
FAST AND ROBUST LOCALIZATION OF
THE HEART IN CARDIAC MRI SERIES
A Cascade of Operations for Automatically Detecting the Heart in Cine MRI Series
Sebastian Zambal, Andreas Sch
¨
ollhuber, Katja B
¨
uhler and Ji
ˇ
r
´
ı Hlad
˚
uvka
VRVis Research Center, Donau-City-Strasse 1, Vienna, Austria
Keywords:
Localization of the Heart, Magnetic Resonance Imaging, Cardiac cine MRI.
Abstract:
This work presents a robust approach for fast initialization of an Active Appearance Model for subsequent
segmentation of cardiac MRI data. The method automatically determines AAM initialization parameters:
position, orientation, and scaling of the model. Four steps are carried out: (1) variance images over time
are calculated to find a bounding box that roughly defines the heart region; (2) circle Hough-transformation
adapted to gray values is performed to detect the left ventricle; (3) thresholding is carried out to determine the
orientation of the heart; (4) the optimal initialization is selected using a mean texture model.
The method was evaluated on 42 MRI short axis studies coming from two MRI scanners of two different
vendors. Automatic initializations are compared to manual ones. It is shown that the proposed automatic
method is much faster than and achieves results qualitatively equal to manual initialization.
1 INTRODUCTION
Segmentation of cardiac structures from magnetic
resonance (MRI) images has been of great interest
in the medical imaging community (S
¨
orgel and Vaer-
man, 1997; Stegmann and Pedersen, 2005; Lelieveldt
et al., 2001; van Assen et al., 2006). The great advan-
tage of model-based segmentation is that it incorpo-
rates prior knowledge about the segmented structures.
Active Appearance Models (AAMs) (Cootes
et al., 1998) are deformable models which describe
possible configurations of shape and gray values by
statistical analysis of a training data set. Several au-
thors have proposed the use of AAMs and their nu-
merous extensions to the problem of segmentation of
cardiac structures. Methods proposed so far com-
prise 3D AAMs (Mitchell et al., 2002), temporal
AAMs (Lelieveldt et al., 2001), and 3D+time AAMs
(Stegmann and Pedersen, 2005).
A concrete instance of an AAM is defined by pa-
rameters comprising: position, scaling, orientation,
shape and texture parameters. Matching the model
to unseen data is equivalent to finding a configura-
tion of parameters that optimally fit the model to the
the unseen data. The common proceeding is to place
the model onto unknown image data. Then deforma-
tions are iteratively applied until a difference mea-
sure such as root mean square (RMS) texture differ-
ence reaches a minimum. A problem often ignored
in literature is robust and fast automatic initialization
of the model, i.e. finding reasonable initial position,
orientation and scaling. A brute-force method itera-
tively tries out each and every configuration. How-
ever this is very time-consuming since the number of
possible initializations is huge. In previous work it
has been suggested to perform AAM Search in par-
allel with multiple different initialization parameters
(Stegmann, 2000). However, this approach is quite
time consuming, especially when dealing with 3D
AAMs. To utilize AAM-based segmentation for car-
diac cine MR in daily clinical practice, a more effi-
cient method for initialization is required.
Recently a method based on sparse Markov Ran-
dom Fields (MRFs) (Donner et al., 2007) has been
proposed for fast initialization of model-based seg-
mentation. However this method relies on feature ex-
traction which is not proven to deliver adequate re-
sults on cardiac cine MR data. Further more the run
times reported for solving the considered MRF are
in the order of a few seconds while the method we
341
Zambal S., Schöllhuber A., Bühler K. and Hladuvka J. (2008).
FAST AND ROBUST LOCALIZATION OF THE HEART IN CARDIAC MRI SERIES - A Cascade of Operations for Automatically Detecting the Heart in
Cine MRI Series.
In Proceedings of the Third International Conference on Computer Vision Theory and Applications, pages 341-346
DOI: 10.5220/0001074503410346
Copyright
c
SciTePress

present in this paper delivers the result after about one
second.
