
BIODEVICES BASED ON SHAPE-MEMORY POLYMERS
Current Capabilities and Challenges
Andrés Díaz Lantada, Pilar Lafont Morgado, Héctor Lorenzo-Yustos, Vicente Lorenzo Esteban
Julio Muñoz-García, José Luis Muñoz Sanz, Javier Echavarri Otero and Juan Manuel Munoz-Guijosa
Grupo de Investigación en Ingeniería de Máquinas – E.T.S.I. Industriales – Universidad Politécnica de Madrid
Grupo POLímeros, Caracterización y Aplicaciones (POLCA) – Universidad Politécnica de Madrid
C/ José Gutiérrez Abascal, nº 2. 28006 – Madrid, Spain
Keywords: Shape-memory polymers (SMPs), Biodevices, Synthesis, Characterization, Processing, Complete
development.
Abstract: Shape-memory polymers are active materials with thermomechanical coupling and a high capability to
recover from high levels of deformation, which, combined with their low cost and density has favoured the
appearance of numerous applications, particularly those linked to the Medical Industry. In many cases, these
materials are of medical standard, which increases the chances of obtaining biocompatible devices.
In the last decade enormous progress has been made on many areas, regarding these materials, such as
synthesis, characterization, activation and others, aimed at improving their applicability. However, various
spheres of action still remain that require more in depth research to promote the production start-up of
various shape-memory polymer-based devices that have had laboratory validation.
This work sets out the potential these materials provide for developing biodevices and the main advances
achieved. Also shown are various medical devices just being developed, as well current study needs and
trends.
1 INTRODUCTION TO
SHAPE-MEMORY POLYMERS
(SMPS)
Shape-memory polymers (SMPs) are materials that
show a mechanical response to external stimuli,
usually to changes of temperature. When these
materials are heated above their “activation”
temperature, there is a radical change from rigid
polymer to an elastic state that will allow
deformations of up to 400%. If the material is cooled
down after manipulation it retains the shape
imposed; the said structure is “frozen” and returns to
a rigid but “non-equilibrium” state. If the material is
again heated above its vitreous transition
temperature or “activation temperature” it recovers
its initial non-deformed state. The cycle can be
repeated numerous times without degrading the
polymer and most suppliers can formulate different
materials with activation temperatures ranging from
–30 ºC to 260 ºC, depending on the application
required. Of
They are therefore active materials that present
thermomechanical coupling and a high capability for
recovery from deformation, (much greater than that
shown by shape-memory metal alloys), which
combined with their lower density and cost has
favoured the appearance of numerous applications.
Their properties permit applications for
manufacturing sensing devices or actuators,
especially for the aeronautics, automobile and
medical industry.
2 POTENTIAL FOR BIODEVICES
2.1 Some Advantages
As polymers, SMPs can be easily conformed into
different complex shapes and their properties
designed or adapted to specific applications and can
also be integrated with other microelectromechanical
sensors (MEMS) to produce “intelligent”
bioactuators and biodevices.
137
Díaz Lantada A., Lafont Morgado P., Lorenzo-Yustos H., Lorenzo Esteban V., Muñoz-García J., Luis Muñoz Sanz J., Echavarri Otero J. and
Munoz-Guijosa J. (2009).
BIODEVICES BASED ON SHAPE-MEMORY POLYMERS - Current Capabilities and Challenges.
In Proceedings of the International Conference on Biomedical Electronics and Devices, pages 137-144
DOI: 10.5220/0001123901370144
Copyright
c
SciTePress

Compared to other shape-memory alloys used in
numerous medical devices, SMPs show a far greater
capability for changing their geometry during
activation.
They are also much cheaper to synthesise and
their large scale mass production costs are reduced
by using technologies such as injection moulding.
All this makes them very versatile active
materials with a high potential for industry, provided
they overcome some of the limitations set out in the
following sections.
2.2 Proposed Devices
Bellow are explained some specific proposals for
developing medical devices based on the use of
shape-memory polymers, most of which have
undergone in vitro laboratory testing. After
undergoing in vitro testing and meeting the
requirements for official approval, in some cases
their commercialisation is subject to their attaining
the goals described at the end of this paper.
