
Evaluation of Automatic Identification Technologies
(Auto-ID) to be Implemented in the Health Care Sector
Mike Krey
Center of Business Information Systems, School of Management
Zurich University for Applied Sciences, PO Box 958, St. Georgenplatz 2
8401 Winterthur, Switzerland
Abstract. The tasks and objectives of automatic identification (Auto-ID) are to
provide information on goods and products. It has already been established for
years in the areas of logistics and trading and can no longer be ignored by the
German health care sector [3, 9]. Some German hospitals have already discov-
ered the capabilities of Auto-ID. Improvements in quality, safety and reduc-
tions in risk, cost and time are aspects and areas where improvements are
achievable. Privacy protection, legal restraints, and the personal rights of pa-
tients and staff members are just a few aspects which make the heath care sec-
tor a sensible field for the implementation of Auto-ID [4]. Auto-ID in this con-
text contains the different technologies, methods and products for the registra-
tion, provision and storage of relevant data [6] With the help of a quantifiable
and science-based evaluation, an answer is sought as to which Auto-ID has the
highest capability to be implemented in health care business.
1 Introduction
The German health care system can be devided into three main branches [13]:
− Ambulatory Treatment, assured by 43’000 medical practitioners
− Clinical Treatment, assured by 2’200 hospitals and
− Post-operative Treatment, assured by 1’400 rehabilitation centres.
It is financed by the compulsory health insurance and not like in the USA by an op-
tional health insurance [10]. With health services assured through the insurance sys-
tem, current demographic changes are resulting in a steadily increasing demand for
medical and nursing care.
Cost pressure, reforms and quality requirements are three keywords which are men-
tioned in the current debate about German health care. The financial framework of
health care in the Federal Republic of Germany is at its limits [8].
Most of the 2’200 German hospitals are community owned and regulated by public
law. These hospitals are in competition with an increasing number of privately fi-
nanced hospitals. For the regulated hospitals it is difficult to make big financial in-
vestments within the normal budget. The IT budget is regulated for one year and
Krey M. (2008).
Evaluation of Automatic Identification Technologies (Auto-ID) to be Implemented in the Health Care Sector.
In Proceedings of the 2nd International Workshop on RFID Technology - Concepts, Applications, Challenges, pages 157-166
DOI: 10.5220/0001738501570166
Copyright
c
SciTePress

includes e.g. labour
costs, IT maintenance and small investments in hard- and soft-
ware. Large IT projects can hardly be implemented by regulated hospitals without
additional external financial support [3]. In accordance with a survey by McKinsey &
Company, a quarter of German hospitals won’t be able to hold out against cost pres-
sure and will have to close by the year 2020 [10]. For the affected hospitals it is nec-
essary to develop concepts and reforms to save costs and to work more efficiently. As
it has in the fields of logistics and trading, Auto-ID can become an important compo-
nent for the solution of operational planning and optimization of processes in health
care, with the resulting cost reductions [11]. Primarily, Auto-ID can be help to opti-
mize and support the business processes in the medical and non-medical areas of a
hospital [7]. Nurses and doctors will be freed from avoidable and time-consuming
activities - there will be more time for patients. For example nurses are wasting 30%
of their time by organising their workflow and 40% with administrative duties, so that
the time for patient care is reduced to 8 minutes per patient and day [10].
Currently, various technologies are coming into operation. Wireless Local Area Net-
work (WLAN) is already firmly established in German hospitals. Other systems are
still at the trial stage, such as Radio Frequency Identification (RFID) as a procedure
for automatic identification (Auto-ID).
2 Goals of this Study
The evaluation of Auto-ID shows a professional approach to structured, rational se-
lection and procurement of a system in accordance with the requirements of the
health care sector. Various Auto-ID procedures are evaluated on the basis of health
care requirements and their resulting use criteria. The requirements definition is based
on practical experiences of the author and personal interviews with Chief Technology
Officers (CTO) of six German hospitals with experience in the implementation of
Auto-ID in different business contexts. Their experiences have been very different.