This work presents a method that automatically
determines the initial position parameters for an AAM
for segmentation of the human heart in MRI short axis
data. In earlier work S
¨
orgel and Vaerman (S
¨
orgel
and Vaerman, 1997) have introduced a method for
automatic heart localization for initialization of ac-
tive contours. The presented work extends this ap-
proach. Instead of initializing active contours the goal
of this paper is to initialize an Active Appearance
Model. A set of well-established image processing
algorithms is used including morphological operators
(Soille, 2002) and Hough-transformation for circles
(Davies, 1988). In contrast to previous work where
Fuzzy Hough-transformation (Philip et al., 1994b)
was applied to detect the left ventricle (Philip et al.,
1994a) we propose to perform Hough-transformation
directly on gray values as will be outlined later in this
paper.
This paper is organized as follows: In section 2 an
overview of the investigated MRI data is given. The
fully automatic method for localization of the heart is
described in detail in section 3. Validation and results
are presented in section 4 and the paper concludes
with section 5.
2 DATA
The 4D data considered in this work consists of cine
MRI short axis studies of 42 different patients. The
data was captured using two MR scanners from dif-
ferent vendors each one operating at a magnetic field
strength of 1.5 Tesla. Each short-axis study con-
sists of 7 to 13 slices with pixel resolutions ranging
from 1.17mm to 1.68mm. The spacing between slices
ranges from 7.2mm to 12.0mm. Time-resolution lies
in the range of 11 to 27 time steps per patient study.
3 METHOD
Our method takes into account the complete four-
dimensional (3D + time) input data and computes the
initial parameters for the position of the model: po-
sition, orientation and scale-factor. The pipeline is
made up of four steps (see figure 1):
• extraction of the region of interest (ROI)
• localization of the LV
• calculation of LV-RV orientation
• model-based candidate selection
In each step elementary digital image processing al-
gorithms are used. This makes the method transpar-
ent, comprehensible, and easy to implement. In the
following the individual steps of the algorithm are ex-
plained in detail.
3.1 Extraction of the Region of Interest
In order to limit more complex calculations to a re-
stricted ROI, the first step is a detection of the im-
age area which contains the heart. Over the period of
the cardiac cycle position and size of the heart vary
due to contraction of the myocardium. As a result the
strongest variations of gray values appear in the re-
gion inside the heart. The localization is derived from
the variations of gray values over time similarly as
proposed by S
¨
orgel and Vaerman (S
¨
orgel and Vaer-
man, 1997).
For every slice a variance image is calculated. The
gray values of a variance image correspond to the
variance of the according pixel over the complete car-
diac cycle. High gray values indicate strong variance
and thus the according pixels belong to the heart re-
gion with high probability. Figure 2 shows examples
of such variance images for different slices of a single
data set.
It has to be considered, especially for MRI data,
that high variance of a pixel’s gray value might pos-
sibly come from noise or imaging artifacts. To re-
duce the disturbing influence of noise the following
image processing steps are carried out for the indi-
vidual variance images. A threshold is selected such
that the according number of pixels above the thresh-
old approximately cover the area of the heart (roughly
10000mm
2
).
To eliminate single pixels and small pixel areas
a morphological cleaning is applied to each variance
image. The morphological structuring element that
is used is a 5 × 5 mask centered over the considered
pixel. If less than 11 pixels in this mask are set the
pixel is unset. If more than 15 pixels are set the center
pixel is set. Otherwise the old pixel value is kept.