Self-expanding Stents. Like the stent designed by
Boston Scientific Corporation using the polymer
from CRG Industries known as “Veriflex” under its
trade-name, to treat the problems arising when the
arteries become narrow or obstructed and also for
removing obstructions from other “tube-shaped”
body parts, like the uretheres and the bronchial
tubes. The stent is inserted in its temporary form
(reduced) and the body’s own heat causes it to dilate
and become attached to the artery.
They may be used to replace stents based on
shape-memory alloys such as Nitinol, once the
appropriate biocompatibility studies have been
carried out. Developments of self-expanding stents
have also been carried out by using injected
polyurethane (Wache, 2003).
Intelligent Sutures. Like those developed at the
Forschungszentrum in Karlsruhe by Lendlein’s team
and at the M.I.T. by Langer’s team, which have a
temporary linear shape and a permanent shape in the
form of a knot, with the change in geometry being
activated by the body’s own temperature. They have
numerous applications in minimally invasive surgery
and, as they are biodegradable, they have additional
advantages over the use of textile sutures and metal
clips (Lendlein, Kelch, Langer, 2002, 2005).
Thrombectomy Devices. With the recent discovery
that the thermal effect of shape-memory can be
activated by a laser, part of whose energy is
absorbed by the polymer, devices with special
geometries have been proposed for removing clots
(Wilson, 2006). The polymer is shaped in a spiral
mould and then heated and stretched to give it its
temporary shape. When the laser light passes
through the polymer, the shape-memory effect is
activated and the device recovers its spiral shape
trapping the clot which can then be removed.
Active Catheters. By using shape-memory
polymers for the distal point of catheters together
with a subsequent activation of the memory effect
by laser light or body heat, different drugs and
antitumoral agents can be released. The presence of
an active catheter point can also help reach zones
that are difficult to access in minimally invasive
surgery tasks (Yackaki, 2007).
Drug Release Devices. If biodegradable shape-
memory polymers are used for implantable medical
devices, drug supply reservoirs can be incorporated
into the device itself. After implant, the polymer
begins to be absorbed by the organism and the drug
is released. Patents have been taken out in this
respect for self-expanding coronary stents or intra-
urethral stents (Boston Scientific Co. and Surmodics
Inc.). The possibility of obtaining temporary
geometries with micro-reservoirs for drug storage
has also been studied. The drugs would then be
released on activation of the shape-memory effect by
body heat (Gall, 2004).
Active Annuloplasty Rings. Aimed at obtaining a
progressive postoperative treatment of mitral
insufficiency, they are based on the use of a
polymeric ring with heating resistances distributed
around the inside to activate the shape-memory
effect by Joule effect. This activation must allow the
cross section of the mitral ring to be gradually
reduced and, therefore, the mitral insufficiency
improved.
Figure 1 shows a schematic design of such a
device proposed by our group at Universidad
Politécnica de Madrid. Prototypes of these rings,
with different geometries and materials, have been
developed and tested “in vitro” in pig’s hearts (Díaz
Lantada, Lafont Morgado, 2008).
The different devices explained will provide
considerable therapeutic benefits compared to
conventional devices, due to their capability to act
inside the body thanks to the use of shape-memory
polymers.
BIODEVICES 2009 - International Conference on Biomedical Electronics and Devices
138

Figure 1: Active annuloplasty ring design. SMP with
internal heating resistances. Biodevices 2008.
3 CURRENT CAPABILITIES
Set out below are some of the main advances
achieved in the last decade concerning shape-
memory polymers and the technologies associated
with their use.
3.1 Synthesis
In recent years particular emphasis has been placed
on obtaining new formulations of polymers with
shape-memory properties, by changing the
proportions of monomers, including additives, and
inducing multiple crosslinkings and working on
previously known formulations whose shape-
memory properties have been boosted. (Lendlein,
2002, Liu, 2007).
