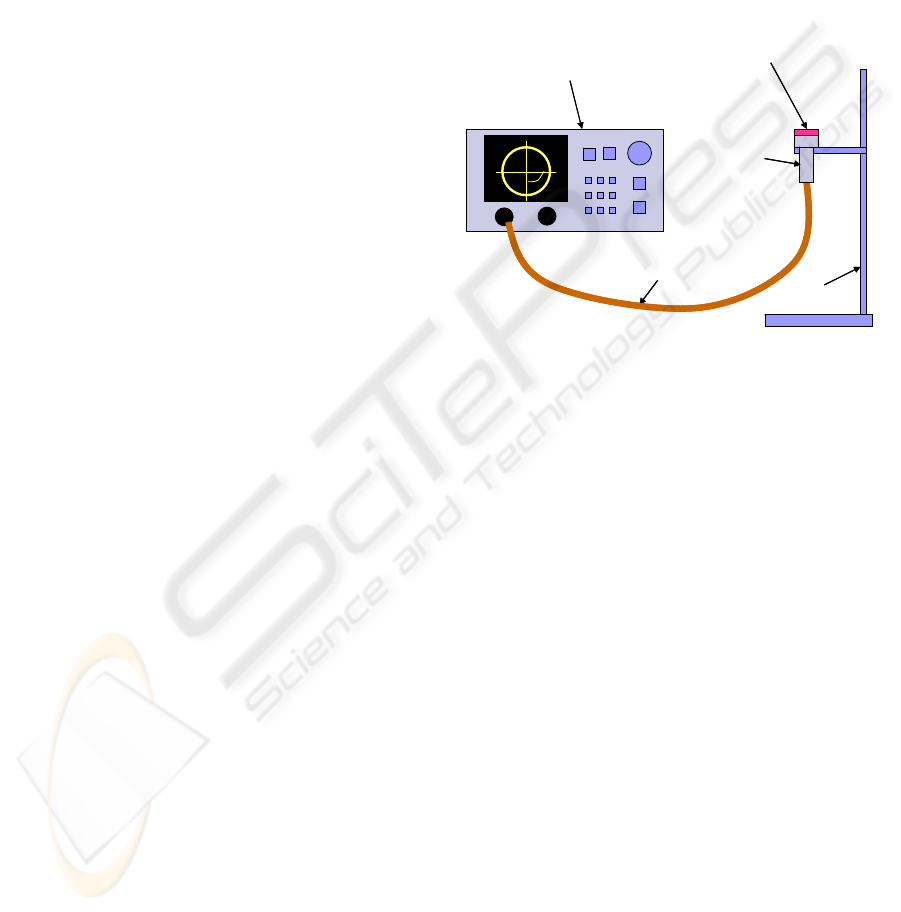
2.3 Experimental Set-up
2.3.1 Experimental Material
Dielectric spectroscopy was performed using a
vector network analyser (model 8753E, Agilent
Technologies) operated on the frequency range
200 MHz – 2 GHz connected to a flanged open-
ended coaxial probe (dielectric probe, Agilent
model 85070) via a coaxial cable.
The complex permittivity of the sample is
actually deduced by calculation from the
measurement of the complex reflection coefficient at
the tip of the probe. Indeed the reflection coefficient
is linked to the impedance or admittance seen at the
tip of the probe, which is itself directly related to the
complex permittivity of the sample by a suitable
model (implemented by Agilent’s software).
In theory, the model supposes that the sample is
semi-infinite (i.e. covers a half space) isotropic and
homogeneous. In practice, if the sample is not
homogeneous (our case), the result is an average
value weighted by the pattern of intensity of the
electric field (which is highest at the centre of the
probe tip). Besides, the sample is always of finite
size. In the probe supplier’s data sheet, the diameter
of the sample must be at least that of the probe, and
its minimal thickness is given by a simple formula
related to the permittivity, which in our case yields
about 2.5 mm. This is in good agreement with values
found in scientific papers to measure biological
tissues with probes of similar dimensions (Semenov,
2000; Hagl, 2003). Indeed, some researchers have
shown that measurement errors are small when the
sample thickness is at least as big as the outer
conductor radius of the probe (Fan, 1990; De
Langhe, 1994; Hoshina 2001), which is 1.5mm in
our case. We therefore decided to prepare 3mm-
thick collagen gels.