This improves the results significantly but in some
cases there still remain misleading pixels set. To in-
crease robustness all masks from all slices are consid-
ered jointly. A new mask is generated by summation
of the individual variance images. This gives a result
as depicted in figure 3(a). Outliers where misleading
variances appear in individual slices only are removed
with the following operation: All pixels which are set
in less than 25% of all slices are deleted. Figure 3(b)
shows an example of the outcome of this step. To fur-
ther reduce artifacts only the largest connected region
in the mask is considered (figure 3(c)). A bounding
VISAPP 2008 - International Conference on Computer Vision Theory and Applications
342
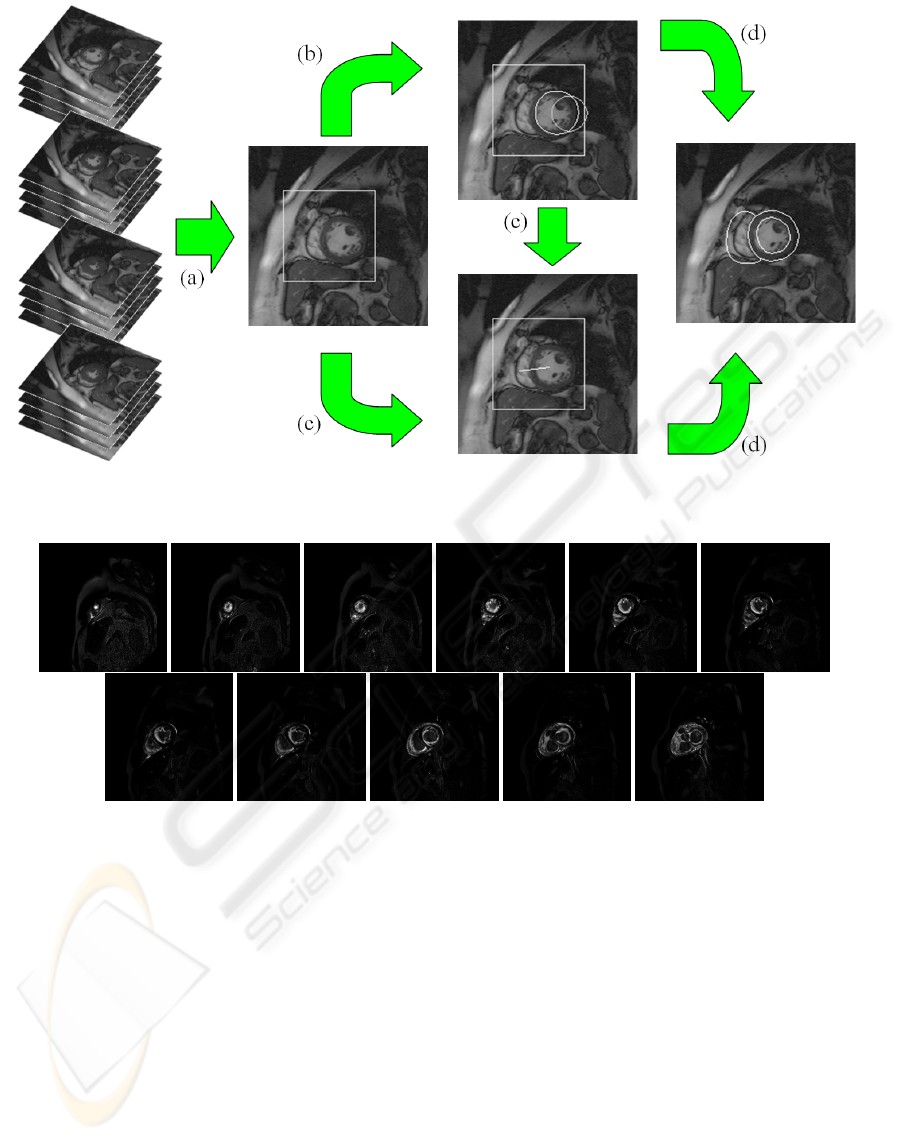
Figure 1: The pipeline: (a) ROI extraction, (b) LV localization, (c) LV-RV orientation, (d) model-based candidate selection.
Figure 2: Variance images for individual slices of a single data set.
box around it defines the ROI as shown in figure 3(d).
Since information from different time steps and dif-
ferent slices is combined it is argued that the resulting
mask robustly identifies the region of the heart.
3.2 Localization of the Left Ventricle
While the first step in the algorithm takes the full
4D data into account the rest of the algorithm is per-
formed on the central slice of end-diastole only. The
end-diastole is typically known since the individual
time steps are delivered as volumes sorted by time,
starting with the end-diastolic volume. Since the cap-
tured volume typically covers the left ventricle from
apex to base the center slice of a volume is taken for
further refined localization of the heart.
The myocardium of LV has approximately the
shape of a circle. This fact motivates the use of
a Hough-transformation for circles (Davies, 1988).
To reduce the computational burden the Hough-
transformation is restricted to the ROI calculated in
the previous step.
Typically the first step in Hough-transformation is
edge detection. In the experiments carried out on car-
diac MRI data it turned out that standard edge detec-
tion algorithms like Canny Edge Detection (Canny,
1983) give very poor results for many data sets. On
the one hand this is due to properties of MRI data.