Different prestigious laboratories have conducted
exhaustive work on synthesis and subsequent
classification in accordance with the molecular
structure of the polymers.
The recent synthesis of polymers capable of
remembering two pre-set shapes using two
programming stages (triple shape effect) has brought
new possibilities for future medical devices, due to
the fact that two postoperative changes can be made
to the geometry (Bellin, 2006).
In spite of the numerous formulations for the
shape-memory polymers that have been synthesised
recently, the main problems for obtaining
commercial biodevices based on these materials are:
the small number of commercial formulations, the
toxicity of many of them and problems regarding
thermomechanical properties, as will be explained in
the following sections.
3.2 Characterization
During the last decade most experiments linked to
characterizing different shape-memory polymer
properties have attempted to compare the
thermomechanical response of different
formulations.
At the Langley Research Centre the results of
tests using thermomechanical analysers (TMA) has
been compared with those obtained by using
differential scan calorimeters (DSC) to obtain
precisely the vitreous transition temperature in
shape-memory polymers (Volk, 2005). This research
also explains deformation recovery tests conduced
by heating under constant deformation and under
constant stress, for which MTS Alliance RT1
traction machines and a heating chamber are used.
Three-point bending tests have also been used in
heated chambers in order to evaluate the geometric
recovery capability of these materials subjected to
different levels of stress and deformation (Lendlein,
2002, Tobushi, 2008).
Dynamic mechanical analyses (DMTA) have
been used basically to evaluate the elastic modulus
of these materials according to temperature. They
also enable the vitreous transition temperatures of
the materials to be found (Mather, 2002, Liu, 2003,
2006, Huang, 2006, Yakacki, 2007). This is a
supplementary technique to DSC tests (which are
usually used for the study of vitreous transitions,
polymorphisms, crystallisations and aging).
All these experiments and many others have
helped to provide basic knowledge concerning the
thermomechanical behaviour of these materials,
which is decisive for future developments.
3.3 Processing Technologies
3.3.1 CAD-CAE-CAM Tools
Computer-aided design, calculation and
manufacturing technologies (CAD-CAE-CAM),
have become essential tools for product
development. They let 3D geometries and alternative
designs be obtained rapidly. Calculations on stress,
deformations, ergonomics, dynamic response and
other aspects including material comparison and
design can also be performed for design
optimization.
The numerous benefits of these technologies for
developing conventional products can also be
applied to the development of shape-memory
polymer-based medical devices.
The recent use of programs such as MIMICS for
processing medical image technique files (TAC,
RMN and others) enables biodevices to be made-to-
measure (Harrysson, 2007). With these programs
three-dimensional geometries of parts of the human
body can be obtained and exported to other CAD-
BIODEVICES BASED ON SHAPE-MEMORY POLYMERS - Current Capabilities and Challenges
139

CAE-CAM programs to perform the customised
designs and obtain prototypes by using techniques
that we will now explain. They also contribute new
possibilities to the design of customised implants
that benefit from the use of SMPs.
3.3.2 Rapid Prototyping Technologies
These new technologies mean that physical parts can
be obtained in a short time (days) directly from the
computer-aided designs. They are of great help in
optimising design iterations, improving end quality
and speeding up production.
Rapid prototyping systems first appeared in 1987
with the American company, 3D Systems’
stereolithography, currently the most widespread
technology. It is based on being able to activate a
polymerisation reaction in a liquid state epoxy resin
by means of laser beam projection with a power and
frequency suited to the type of resin, which “draws”
the required geometry layer by layer. By using this
technology, epoxy resin prototypes with shape-
memory properties can be obtained directly.
Several results of our investigations at Product
Development Laboratory – Universidad Politécnica
de Madrid related with the application of these
technologies to the development of SMP-based
devices are shown below.
Figure 2 shows how a pincer-shaped end for an
active catheter can be obtained from its 3D geometry
in a CAD file. An SLA-3500 machine was used to
polymerise a 3D Systems epoxy resin sold under the
trade name of Accura 60.
The pincer can be made to open by hot
deformation during the shape-memory “training”
process. By then heating it, the pincer closes, as
Figure 3 shows.
