
SINGLE PARTICLE DETECTION
A Diagnostic Tool for Particle Associated Diseases like Alzheimer’s Disease and
Creutzfeldt-Jakob Disease
Eva Birkmann*
;
**
,a
, Susanne Aileen Funke**
,a
, Detlev Riesner* and Dieter Willbold*
,
**
*Institut fuer Physikalische Biologie, Heinrich-Heine-Universitaet, Universitaetsstr. 1,40225 Dueselorf, Germany
**INB-2, Forschungszentrum Juelich, Juelich, Germany;
a
These authors contributed equally to this work.
Keywords: Fluorescence correlation spectroscopy, Alzheimer’ disease, Creutzfeldt-Jakob disease, single particle
detection.
Abstract: Neurodegenerative diseases like Alzheimer’s disease (AD), prion diseases and others are progressive and
lethal. High-molecular weight aggregates of the Amyloid-β-peptides (Aβ) or of the misfolded prion protein
(PrP) are found in patients afflicted by AD or prion diseases, respectively. Despite of many attempts, neither
a therapy for recovery, nor an early diagnosis at preclinical stages are available. Psychological tests and
imaging approaches not directly related with a secure disease marker are in use only for late stages of the
disease. The Creutzfeldt-Jakob-disease (CJD), a human prion disease, is caused by accumulation of
aggregates consisting of an abnormally shaped version of PrP. CJD is diagnosed with certainty only by
neuropathology post mortem. In this study a multidisciplinary development of a novel mode of single
particle counting of immobilized Aβ and PrP aggregates as the most direct biomarkers for Alzheimer’s
disease and Prion diseases, respectively, is introduced. For ultrasensitive detection of aggregates, the
suitable instrumentation as well as data acquisition and data analysis are developed using single molecule
detection and advanced laser scanning fluorescence techniques. In the novel assay development effort
biochemistry, detection and analysis were improved to detect single aggregates immobilised on a surface.
First results show the improvement of single particle detection of PrP-aggregates of TSE-afflicted cattle and
hamsters as well as synthetic Aβ-aggregates.
1 INTRODUCTION
In many neurodegenerative diseases e.g. prion
diseases, Alzheimer’s disease, Parkinson’s disease,
Huntington’s Disease, protein aggregates are formed
in the very beginning or in the progress of disease
(Lee et al., 2005). Up to now it is not known, if these
aggregates are causative agents or symptoms of the
respective disease, but many studies show, that the
aggregates or even oligomers of the according
proteins are neurotoxic and therewith a reason of
neurodegeneration. (Selkoe, 2003)
To understand the progression of these diseases,
as well as disease associated or causative
mechanisms and to monitor potential therapeutically
approaches an ultrasensitive tool to quantify these
disease related aggregates is required. A challenge
for the analytic system is to reliably count only those
aggregates or oligomers that consist of the specified
protein or peptide. Monomeric molecules need to be
clearly distinguished because they are present in
healthy organism as well.
We developed a new method, which is able to
count single protein aggregates bound by a capture-
antibody to a surface (surface-FIDA) (Birkmann et
al., 2007). Our new test system is based on
fluorescence correlation spectroscopy (Eigen and
Rigler, 1994). It is quantifying the number and size
of aggregates simultaneously labelled by two
different antibodies for dual colour fluorescence
intensity distribution analysis (2D-FIDA) (Birkmann
et al., 2006). Only aggregates and oligomers but not
monomeric proteins are counted. To increase the
sensitivity, particles were concentrated in the two-
dimensional space by immobilizing it to capture
antibodies on the surface of the slide. Laser beams
are scanning the surface systematically, so even
single particles are detected (Birkmann et al., 2007).
431
Birkmann E., Aileen Funke S., Riesner D. and Willbold D. (2008).
SINGLE PARTICLE DETECTION - A Diagnostic Tool for Particle Associated Diseases like Alzheimer’s Disease and Creutzfeldt-Jakob Disease.
