
SURFACE MODIFICATION OF DENTAL DEVICES
Surface Analysis of Plasma-based Fluorine and Silver Ion Implanted & Deposited
Acrylic Resin
Yukari Shinonaga, Kenji Arita and Milanita E. Lucas
Department of Pediatric Dentistry, Institute of Health Biosciences, The University of Tokushima Graduate School
Tokushima, Japan
Keywords: Surface modification, Dual ion implantation & deposition, Dental device, Fluorine, Silver, XPS, Contact
angle, Surface free energy, Abrasion.
Abstract: The aim of this study was to modify acrylic resin (PMMA) with fluorine (F) and silver (Ag) dual ions by a
hybrid process of plasma-based ion implantation & deposition (PBII-D), and to enable the surface of the
devices inhibition of bacterial adhesion. The surface characteristics, hydrophobic property and brushing
abrasion resistance were evaluated by XPS analysis, contact angle measurement and brushing abrasion test.
F and Ag were implanted-deposited and have formed carbon-fluoride and Ag-deposited layer on the surface
of the PMMA plate. The contact angle of F+Ag implanted-deposited PMMA was increased compared with
non-treated control and F only deposited PMMA. After 60,000 brushing strokes, the contact angle of
modified PMMA remained to be higher than that of the control PMMA. This study indicated that F+Ag
implantation-deposition has improved the hydrophobic property of acrylic resin and was sustained even
after routine tooth brushing.
1 INTRODUCTION
Bacterial colonization and subsequent device
infection are common complications of medical and
dental devices (Gristina, 1987). Especially, acrylic
base plates for prosthodontics, orthodontics and
pedodontics are exposed to oral microbial flora to
include bacteria, viruses, and fungi, and are
susceptible to adhesion of bacterial plaque. Oral
bacteria can be released from denture plaque into
salivary secretions and then aspirated into the lower
respiratory tract causing pneumonia (Sumi, 2007).
Among patients, the most general method for the
removal of denture plaque is brushing. However,
effective plaque removal requires a degree of
manual dexterity that is often lacking especially
among elderly and individuals with disabilities.
Therefore, it is important to modify the acrylic resin
denture surface to enable inhibition of oral bacterial
adhesion.
Plasma-based ion implantation (PBII) is a
promising method for the surface modification of
three-dimensional materials (Conrad, 1987). In
particular, the ion deposition with simultaneous ion
implantation (plasma-based ion implantation and
deposition: PBII-D) is desirable for efficient
processing and has an advantage over conventional
methods (Kuze, 2002).
Recently, several researchers have carried out the
surface modification using fluorine (F) ion and
found it to be a useful means of inhibiting bacterial
adhesion (Zhao, 2007 and Nurhaerani, 2007). In
addition, it is well known that silver (Ag) possesses
the antibacterial property without any toxic effects in
comparison to other heavy metal ions. We
developed the new technology to simultaneously
implant and deposit both F and Ag ions into dental
and medical devices using PBII-D. The aim of this
study was to examine the effectiveness of both F and
Ag ions implanted-deposited into acrylic resin by
evaluating the surface characteristics and brushing
abrasion resistance.
2 MATERIALS AND METHODS
2.1 Preparation of Specimens
Poly methyl methacrylate (PMMA) (Clarex 000,
Nitto Jushi Kogyo, Co., Ltd., Tokyo, Japan) plates
157
Shinonaga Y., Arita K. and E. Lucas M. (2009).
SURFACE MODIFICATION OF DENTAL DEVICES - Surface Analysis of Plasma-based Fluorine and Silver Ion Implanted & Deposited Acrylic Resin.
In Proceedings of the International Conference on Biomedical Electronics and Devices, pages 157-160
DOI: 10.5220/0001424101570160
Copyright
c
SciTePress
with measurements of 10mm×10mm×1mm were
used. The PMMA plates were modified by plasma-
based ion implantation-deposition equipment at
Plasma Ion Assist Co., Ltd., Kyoto, Japan. Fluoride
gas used for F ion implantation-deposition was
perfluoropropane (C
3
F
8
). For Ag ion implantation-
deposition, a 99.8 % Ag mesh cover was set 10mm
above the plates and sputtered by C
3
F
8
gas. The
conditions of plasma-based F and Ag dual ion
implantation-deposition are shown in Table 1.