This ability can be used in similar devices, with
different geometries and materials, to extract foreign
bodies and in minimally invasive surgery.
Using silicone mould vacuum casting,
polyurethane prototypes with shape-memory can be
obtained. Figure 4 shows a ring device obtained with
this technology using a type of polyurethane sold
under the trade name of MCP 3115, whose
capability for recovering shape through heating is
also shown. Similar devices can be used to change
soft tissue geometry (annuloplasty, sutures,
cerclages and others).
The main benefits of these technologies are:
designs and functionalities can be efficiently
compared and the associated products more rapidly
developed. Being able to apply these technologies to
SMP-based devices is of great importance to their
becoming widespread in industry.
Figure 2: Active pincer design and prototypes.
Figure 3: Geometric activation in an epoxy resin pincer.
Figure 4: PU geometric cerclage activation.
3.3.3 Microfabrication
Being able to use microfabrication technologies with
SMPs provides a new line of use for these materials
BIODEVICES 2009 - International Conference on Biomedical Electronics and Devices
140

inside the medical device industry, particularly in
lab-on-a-chip and controlled drug delivery systems.
Typical devices for these applications require
surface channels and microtextures with micrometric
geometries that can be obtained in polymeric
materials by using technologies such as “hot-
embossing”, “micro injection moulding” or “LIGA”.
To be precise, by applying hot-embossing to
SMPs, surface microtextures and microreservoirs
with temporary geometry can be achieved (Gall,
2004).
The possible use of physical or chemical vapour
deposition technologies, combined with the ability to
produce protective masks by applying UV
photolithography, enables surface embossing to be
performed on very different materials (metals, alloys
and ceramics) using shape-memory polymers as
substrate (Paumier, 2008).
Thus, by making connection microtracks, these
can be used to send an order to certain parts of a
device to activate a geometric change by heating an
adjacent resistance. In some cases, the connection
track itself can be used as a heating element if its
cross section is sufficiently small and its electrical
resistance, therefore, high enough.
This ability to manufacture by layers and
combine different materials enormously strengthens
the capability to integrate certain SMP-based active
parts into complex systems (such as implantable
medical devices).
3.4 Shape-memory Effect Training
The shape-memory effect training process is usually
conducted through heat deformation of the device
manufactured in SMP and subsequent cooling to
maintain the deformation, thereby obtaining the
temporary shape.
To increase the length of the temporary shape
devices traction machines with heated chambers are
used. To produce temporary surface marking hot
compression moulding presses are used.
Recently, the use of cone-shaped countershapes
has been proposed to obtain ring devices with a
temporarily enlarged diameter (Díaz Lantada, Lafont
Morgado, 2008).
3.5 Activation
Another aspect where most progress has been made
is the activation of the memory effect by various
methods, especially:
Joule Effect Activation. Based on distributing
heating resistances at the core of the polymer where
the passing of an electric current generates the
necessary heat.
Light or Laser Activation. Based on projecting a
laser through a shape-memory material with a
similar absorption frequency to that of the laser
used, which produces heating (Lendlein, 2005,
Wilson, 2006).
Magnetic Activation. Based on heating by
induction of magnetic or metallic microparticles,
distributed at the core of the polymer while it is
being conformed to its shape (Buckley, 2006).
However, the biocompatibility of the associated
devices needs to be further optimised.
Support Technologies. Progress in the field of
wireless communications means that devices can
now be remotely activated, which is promoting the
appearance of new active implantable biodevices.
3.6 Commercial Formulations
The promising applications of these materials,
particularly in the field of medicine, together with a
growing industrial demand, has led to departments
dedicated to the synthesis of shape-memory
polymers being set up in large companies and the
appearance of some spin-off. The major ones are:
• Mitsubishi Heavy Industries Ltd.
• DIAPLEX.
• mNemoscience GmbH.
• CRG Industries LLC.
Most of these recently set up companies and
departments offer “a la carte” design work and
prototyping applications using SMP. They also
commercialise their developments, both synthesised
materials and products based on those materials.