In Proceedings of the First International Conference on Bio-inspired Systems and Signal Processing, pages 431-436
DOI: 10.5220/0001065104310436
Copyright
c
SciTePress

We report on the successful use of surface-FIDA
as diagnostic tool for prion diseases. The infectious
agents of prion diseases are composed primarily of
the pathogenic isoform of the prion protein
designated PrP
Sc
, which is generated by a
conformational change of the cellular isoform PrP
C
.
In contrast to its cellular isoform, the pathogenic
isoform PrP
Sc
forms insoluble aggregates. Hitherto
accredited prion tests use the PK-resistance of PrP
Sc
as a marker for the disease. Because of varying
portions of disease related aggregated PrP, which is
not PK-resistant, these prion tests offer only a
limited sensitivity. Therefore prion protein aggregate
detection, which does not rely on PK-digestion, is
favourable for sensitive diagnosis. It allows
detection of both, PK-resistant and PK-sensitive
PrP
Sc
aggregates.
Up to now, we could successfully verify the
novel test system for correct diagnosis of Scrapie-
infected hamsters as well as BSE-infected cattle in
the clinical stages of diseases (Birkmann et al.,
2007). Furthermore, we were able to detect PrP
aggregates in the cerebrospinal fluid of cattle of
BSE-infected cattle for the first time (Birkmann et
al., 2007). During the next steps we will adopt the
highly sensitive test system for diagnosis of human
prion diseases like Creutzfeldt-Jakob disease and
other aggregate related diseases, especially
Alzheimer’s disease.
In this study we apply surface-FIDA to different
disease associated aggregates. First we show the
single aggregate detection of prion protein
aggregates purified from brain homogenates of
Scrapie-infected hamsters and BSE-infected cattle to
demonstrate the principal of surface-FIDA to detect
single particles. In the second part of the work we
show the transfer of surface-FIDA to the detection
of single Aβ aggregates as diagnostic approach for
Alzheimer’s disease. Therefore we compared the
detection of Aβ aggregates in solution with the
application of surface-FIDA.
2 MATERIALS AND METHODS
2.1 Fluorescence Labelling of
Antibodies
Antibodies R1 were kindly provided by S.B.
Prusiner, UCSF, USA (Williamson et al., 1998).
Antibodies 12F10 and Saf32 were obtained from
SpiBio (Massy Cedex, France); antibody D18 was
obtained from InPro (San Francisco, USA). For the
detection of Aβ aggregates, antibodies 6E10 (Sigma
Aldrich, Hamburg, Germany), 8G7, 19H11 and 4G8
(Calbiochem) were purchased.
Antibodies were labelled in free amino groups
via reactive succinimidyl ester groups of Alexa-633
and Alexa-488 (Molecular Probes, Oregon, USA).
For labelling, approximately 50 µg antibodies were
incubated with 5 µg dye in carbonate buffer, pH 8.4
in a total volume of 100 µl for 1 hour. Conjugates
were separated from free dye by gel filtration via
NAP5-column (Pharmacia) with 10 mM TBS, pH
7.2 and 0.2 M NaCl as elution buffer. Labelled
antibodies were stored in the dark at 4°C.
2.2 Fluorescence Correlation
Spectroscopy
In fluorescence correlation spectroscopy (FCS) the
fluorescence intensity is recorded in a very small
volume, i.e. in the femtoliter range. Measurements
were performed with the instrument FCS Olympus
IX 50 (Evotec OAI, Hamburg, Germany) with a
beam scanner unit in dual-colour mode with an
Argon ion laser (excitation wavelength 488/514 nm)
and a helium-neon laser (excitation wavelength 633
nm). The beam scanner unit allows the scanning of
the sample for aggregates. In practice the detection
volume is moved through the sample in horizontal
and vertical dimensions. The beam scanner was used
by moving 1 mm in one direction a rectangular
deviation of 100 µm with a frequency of 50 Hz and
an integration time of 50 µs. A piezo element was
integrated in the optic of the FCS Olympus IX 50,
which allowed a precise z-positioning of the focus in
the 100 nm range.