Nevertheless, a small sample size can be
problematic in the microwave range especially when
its permittivity is high and its losses relatively low,
because cavity resonances could occur. This
phenomenon explained by electromagnetic cavity
theory has been pointed out by some investigators
using the same kind of probe (Grant, 1989; De
Langhe, 1994; Sheen, 1999). To avoid potential
resonance effects, we chose 2 GHz as the upper
frequency (until which we did not observe any
resonance effect).
Besides, the frequency range of the probe using a
network analyser and the supplier’s software is
guaranteed from 200 MHz to 20 GHz. As a result,
we chose the frequency range 200 MHz – 2 GHz.
As shown in Figure 1, gel samples were actually
put onto the probe and fitted its dimension, as the
outer diameter of the probe flange is 19mm. Several
attempts were made to measure the gels from the top
with the probe upside down (compared to Figure 1),
but the repeatability of the measurement was very
poor. Moreover, the gels measured from the top got
squashed. On the contrary, putting the gel onto the
probe keeps its integrity and allows for a good
control of the contact between the gel and the probe,
and improves the repeatability of the measurement.
Figure 1: Diagram of the experimental set-up.
2.3.2 Experimental Method
For each experiment, 7 gels with cells, and 7 gels
without cells (‘controls’) were made and measured.
Each gel was measured 7 times in order to address
the repeatability issue. Prior to measurement, the
system was calibrated using a standard procedure, in
which the standards are air, a short circuit and
deionised water. The latter was measured in a
250 mL beaker to avoid resonance phenomena, in
accordance with (Blackham, 1997).
The measurement method was as follows. The
wells containing the gels were taken out of the
incubator and placed in a water bath at 37°C. Each
gel was then put onto the probe, which was at room
temperature. To achieve as good repeatability as
possible, a few minutes were necessary to get very
stable results. On the one hand, while the gels cool
down towards room temperature, we observed that
the real part of the permittivity ε’ increased slightly,
and the imaginary part ε’’ decreased slightly on the
whole chosen frequency range. On the other hand,
when left several tens of minutes at room
temperature, they start to dry out and we observed
that ε’ started to decrease and ε’’ started to increase
on the whole chosen frequency range. Measurements
were taken at the time when the two aforementioned
Network
analyser
Open-ended
coaxial probe
coaxial cable
stand
collagen gel sampleNetwork
analyser
Open-ended
coaxial probe
coaxial cable
stand
collagen gel sample
BIODEVICES 2008 - International Conference on Biomedical Electronics and Devices
158
phenomena compensate each other. Thus, excellent
repeatability was achieved: at the very most, the
fractional error (standard deviation divided by the
mean of 7 measurements on the same gel) was
0.25% for ε’ and 1% ε’’. If measurements are taken
before stabilization, these figures become
respectively 0.6% and 2.5% at the very most.
3 RESULTS AND DISCUSSION
The measurement reproducibility was assessed for
each volume fraction tested by calculating the
fractional error (standard deviation divided by the
mean, both calculated on 7 gels measured 7 times,
i.e. on 49 measurements) as a function of frequency.
At the very most, it reached 0.9% for ε’ and 5% for
ε’’. The latter was higher in the lower part of the
explored frequency range, where the conductivity is
particularly high. However, it could reach 3.3%
around 2 GHz.