4 CHALLENGES
This section deals with the main fields where more
in-depth study is particularly important, in order to
facilitate the industrial expansion of shape-memory
polymers as an integral part of active implantable
medical devices.
BIODEVICES BASED ON SHAPE-MEMORY POLYMERS - Current Capabilities and Challenges
141

4.1 Thermomechanical Response
Unfortunately, the shape-memory polymer materials
developed up to now only let forces of
approximately 3 MPa be withstood during
activation, which is insufficient for certain medical
applications intended for use as actuators, specially
when wishing to change the geometries of biological
tissues.
Enhancing the activation forces requires greater
understanding of the basic physical-chemical
principles of these phenomena. To this end,
computational models can be used that help apply a
combined knowledge of materials science,
thermodynamics, mechanics and heat transmission
(Conti, 2007).
4.2 Modelling and Simulation
Using the data obtained from the characterization
tests, (of materials and specific applications),
behaviour models can be obtained that facilitate the
development of new applications with the same
material or similar applications with other polymers.
The possibility of combining models that are
developed ad hoc and the multivariable simulations
that allow finite element calculation programs will
help simplify the design of training systems and the
heat activation of SMP-based devices.
4.3 Stability of Properties
In general, the variation in the properties of
polymeric materials through aging has major
economic implications as it affects in-service
performance. Particularly in the case of shape-
memory implants, any change in the vitreous
transition temperature (or activation temperature)
can cause problems when activating the necessary
geometric changes.
It is also necessary to study the changes to the
mechanical properties of these polymers (elasticity
modulus, hardness, and resilience), due to their
being implanted in the human body. Figure 5 shows
an example of an study (carried out by our group at
Universidad Politécnica de Madrid) on how the
hardness of an aging shape-memory polyurethane
evolves at 40ºC for 80 days. A Vickers
microhardness tester was used with a 0.98 N load
and a 15 s contact time.
114
116
118
120
122
124
126
128
130
132
-1 -0,5 0 0,5 1 1,5 2
log (time)
Vickers Microhardness MPa
Figure 5: Evolution of hardness in a shape-memory
polyurethane.
Changes in the mechanical properties like that
shown can considerably affect the applicability of
devices based on these materials. The use of
additives and the synthesis of new formulations that
help minimise the changes to properties with the
passage of time will be highly useful for optimising
devices that can be commercialised.
However, the effects of moisture on changes in
the properties of SMPs may be determining factors
for making an application invalid. This effect has
been studied on shape-memory polyurethanes by
conducting DMTA and DSC tests with samples
submerged in water for different lengths of time to
age them (Yang, 2004).
4.4 Activation Temperature
Only on rare occasions, in the polymeric products
industry, have materials with vitreous transitions of
between 0 ºC and 50 ºC been used, as in-service
changes to properties are not usually desirable. For
this reason, it is difficult to find commercial
formulations for polymers with Tg in the 25 to 45 ºC
range.
However, for the development of shape-memory
polymer-based active implantable medical devices,
it is precisely temperatures near to the 37 ºC of body
heat that are sought. SMPs with a Tg of around 30
ºC may give rise to devices that change their
geometry on contact with the patient’s body. SMPs
with a Tg of around 45 ºC can be used to develop
implants intended for postoperative activation
through heating to induce geometric changes.
Some laboratories and companies achieve
noticeable changes in the Tg of dual component
SMPs by modifying the proportion of monomers and
additives for cross-linking that are used to synthesise
them. Most formulations still have activation
temperatures that are too high to be used in
BIODEVICES 2009 - International Conference on Biomedical Electronics and Devices
142
implantable devices without causing damage to
surrounding tissues.