2.3 Surface-FIDA
The surface-FIDA assay was carried out as
described by earlier (Birkmann et al., 2007).
Briefly, 0.25 – 1 μg capture antibody was
adhesively bound to poly-D-lysine activated glass
surfaces. After blocking the unspecific binding sites
with 10 % fetal calf serum, potentially present
aggregates were bound to the capture by incubating
20 μl of a sample for at least two hours at 4 °C.
After washing twice with PBS buffer (140 mM
NaCl; 2.7 mM KCl; 10 mM Na
2
HPO
4
, pH 7.4), the
fluorescence labelled detection antibodies were
applied (0.1 μg/μl) and incubated for 1 h at 20 °C.
After five washing steps with PBST (PBS with 0.1
% (w/w) Tween 20) and two washing steps with
PBS, the measurements were carried out.
BIOSIGNALS 2008 - International Conference on Bio-inspired Systems and Signal Processing
432
2.4 Preparation of Synthetic
Aß-aggregates
Aβ(1-42) was purchased from JPT Peptide
Technologies (Berlin, Germany). For aggregate
preparation Aβ was dissolved in DMSO to 400 µM,
diluted to 66 µM in PBS (140 mM NaCl; 2.7 mM
KCl; 10 mM Na
2
HPO
4
) and incubated for five days
at 37 °C. Aggregate formation was monitored using
Thioflavin T (ThT) assay. For that, 10 mM ThT
(Sigma, Hamburg, Germany) was added to the
samples. Fluorescence was monitored with a
microplate reader at excitation and emission
wavelengths of 440 nm and 490 nm, respectively
(Polarstar Optima, BMG, Offenburg, Germany).
For surface-FIDA, the aggregates were diluted
1:10 in pooled CSF of healthy people. CSF was
obtained by Biochemed Services, Winchester,
USA).
3 RESULTS
3.1 Methodical Setup
The new optical method for detection of protein
aggregates is based on fluorescence intensity
distribution analysis (FIDA). For detection of
pathologic protein aggregates single molecule
detection (SMD) combined with quantification of
single aggregates immobilised on a relatively large
surface was employed to achieve high sensitivity
and specificity.
The new method, therefore, is called surface-