Some implementations had the status of fundamental research in small, autonomous
environments and others are funded by Auto-ID producers so that a clear considera-
tion of costs and needs cannot be made. The focus of this work is a general list of
requirements and makes no claim to be complete. The list will need adaption to a
concrete business context in a hospital. The aim, after the evaluation process, is to
make a valid and objective statement regarding which procedures are appropriate for
application in the health care sector.
3 Scientific Approach and Automatic Identification (Auto-ID)
Technologies Analysed
The following approach to evaluation was executed [12]:
1. Defining requirements. A structured catalogue with wishes and demands on
the Auto-ID was created with the help of the operating and IT department of
the hospital. If necessary additional expert knowledge can help to channel
158

the requirements process. The operating department uses relevant medical
knowledge about the internal processes of the hospital or department where
the Auto-ID should be implemented. The IT department is responsible for
the technical input and uses knowledge for example in the fields of systems
integration, project management or functional range of medical equipment.
2. Establishing criteria. Based on the defined requirements for the Auto-ID, a
list of criteria was created. The different criteria are grouped into levels
which contain on the lowest level qualified and measureable questions with
clear origin which can be answered by the responsible department members.
For a rational and objective execution of the evaluation the criteria will be
weighted.
3. Benefit analysis. The collected detailed information for every technology is
the basis on which every criterion is evaluated in terms of their degree of
performance [12].
4. Conclusion. Based on the result of the benefit analysis a concrete proposal
was developed. The selected Auto-ID has the optimal capability of being
implemented in health care in terms of the weighted criteria. The single
Auto-ID technology with the highest value in the benefit analysis is not nec-
essarily the exclusive optimal proposal. Under certain circumstances it can
be reasonable that two or more Auto-ID technologies can be combined.
3.1 Fields of Application and Requirements to Auto-ID in the Health Care
Sector
The motivation to implement Auto-ID in heath care business ranges from marketing
purposes and advancement of clinical pathways to automatisation of clinical business
processes [3]. The following scenarios have a deep impact on the functional range of
the technology and shows that field of application for using Auto-ID in German
health care may be different [5], [9]. This list of scenarios may not be complete, but it
provides an understanding of the different fields of application in health care business
for the reader. E.g.
− Documentation support in patient treatment processes
− Automation of staff authentication processes
− Completely transparent supply chain process (inventory management)
− Patient identification and location assistance to ensure patient safety (patient track-
ing and prevention of baby theft.
− Control of cleaning intervals of medical devices
− Optimisation in terms of finding the right clinical assets at the right time (asset
tracking)
159

− Control of temperature throughout blood supply chain (Auto-ID in combination
with sensors)
Process engineering/Process control means the logistics business processes in a hos-
pital, such as inventory control, with mechanisms for automatic tracking of goods or
medical products.
The documentation of time, date and cleaner of medical equipment which was used,
for example, in a surgery, is regulated by law and statutory regulations. Authorisation
and documentation processes supported by Auto-ID can help to make these standard
processes easier to handle by combining them with authentication mechanism [9].
The result is a fully automated and paperless process.
Tracking in the health care business initiated a public dispute in Germany. The tech-
nology tells us what asset is in which room or part of the building at what time. The
result is that valuable time previously spent searching for an asset can be reduced or
even eliminated [9]. But data protection officers objected that tracking a patient or a
staff member is an intrusion into privacy which cannot be tolerated by law.
Auto-ID can help to control the ‘viability’ of a patient. If the Auto-ID systems regis-
ter a value below or above a defined reference value, for example, a message will be
sent automatically by mobile phone to the next emergency department. Another pos-
sibility is the combination of Auto-ID with a sensor system, which controls the tem-
perature throughout the supply chain of a blood bottle [9].