For all the cell volume fractions tested, a
significant difference in permittivity was observed
between composite gels (containing cells) compared
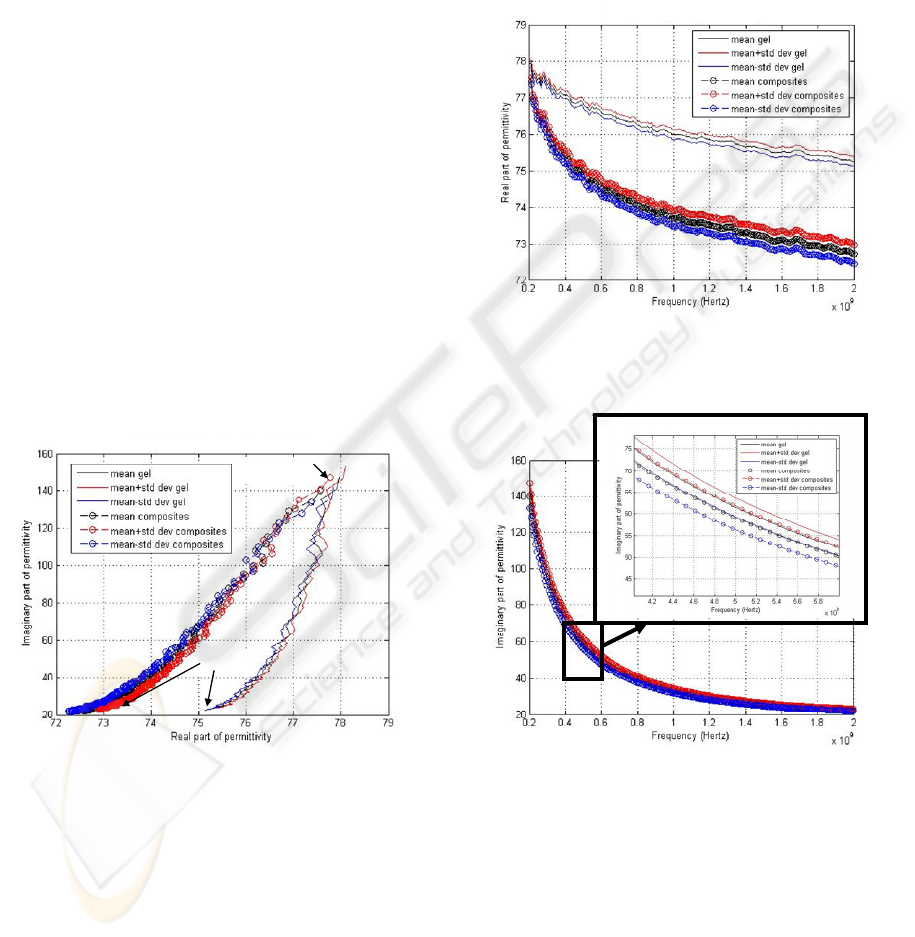
to gels alone. An example of result for a volume
fraction of 4.4% is given on Figure 2 in the complex
plane (Cole-Cole diagram).
200 MHz
2 GHz
200 MHz
2 GHz
Figure 2: Comparison composite gels / pure gels in the
complex plane (mean +/- standard deviation: data obtained
on 7 gel samples of each type); volume fraction: 4.4%.
The real part ε’ was found to be lower when the gels
contained cells rather than when they did not (cf.
Figure 3), and also to consistently decrease with the
cell volume fraction. A statistically significant
difference between composites and pure gels was
found for all volume fractions. This was
demonstrated by a two-tailed Student’s t-test (p-
value lower than 0.05 or even 0.01 on the major part
of the frequency range). Moreover, the difference in
real part between two adjacent volume fractions was
also found to be statistically significant by a two-
tailed t-test despite the close proximity of the
observed variations. It is in good agreement with
(Bagnaninchi, 2004) stating that a variation of 0.5%
volume fraction is detectable for low cell volume
fractions. The p-values were even lower for
differences in volume fractions greater than 1%.
Figure 3: Comparison composite gels / pure gels. Real part
of permittivity (mean +/- standard deviation: data obtained
on 7 gel samples of each type); volume fraction: 4.4%.