FIDA. It is able to count single protein particles
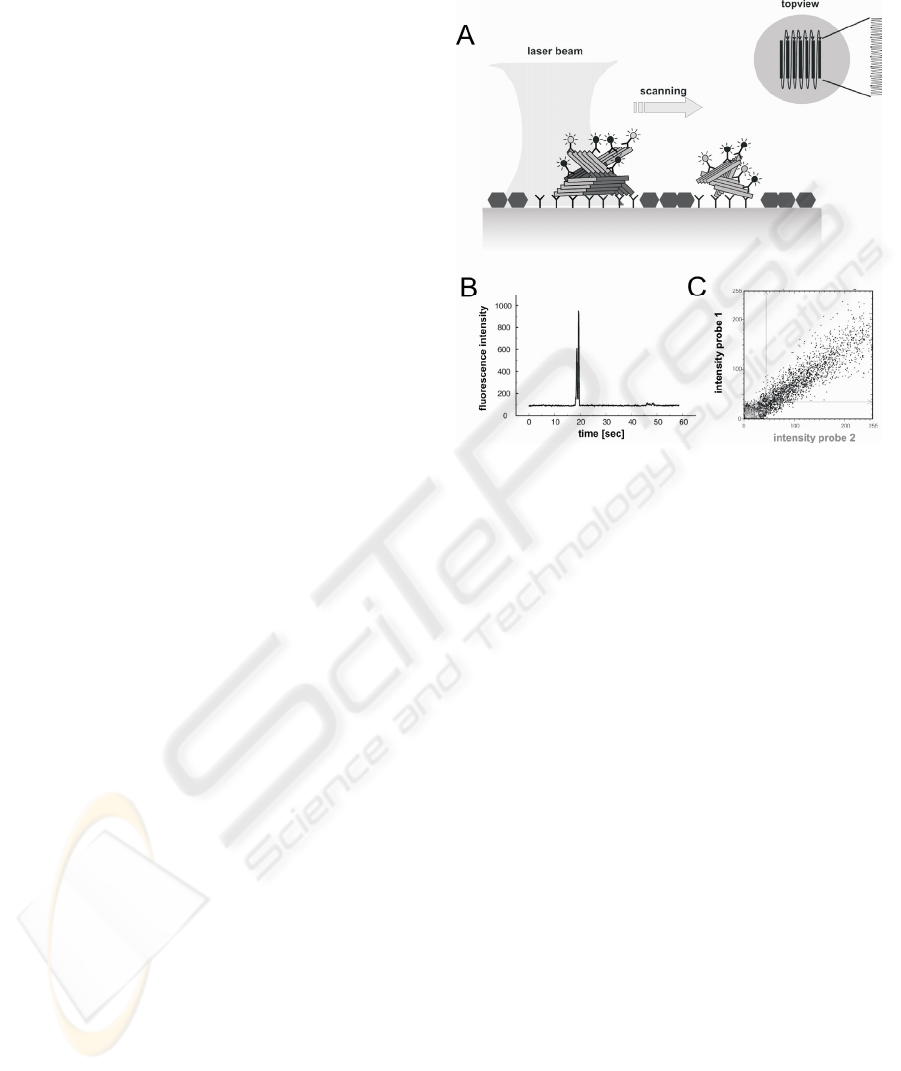
bound to a capture-antibody on the surface (fig. 1a)
(Birkmann et al., 2007). Specific protein-particles
are labelled simultaneously by two different
antibodies for dual colour fluorescence intensity
distribution analysis (2D-FIDA). Among the capture
and both detection antibodies, at least two antibodies
bind the same epitope. Thus, only aggregates but not
monomers are counted. A laser beam scans the two-
dimensional surface systematically in a double-
meander mode. Thus, even single protein-particles
are detected (fig. 1B). By utilising two lasers
simultaneously two different fluorescence labels can
be crosscorrelated. Only if the different labels are
bound to the same aggregate both labels occur to the
same time in small detection focus. A typical
distribution of a coincident signal of double labelled
aggregates is shown in fig. 1C.
3.2 Detection of Pathological Protein
Particles with Surface-FIDA
To observe, if the surface-FIDA setup is able to
detect single aggregates different types of protein
aggregates were tested.
3.2.1 PrP-particles Purified from Brain of
Scrapie Infected Hamsters
Prion Protein aggregates were purified from brain
homogenates of Scrapie infected hamsters in the
clinical state of disease by NaPTA precipitation
(Safar et al., 1998). The antibody R1 (Williamson et
al., 1998) served as capture. The antibodies D13 and
R1 were fluorescence labelled and utilized as
detection probes. Same treated brain homogenates of
healthy hamsters were used as control samples. The
results of 2D-surface-FIDA in different distances to
the surface are shown in fig. 2. In the samples of
Scrapie infected hamsters at all distances between
10 µm and 20µm fluorescence peaks with high
fluorescence intensity in both channels could be
detected. At distances below 10 µm background
signal in the control sample rise and in distances
above 20 µm the signals of the Scrapie samples
descended (data not shown).