Depending on the scenario in which Auto-ID will be implemented, the requirements
on the functional range differs. The following list of questions gives the reader an
understanding of the defined requirements on Auto-ID but may not be complete.
− How can Auto-ID hardware be affixed to medical equipment?
− Is the Auto-ID equipment resistant to water, temperature and chemical substances
in medical treatment processes?
− Are there ways to integrate the technology into the existing hospital information
systems (HIS) via HL-7 or CORBAmed interfaces?
− How safely can personal data be handled (Security features)?
− How does the Auto-ID fulfil the requirements for fire protection in the health care
sector?
− What is the maximum data rate and what data volume can be handled by the tech-
nology?
− How can be guaranteed that the data is valid, persistent reliable and available?
3.2 Auto-ID Analysed
Auto-ID contains the different technologies, methods and products for the registra-
tion, provision and storage of relevant data [6]. Auto-ID can play a part in the im-
160
provement of processes in health care business. With the help of real-time processing
and automatic stock detection, for example in the supply chain, and with its ability to
trace goods, the efficiency of a hospital can be sustainably impoved [4].
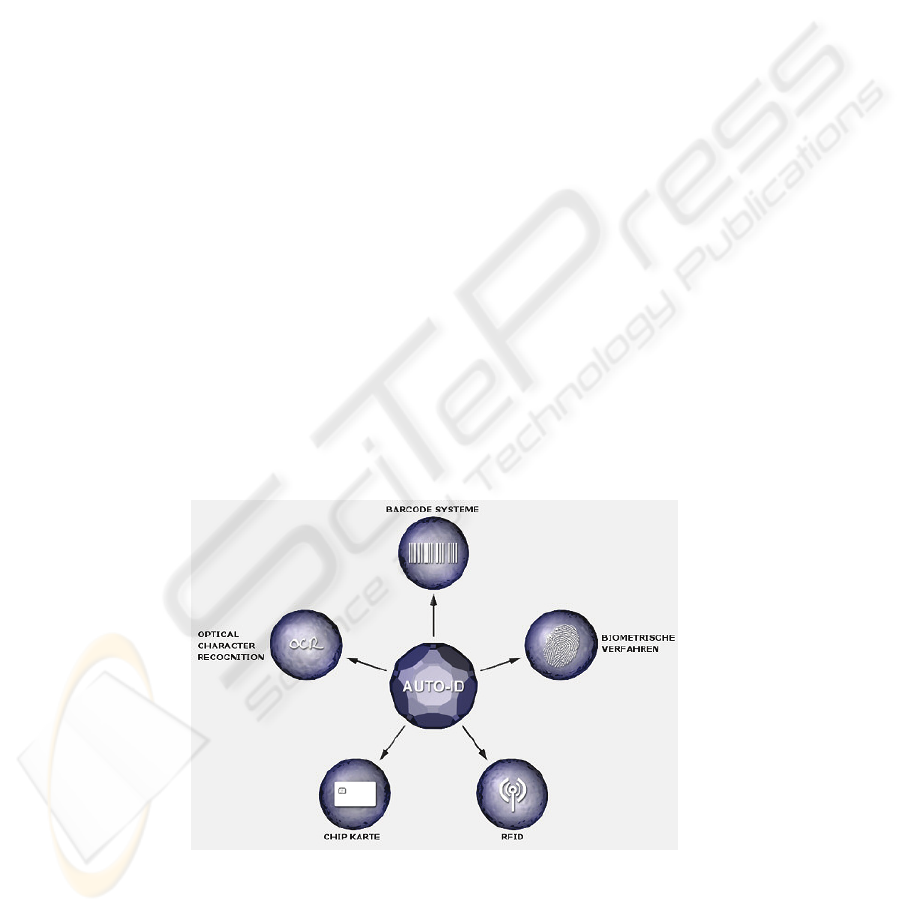
The following Auto-ID technologies were the basis for the evaluation [6], (Fig. 1):
− Bar Code
− Optical Character Recognition (OCR)
− Chip Card
− Biometric Methods
− RFID
A distinction is drawn between four basic principles [2]:
− Mechanical and electromechanical identification systems, which use mechanical
components like pins as binary storage. They are based on capacitive and inductive
mechanisms for scanning.