Figure 4: Comparison composite gels / pure gels for the
imaginary part of permittivity (mean +/- standard
deviation: data obtained on 7 gel samples of each type);
volume fraction: 4.4%.
Regarding the imaginary part of the permittivity, a
group of 3 experiments (out of 6) showed an
increase in ε’’ when comparing composites and pure
gels, and the 3 others showed a decrease (when
considering the means). A two-tailed t-test proved
that 2 variations among them were not statistically
MICROWAVE DIELECTRIC SPECTROSCOPY OF LOW-VOLUME FRACTION HUMAN CANCER CELLS
EMBEDDED IN COLLAGEN GELS - Experimental Feasibility Study with an Open-ended Coaxial Probe
159

significant (one in each group; p>0.1 and 0.3), an
example of which is shown on Figure 4.
This result about the imaginary part ε’’ can be
commented on qualitatively as follows. This
suggests that ε’’ of the composite gels could actually
be of the same order of magnitude as ε’’ of pure gels
because the reproducibility fractional error on ε’’
(which could reach 5% in the lower frequencies) is
not negligible. As gels are quite conductive, ε’’ is a
sensitive parameter whose variability is not
negligible.
Quantitatively we did some modelling to explain
why ε’’ of the composites gels can either be a bit
lower or higher than ε’’ of pure gels. We used
effective medium approximations commonly utilised
with biological cells, such as Maxwell-Wagner or
Looyenga equations (Bordi, 2002; Asami, 2002). A
composite gel is considered as an effective medium
in which cells are inclusions. A basic single-shell
cell model, modelling the membrane and cytoplasm
by their respective permittivities and conductivities
was implemented. The latter were varied even
beyond their commonly accepted values: the
permittivity of the membrane and the cytoplasm
were respectively varied from 2 to 30 and from 30 to
70. The simulation easily shows that for small
volume fractions, the conductivity of the cytoplasm
is decisive: if it is lower (respectively greater) than
the one of pure gel, ε’’ is lower (respectively
greater) for composite than for pure gel. Thus,
owing to the variability of the cytoplasm properties,
ε’’ of composites gels can be a bit lower or higher
than ε’’ of pure gels. Hence, the conductivity of the
cytoplasm of the measured SK-MES cells could be
of the same order of magnitude as that of the
measured gels.
Another study (Bagnaninchi, 2003) carried out
on the same frequency range but with macrophages
put inside chitosan scaffolds filled with a similar
ionic culture medium (RPMI) showed different
results. The addition of cells induced an increase in
ε’ and a decrease in ε’’. However, the effective
dielectric behaviour depends on the particular
dielectric properties of each type of cells and of the
surrounding media. The former constitute the next
step of the study.
4 CONCLUSIONS - PROSPECTS
This study has proven the feasibility of detecting
small volume fractions of lung cancer cells
embedded in collagen gels by microwave dielectric
spectroscopy. The real part of the permittivity was
found to decrease with the presence of cells. The
imaginary part did not significantly show a
consistent variation.
The prospects of this study are mainly threefold.
Firstly, further suitable modelling should be
developed to try to retrieve cell signature and
properties. Secondly, similar experiments should be
carried out with the corresponding normal lung
epithelial cells and results compared to this study.
Thirdly, other DS experiments should also be tried
to analyse the response and effectivnesses of various
anti-cancer treatments such as chemotherapy drugs
on in vitro cell culture samples.
ACKNOWLEDGEMENTS
This work was partially supported by Maxime Hanss
Prize (BBSRC – Alliance Française).
REFERENCES
Asami K., 2002. “Characterization of heterogeneous
systems by dielectric spectroscopy”. Progr. in Polym.
Sci., 27.
Bagnaninchi P-O et al., 2003. “Towards On-Line
Monitoring of Cell Growth in Microporous Scaffolds:
Utilization and Interpretation of Complex Permittivity
Measurements”. Biotech. Bioeng, 84, 3.