Table 1: The condition of fluorine and silver ion
implantation-deposition into PMMA plates.
Group Conditions
Implantation
process
Deposition
process
Control
Voltage (keV)
Time (min)
Ag mesh
0
0
unused
0
0
unused
F deposited
Voltage (keV)
Time (min)
Ag mesh
0
0
unused
-0.5
60
unused
F+Ag
implanted-
deposited
Voltage (keV)
Time (min)
Ag mesh
-5
30
used
-0.5
60
used
2.2 Surface Shemical Analysis by XPS
The surfaces of the control, F deposited and F+Ag
implanted-deposited PMMA were characterized by
X-ray photoelectron spectroscopy (XPS). XPS
spectra were obtained using an X-ray photoelectron
spectrometer (ESCA-850, Shimadzu Co., Kyoto,
Japan) with Al-Kα radiation operated at 30 mA
current and 7 kV accelerating voltage. Specifically,
depth profile analysis for the F+Ag implanted-
deposited PMMA was performed using Ar etching
under the pressure of 5×10
-4
Pa. The Ar etching rate
was approximately 6 nm/min on Ag.
2.3 Contact Angle Measurements
Specimens were ultrasonically cleaned in distilled
water for 10 minutes, and then dried at room
temperature before contact angle measurement.
Static contact angle measurements were conducted
by the sessile drop technique using a contact angle
meter (CA-DT, Kyowa Kaimenkagaku Co. Ltd.,
Saitama, Japan) with three test liquids: distilled
water, diiodomethane and ethylene glycol at room
temperature. One point per specimen was measured
(N=5/group, i.e. 5 points/group). The surface free
energies of specimens were calculated from the
contact angles with the three test liquids (Liu, 2005).
2.4 Brushing Abrasion Test
The brushing abrasion test machine (MANA-63S,
MASUDA Co., Osaka, Japan) was used. A
commercial toothbrush (Dr. Bee Young ⅡS, Bee
Brand Medico Dental Co., Ltd., Osaka, Japan) was