− Magnetic identification systems, which use magnetic fields e.g. of permanent mag-
net (magnetic card) as storage. They require small distances for accurate scanning.
− Opto-electronical identification systems, which are able to detect the shape of an
item or markings like colours, OCR or bar code.
− Electromagnetic identification systems, which use inductive waves for a touchless
carrier of information. They use electrically programmable microchips to store
data.
Biometric methods are additionally separated into iris identification, fingerprint and
voice identification. The detailed description of every Auto-ID is left out and can be
gleaned at Finkenzeller [6].
Fig. 1. Defined Auto-ID technologies [6].
161
4 Established Criteria and Benefit Analysis
4.1 Established Criteria
To provide an efficient way of using the list of criteria from both, the IT department
and the operating department of the hospital, the collected wishes and demands are
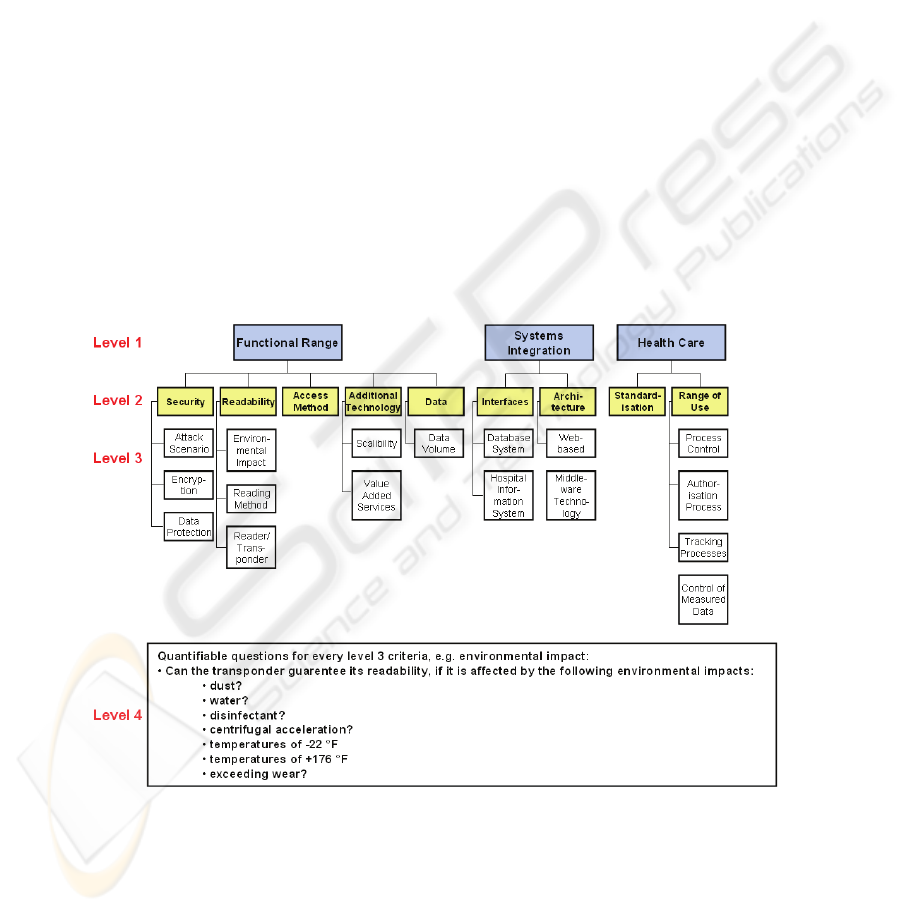
assigned to four criteria levels (level 1 - 4). The highest level (level 1) is divided into
the following and aggregates the lower criteria (Fig. 2):
− Functional range
− Systems integration
− Health care
− General criteria
The criteria in these categories are not directly measureable or rateable and have to be
separated into detailed subcriteria (level 2 & 3), (Fig. 2). Level 4 deals with quantifi-
able questions, measureable by the department members and relating to the require-
ments in the systems specification [12]. By defining one category just for the re-
quirements of heath care we focus on the aim of finding a valid Auto-ID which is
appropriate for application to the health care sector specifically.