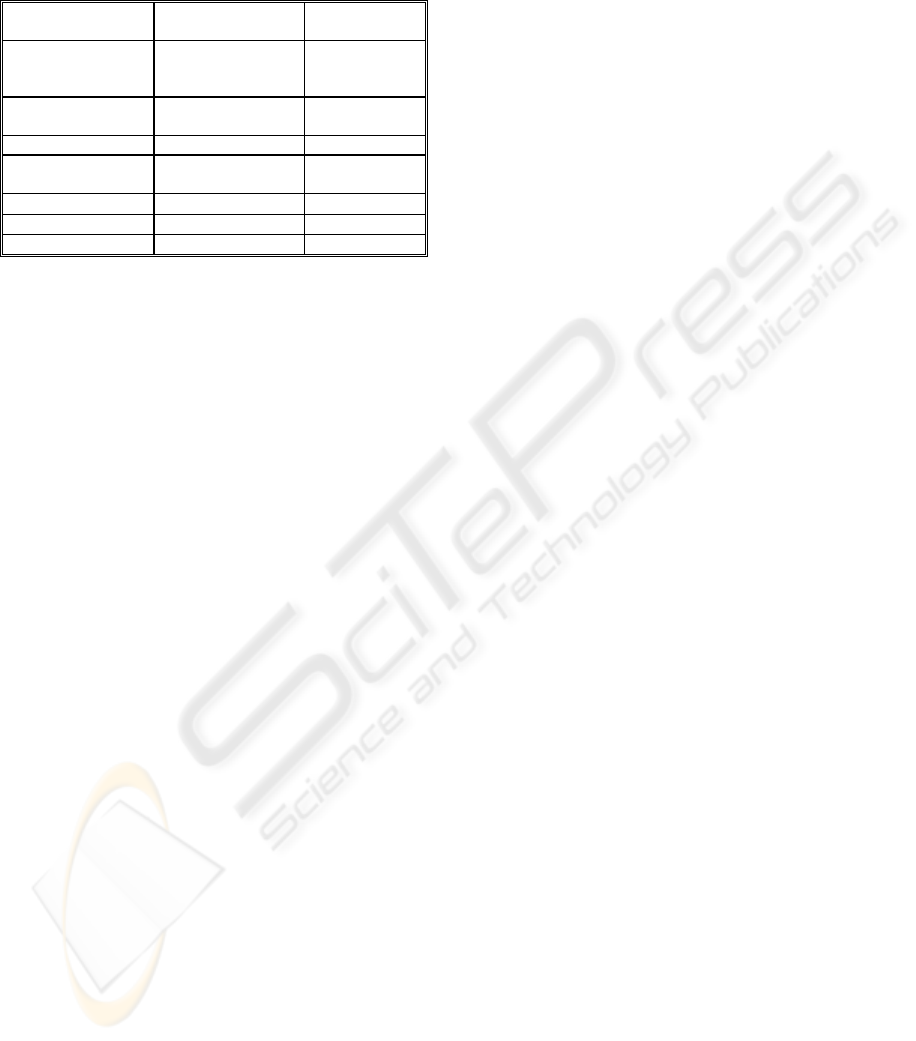
Table 1: Materials and acceptable Tg for biodevices.
Material
Vitreous transition
temperature
Reference
tBA-co-PEGDMA
40 - 52 ºC
(according to % of
cross-linking)
(Yakacki, 2008)
Polynorbornene
(Norsorex
®
)
Around 40 ºC
(Liu, Mather,
2003, 2007)
Polyurethane Room Temp. ±50 ºC (Tobushi, 2008)
Polyurethane Diaplex
MM5520
55 ºC
DIAPLEX Ltd.
(Small, 2005)
Poly(ε-caprolactone)
40 – 59 ºC (Lendlein, 2002)
Epoxy-based 35 – 105 ºC CRG Industries
Styrene-based 45 – 95 ºC CRG Industries
Fortunately, in the last 5 years new SMPs with a
Tg closer to body temperature have been synthesised
and could be used in conjunction with appropriate
protective coatings to develop percutaneous
implants. Table 1 shows some prime examples.
In addition, some considerations, which are set out
below, must be taken into account concerning the
feasibility of using these materials.
4.5 Security Issues
4.5.1 Biocompatibility Improvements
Starting up production of shape-memory-based
polymers is closer than ever as more emphasis is
being placed on improving the biocompatibility of
these devices.