Figure 1: A) Scheme of surface-FIDA; B) fluorescence
peak caused by the labelled aggregate: principal of particle
counting; C) 2D-FIDA:Two probes with different
fluorescence labels were used; Only simultaneous binding
of both probes to the aggregates results in the specific
diagonal signal distribution as shown in the plot
SINGLE PARTICLE DETECTION - A Diagnostic Tool for Particle Associated Diseases like Alzheimer’s Disease and
Creutzfeldt-Jakob Disease
433
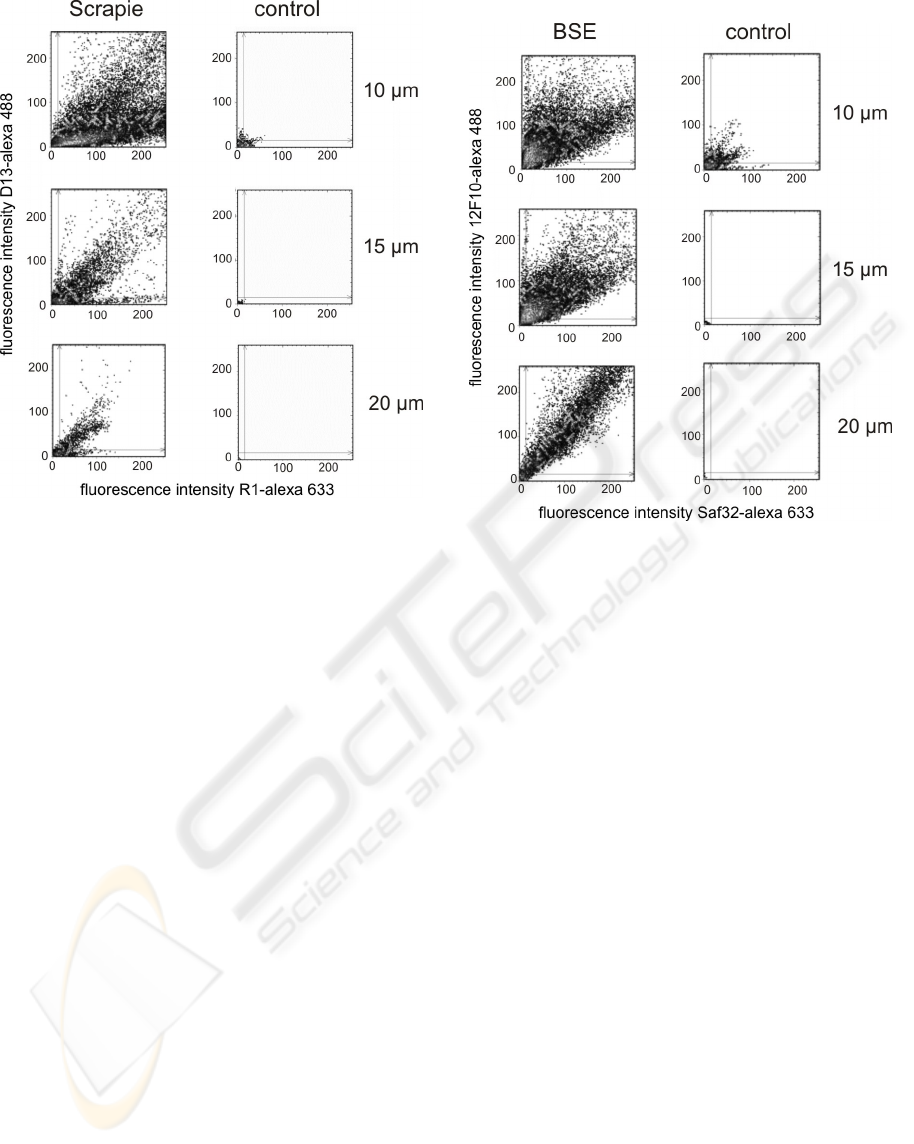
Figure 2: 2D-surface-FIDA of PrP-aggregates purified
from brain homogenate of Scrapie infected hamsters and
same treated brain homogenate of a healthy control in
different distances to the surface 10-20 µm.
3.2.2 PrP-particles Purified from Brain of
BSE Infected Cattle
Prion Protein aggregates were purified from brain
homogenates of BSE infected cattle in the clinical
state of disease by NaPTA precipitation (Safar et al.,
2002). The antibody Saf32 (Krasemann et al., 1999)
served as capture. The antibodies 12F10 and Saf32
were fluorescence labelled and utilized as detection
probes. Same treated brain homogenates of healthy
cattle were used as control samples. The results of
2D-surface-FIDA at different distances to the
surface are shown in fig. 3.
3.2.3 Synthetic Aβ-Aggregates
As a first proof of principle, synthetical Aβ
aggregates were used in the assay described above.