attached to the toothbrush holder in contact with the
F deposited and F+Ag implanted-deposited PMMA
set on the sample holder. Distilled water without
dentifrice was then poured into the vessel, and the
machine was run at 80 rpm with a 200 g load. The
water contact angle measurements of the F deposited
and F+Ag implanted-deposited PMMA were done
every 10,000 strokes up to 60,000 strokes. In the
control PMMA, the contact angle was measured
without brushing. Five points per specimen were
measured.
2.5 Statistical Analysis
The results of the contact angle measurements
before and after the brushing abrasion test were
expressed as the mean ± standard deviation. The
data were analyzed using one-way ANOVA and
Scheffe’s multiple comparison tests (α = 0.05).
3 RESULT
3.1 XPS Analysis
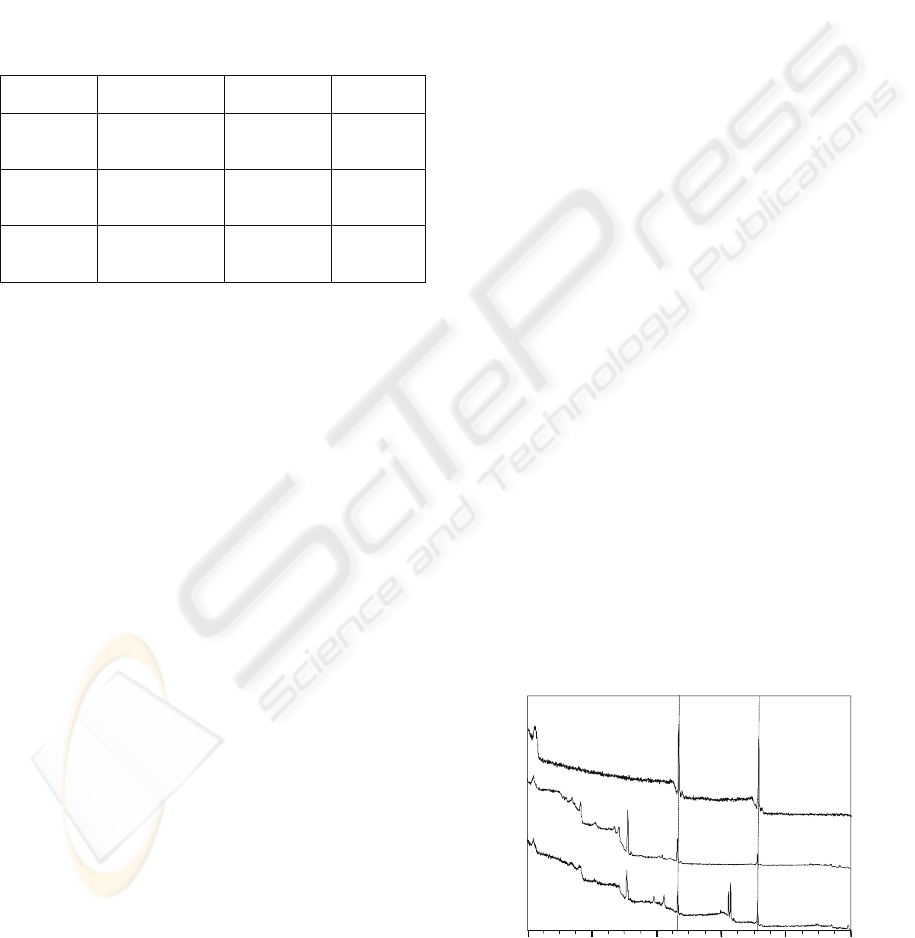
XPS wide-scan spectra of the control, F deposited
and F+Ag implanted-deposited PMMA are shown in
Figure 1. The peaks of C1s and O1s were detected in
the wide-scan spectrum of the control PMMA.
Moreover, F1s peak appeared on the F deposited
PMMA surface; and F1s, Ag3d and Ag3p peaks
appeared on the F+Ag implanted-deposited PMMA
surface. The F1s and Ag3d XPS depth profiles of the
800 600 400 200 0
Binding Energy (eV)
1000
F+Ag
Implanted/deposited
F dposited
Control
F1s
F1s
Ag3p
Ag3d
O1s
C1s
Count (arbitrary unit)
Figure 1: XPS wide-scan spectra of PMMA specimens.
BIODEVICES 2009 - International Conference on Biomedical Electronics and Devices
158
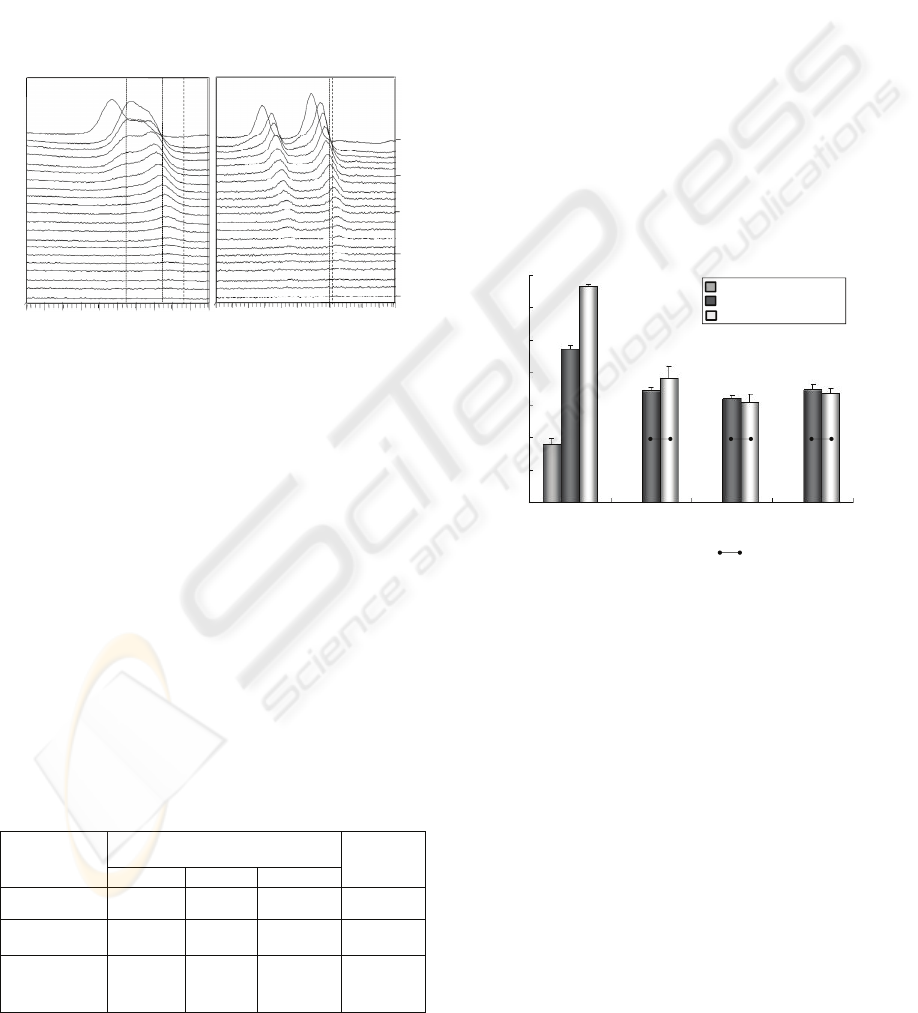
F+Ag implanted-deposited PMMA are shown in
Figure 2. The chemically shifted peaks of F1s from
surface to 7 minutes Ar etching depth are observed
in the higher binding energy region (689-690 eV).