Bagnaninchi P-O et al., 2004. “Complex Permittivity
Measurement as a New Noninvasive Tool for
Monitoring In Vitro Tissue Engineering and Cell
Signature Through the Detection of Cell Proliferation,
Differentiation, and Pretissue Formation”. IEEE
Trans. on Nanobioscience, 3, 4.
Bindu G. et al., 2006. “Active Microwave Imaging For
Breast Cancer Detection”. Prog. In Electromagnetics
Research, PIER 58.
Blackham D.V., Pollard R.D., 1997. “An Improved
Technique for Permittivity Measurements Using a
Coaxial Probe”. IEEE Trans. Instrum. Meas., 46, 5.
Bordi F. et al., 2002. “Dielectric spectroscopy of
erythrocyte cell suspensions. A comparison between
Looyenga and Maxwell-Wagner-Hanai effective
medium theory formulations”. J. Non-Cryst. Sol., 305.
Bulyshev A.E. et al., 2001. “Computational Modeling of
Three-Dimensional Microwave Tomography of Breast
Cancer”. IEEE Trans. Biomed. Eng.,48, 9.
Chaudhary S.S. et al., 1984. “Dielectric Properties of
Normal & Malignant Human Breast Tissues at
Radiowave & Microwave Frequencies”. Indian J.
Biochem. and Biophys., 21.
Chelidze T., 2002. “Dielectric spectroscopy of blood”. J.
Non-Cryst. Sol., 305.
BIODEVICES 2008 - International Conference on Biomedical Electronics and Devices
160

Claycomb J.R. et al., 2002. “Nonlinear Dielectric
Spectroscopy of Living Cell Suspensions”. 2nd joint
EMBS/BMES Conference, Houston, USA.
De Langhe P. et al., 1994. “Design Rules for an
Experimental Setup Using an Open-Ended Coaxial
Probe Based on Theoretical Modelling”. IEEE Trans,
Instrum. Meas., 43, 6.
Duncan L. et al., 2006. “Assessment of the dielectric
properties of drug sensitive and resistant leukaemic
cells before and after ion channel blockers using
dielectrophoresis”. NSTI-Nanotech 2006.
Ermolina I. et al., 2001. “Study of Normal and Malignant
White Blood Cells by Time Domain Dielectric
Spectroscopy”. IEEE Trans. Dielectrics and Electrical
Insulation, 8, 2.
Fan S. et al., 1990. “Static analysis of an Open-Ended
Coaxial Line Terminated by Layered Media”. IEEE
Trans, on Instrum. Meas., 39, 2.
Fear E.C., 2005. “Microwave Imaging of the Breast”.
Tech. Cancer Res. Treatm., 4, 1.
Foster K.R., Schwan H.P., 1996. “Dielectric properties of
tissues” in C. Polk, E. Postow: Handbook of
Biological Effects of Electromagnetic fields, CRC,
Boca Raton, FL.
Gabriel S. et al., 1996. “The dielectric properties of
biological tissues: I. Literature survey - II.
Measurements in the frequency range 10 Hz to
20 GHz - III. Parametric models for the dielectric
spectrum of tissues”. Phys. Med. Biol., 41.
Gascoyne P. R. C. et al., 1997. “Dielectrophoretic
separation of cancer cells from blood”. IEEE Trans. on
industry applic., 33, 3.
Grant J.P. et al., 1989. “A critical study of the open-ended
coaxial line sensor technique for RF and microwave
complex permittivity measurements”. J. Physics E:
Sci. Instrum., 22.
Hagl D.M. et al., 2003. “Sensing Volume of Open-Ended
Coaxial Probes for Dielectric Characterization of
Breast Tissue at Microwave Frequencies”. IEEE
Trans. Microw. Theo. Tech., 51, 4.