Fig. 2. Level 1, 2, 3 & 4 of the established criteria for Functional range, Systems integration
and Health care.
162
The general criteria are consciously left out of this consideration. This criteria level
deals e.g. with general information about cost for the Auto-ID technology (hard &
software) and support or criteria concerning the know-how of the producer in the
health care sector.
4.2 Benefit Analysis
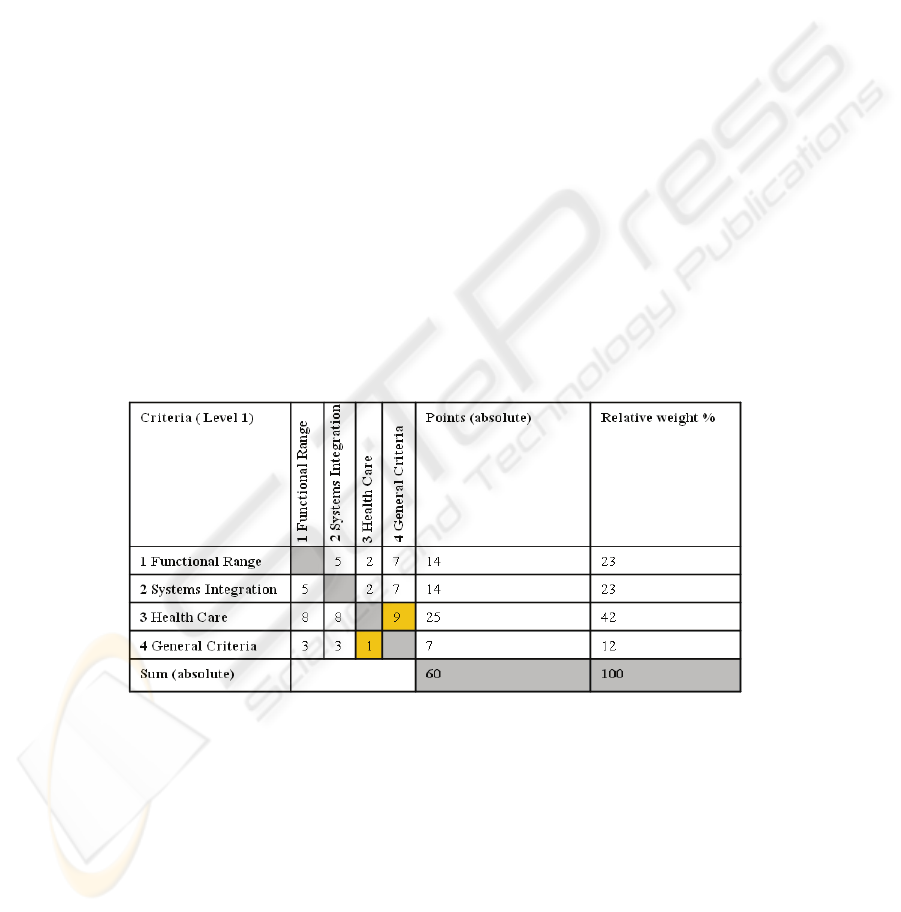
The method of the paired comparison was used to give a valid weighting on every
criterion (table 1). It was used for levels one to three (level 1 - 3). Table 1 shows the
paired comparison carried out for the first level. Every sum of the comparison be-
tween two criteria has the result 10. In this example the ‘general criteria’ are less
important (expresses by ‘1’) than criteria concerning ‘heath care’ (expressed by ‘9’).