First measurements were done in solution without
immobilizing the aggregates. The antibodies 6E10
(N-terminal epitope) and 8G7 (C-terminal epitope)
were fluorescence labelled and used as detection
probes. Experiments were done in PBS. In the
negative control, 0.2 % SDS was used to prevent Aβ
aggregation, as monitored by ThT assay (data not
shown). As expected, only aggregated Aβ resulted in
fluorescence peaks as can be seen in fig. 4a.
Figure 3: 2D-surface-FIDA of PrP-aggregates purified
from brain homogenate of BSE infected cattle and same
treated brain homogenate of a healthy control.
In a next step, Aβ aggregates diluted 1:10 in CSF to
meet realistic conditions were immobilized on the
surface of the slide. Antibody 4G8 (binding to amino
acids 1-17 of Aβ) served as capture. The antibodies
6E10 and 19H11 were fluorescence labelled and
served as detection probes. As both antibodies bind
to the N-terminal part of Aβ, a simultaneous
labelling of Aβ monomers was excluded. As
controls, only CSF without additional Aβ aggregates
was used in the immobilization procedure. The
results of 2D-surface-FIDA are shown in fig. 4b.
The measurements were done at 5 µm distance to
the surface.
4 CONCLUSIONS
The proof of principle for the use of surface-FIDA to
detect aggregates was shown for natural PrP-
aggregates purified from brain of Scrapie infected
hamsters, BSE infected cattle and for synthetic Aβ
aggregates diluted in CSF.
Single particle counting as diagnostic tool is
more sensitive as compared to measuring the
integrated signal of all or many particles. “Single
particle counting” allows measuring of multiple
BIOSIGNALS 2008 - International Conference on Bio-inspired Systems and Signal Processing
434

fluorescence intensity
6H10-alexa 488
fluorescence intensity
19H11-alexa633
A)
B)
control
Aβ aggregates
0 100 200
0
100
200
0 100 200
0
100
200
fluorescence intensity
6H10-alexa 488
0 100 200
0
100
200
0
100
200
0 100 200
fluorescence intensity
19H11-alexa633
fluorescence intensity
8G7-alexa633
fluorescence intensity
8G7-alexa633
Aβ aggregates
control
fluorescence intensity
6H10-alexa 488
fluorescence intensity
19H11-alexa633
A)
B)
control
Aβ aggregates
0 100 200
0
100
200
0 100 200
0
100
200
0
100
200
0 100 200
0
100
200
0 100 200
0
100
200
0
100
200
fluorescence intensity
6H10-alexa 488
0 100 200
0
100
200
0 100 200
0
100
200
0
100
200
0
100
200
0 100 200
0
100
200
0
100
200
0
100
200
0 100 200
fluorescence intensity
19H11-alexa633
fluorescence intensity
8G7-alexa633
fluorescence intensity
8G7-alexa633
Aβ aggregates
control
Figure 4: A) 2D-FIDA of synthetically prepared Aβ
aggregates in solution (concentration 3,3 µM). Aβ, kept
from aggregation by 0.2 % SDS, was used as control.
B) 2D-surface-FIDA of synthetically prepared and in CSF
diluted Aβ aggregates (concentration 6µM). CSF without
additional Aβ aggregates was used as control.
Measurements were done at 5 µm distance to the slide
surface.
parameters of the individual particles are recorded
like size, number of epitopes, different epitopes on
the same particle etc. and those parameters can be
used for improvement of specificity.
When the detection of single particles was
carried out in suspension using the dual colour
fluorescence intensity distribution analysis (2D-
FIDA) (Birkmann et al., 2006), it was done in a
small volume taken from a much larger sample
volume by moving the laser detection focus through
a cuvette. Diffusion of the particles and scanning of
the volume were superimposed so that it was
difficult to account quantitatively for all particles in
the sample. Therefore the immobilisation of the
particles on a surface had a major impact of the
sensitivity of the whole assay, because it allows
searching for the particles in a systematical way.