Hagness S.C. et al., 1998. “Two-dimensional FDTD
analysis of a pulsed microwave confocal system for
breast cancer detection: Fixed-focus and antenna array
sensors”. IEEE Trans. Biomed. Eng., vol. 28.
Hoshina et al., 2001. “A Numerical Study on the
Measurement Region of an Open-Ended Coaxial
Probe Used for Complex Permittivity Measurement”.
IEEE Trans. Magnetics, 37, 5.
Hübner Y. et al., 2005. “Parallel measurements of drug
actions on Erythrocytes by dielectrophoresis, using a
three-dimensional electrode design”. IEE Proc.
Nanobiotechnol., 152, 4.
Jaspard F. et al., 2003. “Dielectric properties of blood: an
investigation of haematocrit dependence”. Physiol.
Meas., 24.
Joines W.T. et al., 1994. “The measured electrical
properties of normal and malignant human tissues
from 50 to 900 MHz”.
Med. Phys., 21, 4.
Lisin R. et al, 1996. “Time domain dielectric spectroscopy
study of human cells. I. Erythrocytes and ghosts”.
Biochim. Biophys. Acta, vol. 1280.
Pietrucha K., Marzec E., 2005. “Dielectric properties of
the collagen–glycosaminoglycans scaffolds in the
temperature range of thermal decomposition”.
Biophys. Chemistry, 118.
Polevaya Y. et al., 1999. “Time domain dielectric
spectroscopy study of human cells. II. Normal and
malignant white blood cells”. Biochim. Biophys. Acta,
1419.
Santini M.T. et al., 1991. “Effects of lonidamine on the
membrane electrical properties of Ehrlich ascites
tumor cells”. FEBS, 291, 2.
Santini M.T. et al., 1995. “A dielectric Relaxation Study
on the Effects of the Antitumor Drugs Lonidamine and
Rhein on the Membrane Electrical Properties of
Ehrlich Ascites Tumor Cells”. Anticancer Res., 15.
Semenov S.Y. et al., 2000. “Microwave Spectroscopy of
Myocardial Ischemia and Infarction. I. Experimental
Study”. Ann. Biomed. Eng., 28.
Semenov S.Y. et al., 2003. “Microwave Tomography for
Detection/Imaging of Myocardial Infarction. I.
Excised Canine Hearts”. Ann. Biomed. Eng., 31.
Sha L. et al., 2002. “A review of dielectric properties of
normal and malignant breast tissue”. IEEE
SoutheastCon conference, Columbia SC, USA.
Shao W. et al., 2005. “UWB Microwave Imaging for
Breast Tumor Detection in Inhomogeneous Tissue”.
27
th
Conf. IEEE EMBS.
Sheen N.I., Woodhead I.M., 1999. “An Open-ended
Coaxial Probe for Broad-band Permittivity
Measurement of Agricultural Products”. J. Agricult.
Eng. Res., 74.
Smith S.R. et al., 1986. “Dielectric properties of VX-2
Carcinoma Versus Normal Liver Tissue”. IEEE Trans.
Biomed. Eng., 33, 5.
Surowiec A.J. et al., 1988. “Dielectric Properties of Breast
Carcinoma and the Surrounding Tissues”. IEEE Trans.
Biomed. Eng., 35, 4.
Tofighi M.R., Daryoush A.S., 2001. “Near field
microwave brain imaging”. Electron. Lett., 37, 13.
Treo E.F. et al., 2005. “Hematocrit Measurement by
Dielectric Spectroscopy”. IEEE Trans. Biomed. Eng.,
52, 1.
Yang Y. et al, 2004. “Monitoring of lung tumour cell
growth in artificial membranes”. Biosensors and
Bioelectronics, 20.
MICROWAVE DIELECTRIC SPECTROSCOPY OF LOW-VOLUME FRACTION HUMAN CANCER CELLS
EMBEDDED IN COLLAGEN GELS - Experimental Feasibility Study with an Open-ended Coaxial Probe
161