Many SMP formulations are toxic; however,
some of them have been shown to be compatible
with human tissues (Cabanlit, 2007, Sokolowsky,
2007), which is hopeful for future developments. In
whatever case, the use of protecting coatings (using
PVD or CVD) may be of considerable help in
improving this aspect.
4.5.2 Sterilization
Before in vivo implantation the devices need to be
sterilised using some of the methods that are usually
applied to polymers (steam, ethylene oxide, gamma
radiation, low temperature plasma “LTP” or the
Noxilizer process).
In spite of the numerous methods that can be
used, it is preferable to choose low temperature
sterilisation (LTP, ethylene oxide or the Noxilizer
method) to avoid activating the memory effect
before implanting the devices.
The influence of these methods on toxicity and
thermomechanical response of these materials has
recently begun to be studied with promising results
(Yakacki, 2008).
4.5.3 Regulations
In order to optimise the safety of devices based on
these materials they must be in compliance with the
guidelines of the “European Directive on Medical
Devices – 93/42/EEC” and the “European Directive
on Active Implantable Medical Devices –
90/385/EEC”. It is also advisable to follow the
recommendations of Standard ISO 13485 on quality
in medical devices as well as specific legislation
concerning materials characterization tests (ISO and
ASTM Standards especially).
4.6 Structured Development Process
If the development of commercial medical
applications based on these devices is to be
promoted, it is important to increase the connection
between all the actors taking part in the different
development stages.
In this way, they could collaborate to establish a
structured design process to combine the tasks of:
synthesis, materials characterization and processing,
mechanical design, prototype manufacture, “in
vitro” and “in vivo” trials, official approval and
subsequent production start-up.
Similar proposals are being successfully applied
to promote developments based on other active
materials, such as electroactive polymers (EAPs),
(Bar-Cohen, 2002, 2006).
5 IMPROVING RESULTS AND
CONCLUSIONS
Shape-memory polymers have emerged with
enormous potential allowing the development of
medical devices with special features and
capabilities for activation hitherto unachievable.
The development of bioactuators based on these
materials at present requires progress in various
scientific-technological aspects to optimise their
possibilities. It will then be possible to obtain
commercialisable medical devices (diagnostic and
therapeutic) that fulfil all the mechanical,
therapeutic, stability and safety requirements.
Recent advances in issues of international co-
operation concerning active materials with the
setting up of specific forums like Scientific.net,
Biomat.net and others, are helping to disseminate
results and exchange opinions. However, it would be
BIODEVICES BASED ON SHAPE-MEMORY POLYMERS - Current Capabilities and Challenges
143

of great interest to create a specific forum on shape-
memory polymers and their applications, where
researchers, universities and enterprises could make
contact in order to fit technological supply with
market requirements, which is of particular
importance for the Medical Industry.
While the new capabilities brought by these
materials give rise to expectations that many medical
devices will become more effective, considerably
more effort still needs to be put into research and
development, so as to obtain robust and effective
actuators based on these materials.
REFERENCES
Lendlein, A., Kelch, S., 2002. Shape-Memory Polymers.
Angewandte Chemie International.
Lendlein, A., Kelch, S., 2005. Shape-Memory Polymers.
Encyclopedia of Materials: Science and Technology.
Liu, C., Mather, P., 2007. Review of progress in Shape-
Memory Polymers. Journal of Materials Chemistry.
Wache, H., 2003. Development of a polymer stent with
shape-memory effect as a drug delivery system.
Journal of Materials Science – Materials in Medicine.
Lendlein, A., Kelch, S., 2005. Shape-Memory Polymers as
Stimuli-sensitive Implant Materials. Clinical
Hemorheology and Microcirculation.
Lendlein, A., Langer, R., 2002. Biodegradable, Elastic
Shape-Memory Polymers for Potential Biomedical
Applications. Science.
Wilson,T., et al., 2006. Shape-memory Polymer
Therapeutic Devices for Stroke. Lawrence Livermore
National Laboratory.