In this evaluation, the significance of the criterion ‘heath care’ can be seen (42%).
The score in this category is influenced by the ability of the Auto-ID process to live
up to context-specific expectations and its ease of integration into existing infrastruc-
ture. The weighing of the criteria was made by the author. It should be mentioned that
no concrete project with predefined requirements has been the basis for the weighing
decisions and the result of the paired comparison is just valid for this work and has to
be reviewed and adapted if the requirements and the field where the project takes
place should change. It is possible if the evaluation takes place in another context, for
example when Auto-ID should be implemented in an existing environment in a hospi-
tal the criteria of ‘systems integration’ should get more emphasis.
Table 1. Result of the paired comparison for the first level (level 1).
The second step, after carrying out the paired comparison, is to calculate the value of
benefit. In this calculation, the weight of a level obviously determines the influence of
the next level down. This method guarantees that all relevant criteria are rated and
aggregated to the level above. The detailed description of this method is left out and
can be gleaned at Schreiber [12].
163
5 Conclusions
The conclusion presents an interpretation of the most important results of the evalua-
tion and makes no claim to be complete.
The aim of the evaluation was to find a valid statement as to which procedure, in
accordance with requirements, is appropriate for application to the health care sector.
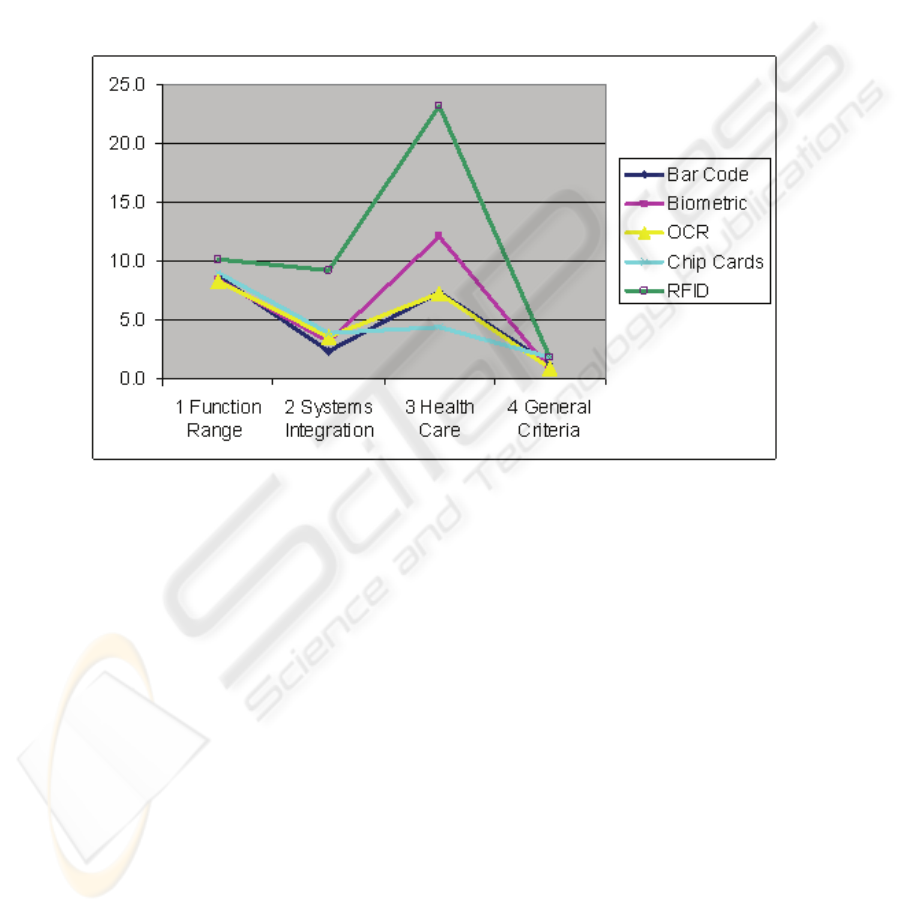
RFID shows the highest benefit value in every aggregated level of achievement on
level 1 and has good potential to place lasting emphasis on clinical processes and
pathways (Fig. 3).