On the other hand fuzzy anatomical structures such as
papillary muscles and trabeculae make it very difficult
FAST AND ROBUST LOCALIZATION OF THE HEART IN CARDIAC MRI SERIES - A Cascade of Operations for
Automatically Detecting the Heart in Cine MRI Series
343

(a) (b) (c) (d)
Figure 3: Towards the ROI: (a) Sum of variations, (b) thresholded, (c) largest region extracted, (d) and resulting bounding
box.
to calculate meaningful image gradients that clearly
represent transitions between objects. Furthermore in
experiments it was observed that gray value distribu-
tions for MRI images fluctuate significantly inter and
even intra patient studies. This makes it very hard to
select generic parameters for an elaborate edge detec-
tion algorithm.
More elaborate approaches like Fuzzy Hough-
transformation (Philip et al., 1994b) try to circum-
vent the problem of structures deviating from perfect
circles. Anyway the problem of strongly misleading
edges in the region of papillary muscles remains.
In order to overcome the problems of edge de-
tection, the Hough-transformation is adopted to take
original gray-values rather than edge information as
input. The assumption is made that gray values of the
myocardium are significantly darker than those of the
blood inside the ventricles. Thus, for transforming
the image into Hough-space low gray values in the
image are assumed to belong to the myocardium with
high probability. Using this approach the detected cir-
cle does not lie on the boundaries of the myocardium
but somewhere in between. As a result the circle is
detected robustly even if the shape of the left ventri-
cle deviates from the perfect circle. Compared to the
Fuzzy Hough-transformation the computational com-
plexity is even reduced (no gradient calculation is re-
quired). Note that the even darker gray values in the
lung region were excluded since they lie outside the
previously calculated ROI.
The Hough-space considered is a three-
dimensional space of parameters. Its axes are x,
y (position of the circle’s center) and r (radius of
the circle). x and y are constrained by the bounding
box defining the ROI. r is restricted to an interval of
25mm to 40mm – a typical range of radii for LVs.
Although Hough-transformation as we have de-
scribed it works quite robustly there is still a small
chance that the highest evidence for a circle is not
correctly describing the LV. Our experiments showed
that the correct contour of the LV always corresponds
to one of the first two largest peaks in Hough-space.
Thus the first two most prominent candidates for po-
sition and scaling of the LV are considered at the last
stage of the algorithm.
3.3 Heart Orientation
From previous steps two possible candidates for the
left ventricle are extracted. Each of these candidates
described by position and size of the two Hough cir-
cles. What remains is to determine the orientation of
the heart, i.e. where the right ventricle is located rela-
tive to the left ventricle.
The ROI computed in section 3.1 is thresholded
such that the 20% brightest pixels remain. As exper-
iments showed, the two largest connected regions ro-
bustly correspond to the blood inside LV and RV. The
centroids of these regions already indicate the spa-
tial relation between the ventricles. It is however not
known which region corresponds to the left ventricle
and which to the right one. To resolve this ambiguity
the region with its centroid closer to the center of a
Hough candidate is identified as the left ventricle.
This way a unique orientation is assigned to both
Hough-candidates.
3.4 Model-based Candidate Selection
The preceding steps reduced the initialization search
space from millions (possibly every pixel with mul-
tiple different orientations) to two candidates: two
Hough circles for the myocardium of the LV together
with estimates of LV-RV orientation. Each of the two
candidates defines position, scaling, and orientation
for a possible initialization of the model.
To select the optimal candidate the root mean
squared (RMS) texture errors between model and
both initialization candidates are calculated. The can-
didate which produces the smaller error is identified
as the final result.
VISAPP 2008 - International Conference on Computer Vision Theory and Applications
344

4 VALIDATION AND RESULTS
The method has been evaluated for a total of 42 MRI
studies. Automatic initializations have been com-
pared to manual ones: five users interactively initial-
ized the model. To assess the quality of the automatic
method three figures of merit have been evaluated:
average point-to-surface distance, texture error, and
time performance.
The average point-to-surface (PTS) distance is
calculated for the manually and automatically placed
mean model shape relative to the accurately done
manual ground truth segmentation. This was only
possible for a subset of 31 data sets where ground
truth segmentation was available. Figure 4 (top) sum-
marizes PTS measures achieved by users and the au-
tomatic method. It is observed that automatic initial-
izations come close to the manual ones. Please note
that the average discrepancy of 6mm only refers to
rigid initialization of the mean model. In this work
only initialization is investigated – no subsequent de-
formations of the model are applied in attempt to
achieve final segmentations.
In order to evaluate initializations for which no
ground truth was given, the texture difference be-
tween mean model and image data was determined.