Small, W., et al., 2005. Laser-activated Shape-Memory
Polymer Intravascular Thrombectomy Device. Optics
Express.
Yakacki, C.M. et al., 2007. Unconstrained Recovery of
Shape-Memory Polymers Networks for
Cardiovascular Applications. Biomaterials.
Yakacki, C.M. et al., 2008. Deformation Limits in Shape-
Memory Polymers. Advance Engineering Materials.
Gall, K.; Kreiner, P. et al., 2004. Shape-memory Polymers
for MEMS Systems. Journal of
Microelechtromechanical Systems.
Díaz Lantada, A., Lafont, P. et al., 2008. Treatment of
Mitral Valve Insufficiency by Shape-Memory Polymer
Based Active Annuloplasty. Biodevices 2008 –
International Conference onBiomedical Electronics
and Devices. INSTICC Press.
Lafont, P., Díaz Lantada et al., 2006. Patent Document
P200603149: Sistema activo de anuloplastia para
tratamiento de la insuficiencia mitral y otras patologías
cardiovasculares. Oficina Española de Patentes y
Marcas.
Bellin, I., et al., 2006. Polymeric Triple-Shape Materials.
Proceedings of the National Academy of Sciences.
Volk, B. et al., 2005. Characterization of Shape-memory
Polymers. NASA Langley Research Centre. Texas
A&M University.
Tobushi, H. et al., 2008. Shape Recovery and
Irrecoverable Strain Control in Polyurethane Shape-
Memory Polymer. Science and Technology of
Advanced Materials.
Liu, C. and Mather, P., 2002. Thermomechanical
Characterization of a Tailored Series of Shape-
memory Polymers. Journal of Applied Medical
Polymers.
Liu, Y. et al., 2003. Thermomechanical Recovery
Couplings of Shape-memory Polymers in Flexure.
Smart Materials and Structures.
Huang, W. and Lee, C., 2006. Thermomechanical
Behaviour of a Polyurethane Shape-memory Polymer
Foam. Journal of Intelligent Material Systems and
Structures.
Liu, Y. et al., 2006. Thermomechanics of shape-memory
polymers: Uniaxial experiments and constitutive
modelling. Int. Journal of Plasticity.
Yakacki, C.M. et al., 2007. Unconstrained recovery of
shape-memory polymer networks for cardiovascular
applications. Biomaterials.
Harrysson, O., et al., 2007, Custom-designed orthopaedic
implants evaluated using FEM analysis of patient
computed tomography data. BMC Musculoskeletal
Disorders.
Paumier, G., et al., 2008. Thermoresponsive Polymer-
Based Microdevice for Nano-Liquid Chromatography.
Biodevices 2008 – Int. Conference on Biomedical
Electronics and Devices. INSTICC Press.
Lendlein, A., et al., 2005. Light-induced shape-memory
polymers. Nature.
Buckley, P., et al., 2006. Inductively Heated Shape-
memory Polymer for the Magnetic Actuation of
Medical Devices. IEEE Transactions on Biomedical
Engineering.
Conti, S., et al., 2007. Modelling and Simulation of
Magnetic Shape-Memory Polymer Composites.
Journal of Mechanics and Physics of Solids.
Yang, B., et al., 2004. On the effects of moisture in a
polyurethane shape-memory polymer. Smart Materials
and Structures.
Yakacki, C.M. et al., 2008. Cytoxicity and
Thermomechanical Behaviour of Biomedical Shape-
Memory Polymer Networks Post-sterilization.
Biomedical Materials.
Cabanlit, M., et al., 2007, Polyurethane Shape-Memory
Polymers Demonstrate Functional Biocompatibility In
Vitro. Macromolecular Bioscience.
Sokolowsky, W., et al., 2007, Medical Applications of
Shape-memory Polymers. Biomedical Materials.
Bar-Cohen, Y., 2006. Artificial Muscles using
Electroactive Polymers (EAP): Capabilities,
Challenges and Potential. SPIE Press.
BIODEVICES 2009 - International Conference on Biomedical Electronics and Devices
144