In the near future, we will develop surface-FIDA
into an ultrasensitive diagnostic assay for particle
associated disease, especially CJD and Alzheimer’s
disease. Such an assay will allow early diagnosis of
AD and CJD using a minimally invasive approach in
the living patient. In addition, such a diagnostic tool
will be crucial for on line monitoring of disease
progression and progress of a therapeutic approach.
Table 1: Sensitivity and Specificity Characteristics of
surface-FIDA.
sensitivity specificity
Concentration of
particles on two
dimensional surface
Simultaneous binding of
three probes (one capture,
two detection probes)
Single particle detection Adjustable washing steps
Reproducible and
complete counting of all
aggregates by surface
scanning
Detection of protein
aggregates only, no
monomers
ACKNOWLEDGEMENTS
This work was supported by the Ministry for
Environment and Nature Protection, Agriculture and
Consumer Protection of North Rhine-Westphalia
(grant VI-1-17.90.01), Forschungs- und
Innovationfonds of the Heinrich-Heine-University of
Duesselsdorf and EU NoE Neuroprion. We
gratefully acknowledge Dr. Prusiner for supplying
the antibody R1, D13 and the scrapie brain material
with negative controls. We would also like to thank
Dr. M. Groschup, Friedrich-Loeffler-Institut (FLI),
Institute for Novel and Emerging Infectious
Diseases, for supplying BSE material of infected
cattle and negative controls.
REFERENCES
Birkmann, E., Schäfer, O., Weinmann, N., Dumpitak, C.,
Beekes, M., Jackman, R., Thorne, L., Riesner, D.,
2006. Detection of prion particles in samples of BSE
and scrapie by fluorescence correlation spectroscopy
without proteinase K digestion. Biol. Chem. 387, 95–
102.
Birkmann, E., Henke, F., Weinmann, N., Dumpitak, C.,
Groschup, M., Funke A., Willbold, D., Riesner, D.
2007. Counting of single prion particles bound to a
capture-antibody surface (surface-FIDA)
Vet. Microbiol. (2007), in press
Eigen, M. and Rigler, R. (1994). Sorting single molecules
– application to diagnostics and evolutionary
biotechnology. Proc. Natl. Acad. Sci. USA 91,
5740–
5747.
Krasemann S, Jurgens T, Bodemer W., 1999. Generation
of monoclonal antibodies against prion proteins with
an unconventional nucleic acid-based immunization
strategy. J. Biotechnol. 73, 119-29.
Lee C, Yu MH., 2005 Protein folding and diseases.
J Biochem Mol Biol. 2005; 38(3), 275-80
Safar, J., Wille, H., Itri, V., Groth, D., Serban, H., Torchia,
M., Cohen, F.E., Prusiner, S.B., 1998. Eight prion
SINGLE PARTICLE DETECTION - A Diagnostic Tool for Particle Associated Diseases like Alzheimer’s Disease and
Creutzfeldt-Jakob Disease
435

strains have PrP(Sc) molecules with different
conformations Nat. Med. 10, 1157-1165.
Safar, J.G., Scott, M., Monaghan, J., Deering, C.,
Didorenko, S., Vergara, J., Ball, H., Legname, G.,
Leclerc, E., Solforosi, L., Serban, H., Groth, D.,
Burton, D.R., Prusiner, S.B., and Williamson, R.A.,
2002. Measuring prions causing bovine spongiform
encephalopathy or chronic wasting disease by
immunoassays and transgenic mice. Nat. Biotechnol.
20, 1147-1150.
Selkoe, D.J. 2003, Folding proteins in fatal ways
Nature 426; 900-904
Williamson, R.A., Peretz, D., Pinilla, C., Ball, H.,
Bastidas, R.B., Rozenshteyn, R., Houghten, R.A.,
Prusiner, S.B., Burton, D.R., 1998. Mapping the prion
protein using recombinant antibodies. J. Virol. 72,
9413-8.
BIOSIGNALS 2008 - International Conference on Bio-inspired Systems and Signal Processing
436