Figure 4 (middle) shows the quality of matches for
all (unsegmented) 42 data sets after manual/automatic
initializations with respect to texture difference. It is
again concluded that the automatic method generates
initializations qualitatively comparable to those of the
users. The final results – the initializations – for all
validation data sets are visualized in figure 5 for the
central slices of end-diastole.
While similar in quality figure 4 (bottom) proves
another advantage of the automatization over user in-
teraction – the speed-up. The average initialization of
1 second has been achieved by a Java implementation.
5 CONCLUSIONS
This work has introduced an automatic and robust
method for localization of LV and RV in 4D cardiac
MRI data. The method has been designed with help
of few elementary image processing operators. The
Hough-transformation for circles was adapted to op-
erate on original image gray values instead of gradi-
ent magnitudes which makes the detection of the LV
highly robust. The overall quality of initialization has
been assessed by a user study. Time performance of
the method indicates a high potential for daily clinical
use.
REFERENCES
Canny, J. F. (1983). A variational approach to edge detec-
tion. In Proceedings of the Third National Conference
on Artificial Intelligence (AAAI), pages 54–58.
Cootes, T. F., Edwards, G. J., and Taylor, C. J. (1998). Ac-
tive appearance models. In ECCV, volume 2, pages
484–498.
Davies, E. (1988). A modified Hough scheme for general
circle location. Pattern Recogn., 7(1):37–43.
Donner, R., Micu
ˇ
s
´
ık, B., Langs, G., and Bischof, H. (2007).
Sparse MRF appearance models for fast anatomical
structure localisation. In British Machine Vision Con-
ference.
Lelieveldt, B. P. F., Mitchell, S. C., Bosch, J. G., van der
Geest, R. J., Sonka, M., and Reiber, J. H. C. (2001).
Time-continuous segmentation of cardiac image se-
quences using active appearance motion models. In
Information Processing in Medical Imaging (IPMI),
pages 446–452.
Mitchell, S. C., Bosch, J. G., Lelieveldt, P. F., van der Geest,
R. J., Reiber, J. H. C., and Sonka, M. (2002). 3D ac-
tive appearance models: Segmentation of cardiac MR
and ultrasound images. IEEE Transactions on Medi-
cal Imaging, 21(9):1167–1178.
Philip, K., Dove, E., McPherson, D., Gotteiner, N., Stan-
ford, W., and Chandran, K. (1994a). Automatic detec-
tion of myocardial contours in cine-computed tomo-
graphic images. IEEE Transactions on Medical Imag-
ing, 13:241–253.
Philip, K., Dove, E., McPherson, D., Gotteiner, N., Stan-
ford, W., and Chandran, K. (1994b). The fuzzy hough
transform-feature extraction in medical images. IEEE
Transactions on Medical Imaging, 13:235–240.
Soille, P. (2002). Morphological Image Analysis. Springer
Verlag Berlin.
S
¨
orgel, W. and Vaerman, V. (1997). Automatic heart local-
ization from a 4D MRI dataset. In Proceedings SPIE
Medical Imaging.
Stegmann, M. B. (2000). Active appearance models: The-
ory, extensions and cases. Master’s thesis, Informatics
and Mathematical Modelling, Technical University of
Denmark, DTU.
Stegmann, M. B. and Pedersen, D. (2005). Bi-temporal 3D
active appearance models with applications to unsu-
pervised ejection fraction estimation. In The Interna-
tional Symposium on Medical Imaging, volume 5747.
van Assen, H., Danilouchkine, M., Frangi, A., Ords, S.,
Westenberg, J., Reiber, J., and Lelieveldt, B. (2006).
SPASM: a 3D-ASM for segmentation of sparse and
arbitrarily oriented cardiac MRI data. Medical Image
Analysis, 10(2):286–303.
FAST AND ROBUST LOCALIZATION OF THE HEART IN CARDIAC MRI SERIES - A Cascade of Operations for
Automatically Detecting the Heart in Cine MRI Series
345

Figure 4: User study at a glance: Averaged point-to-surface distances for 41 data sets (top left), RMS texture errors for all
validation data sets (top right), and time performance for all validation data sets (bottom).
Figure 5: The result: Initialization of the AAM’s mean at the central slices.
VISAPP 2008 - International Conference on Computer Vision Theory and Applications
346