Fig. 3. Conclusion of the evaluation (Level 1).
It became clear that for a concrete project a new arrangement of the weightings has to
be devised to cover all relevant project requirements as well as the context in which
the project is to be implemented in the hospital. But the evaluation showed as well
that it is not possible to make a single statement as to which method is the most useful
for every field of application in the health care sector and should generally be imple-
mented. If we take a look at level 2, RFID in authorisation (security and data) proc-
esses is not the only useful solution (Fig. 4). Biometic methods have the highest bene-
fit value in these fields. Based on this awareness a useful combination of two methods
- RFID and biometric methods - could guarantee a holistically successful, sustainable
and well-accepted project in health care. The developed list of criteria is an important
orientation guide for decision making but has to be reengineered if the requirements
are changed.
164
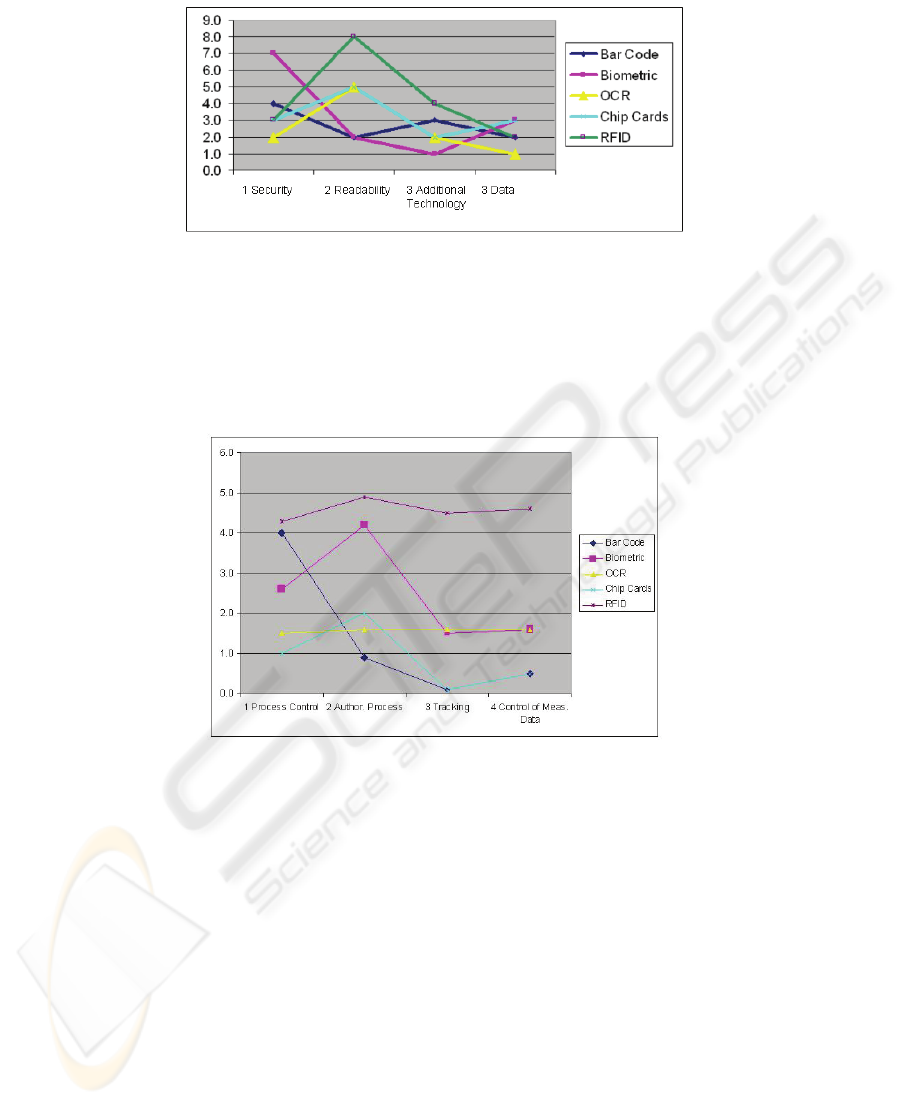
Fig. 4. Conclusion of the evaluation (Level 2).
If we take a look at level 3 - RFID has by far the highest impact in the field of appli-
cations for controlling measurable data and tracking items (Fig. 5). If the field of
application ‘Process control’ is concerned, the bar code has nearly the same impact
and can have a lasting effect in areas of logistics in a hospital. That result shows that
RFID keeps its relevance for the area of logistics as well for the health care business.
Fig. 5. Conclusion of the evaluation (Level 3).
The evaluation showed that RFID offers significant benefit potential in terms of sup-
porting quality processes and saving money and time. It is not, however, a technical
view on RFID. It should be treated as an instrument to accelerate administrative and
clinical business processes and to design them in a more efficient way.
Most of the German health care institutions implementing RFID projects have the
status of “early adopters”. This denomination will soon no longer be applicable, as
German hospitals are forced to make their services more attractive to patients. The
increase of cost pressure in the health care system leads managers to lay more empha-
sis on return on invest (ROI) and repayment strategies. Processes have to be opti-
mised so that costs can be saved. In this regard RFID can play an important role.
165

References
1. AeroScout (2007): Case Studies online, http://www.aeroscout.com/content.asp
?page=case%20 studies, 02.03.2008
2. Arnold, D. et al. (2003): Handbuch Logistik, Berlin 2003
3. Becker-Berke, St. & Lautwein-Reinhard, B. (2004): Gesundheitswesen. Ein Lexikon für
Einsteiger und Insider, Bonn 2004
4. Bullinger, H.J. & ten Hompel, M. (ed.) (2007): Internet der Dinge, Berlin 2007
5. Bultmann, M. et al. (2002): Datenschutz und Telemedizin. Anforderungen an
Medizinnetze, http://www.datenschutz-bayern.de/verwaltung/datenschutzTelemedizin.pdf,
26.03.2008
6. Finkenzeller, K. (2006): RFID-Handbuch,4., Muenchen 2006
7. Gillert, F. & Hansen, W.-R. (2007): RFID für die Optimierung von Geschäftsprozessen,
Muenchen 2007
8. Jaeckel, A. (2004): Telemedizinfuehrer Deutschland. Deutsches Medizin Forum, Ober-
Moerlen 2004
9. Kern, Ch. (2006): Anwendungen von RFID-Systemen, 2., Berlin 2006
10. McKinsey & Company (2006): Studie Perspektiven der Krankenhausversorgung in
Deutschland, http://www.mckinsey.de/presse/060502_business_breakfast.htm, 30.03.2008
11. Middendorf, C. (2005): Klinisches Risikomanagement. Implikationen, Methoden und
Gestaltungsempfehlungen für das Management klinischer Risiken in Krankenhaeusern,
Muenster 2005
12. Schreiber, J. (2003): Beschaffung von Informatikmitteln, 4., Bern 2003
13. Statistisches Bundesamt (2004): Daten zur Bevölkerung, http://www.destatis.de/
jetspeed/portal/cms/Sites/destatis/Internet/DE/Navigation/Publikationen/Querschnittsveroef
fentlichungen/Querschnitt,templateId=renderPrint.psml, 18.04.2008
166