The higher values was close to the reported binding
energy of p-(CF
2
=CF
2
) (689 eV) (Briggs and Seah,
1990).The F1s peak decreased as the Ar etching
increased and was not detected after 15 minutes of
Ar etching. Ag3d peak was also detected after Ar
etching for about 15 minutes in Ag3d depth profile.
In F1s and Ag 3d depth profiles, the peaks of AgF
were not detected.
700 696 692
688
684
680
Binding Energy (eV)
380
376
372
368
364
360
Ar etching time (min)
0
5
10
15
20
Binding Energy (eV)
(A) F1s
(B) Ag3d
p-(CF
2
=CF
2
)F
Ag
AgF
AgF
Count (arbitrary unit)
Figure 2: F1s (A) and Ag3d (B) XPS depth profiles of the
F+Ag implanted-deposited PMMA. Dashed lines show the
binding energy value of p-(CF
2
=CF
2
): 689.0 eV, F: 685
eV and AgF: 682.7 eV in the F1s depth profile (A), and
Ag: 368.2 eV and AgF: 367.7 eV in the Ag3d depth
profile (B). The values were based from Briggs and Seah,
(1990).
3.2 Surface Energy Analysis
The contact angles of three test liquids: distilled
water (W), diiodomethane (Di) and ethylene glycol
(EG) and the calculated surface free energy values
of control, F deposited and F+Ag implanted-
deposited PMMA are shown in Table 2. The contact
angles of all test liquids on the F deposited and
F+Ag implanted-deposited PMMA were
significantly higher than that of the control PMMA
Table 2: Contact angle and surface free energy of PMMA
specimens.
(p<0.01). Moreover, the contact angle of all test
liquids on the F+Ag implanted-deposited PMMA
was higher than that of the F deposited PMMA
(p<0.001). The surface free energy of the F+Ag
implanted-deposited PMMA was lower than that of
the control and F deposited PMMA.
3.3 Brushing Abrasion Test
In the F deposited and F+Ag deposited PMMA, the
contact angles after 10000, 30000 and 60000
brushing strokes were significantly lower than
before brushing (p<0.001). The contact angle of the
F+Ag implanted-deposited PMMA was not
significantly different compared with that of the F
deposited PMMA for the same number of brush
strokes. However, the contact angles of the F
deposited and F+Ag implanted-deposited PMMA
after the brushing abrasion test resulted in a
significantly higher contact angle compared to that
of the control PMMA (p<0.001).
0 10000 30000
60000
Number of brush strokes
50
60
70
80
90
100
110
Contact angle (degree)
120
Control
F deposited
F+Ag implanted/deposited
:not significant
ANOVA/Scheffe, α=0.05
Figure 3: Contact angles of distilled water on the F
deposited and F+Ag implanted-deposited PMMA
before/after the brushing abrasion test. Horizontal lines
indicate no significant differences (ANOVA/ Scheffe,
α=0.05).
4 DISCUSSIONS
In this study, we have attempted to modify the
surface of PMMA by the simultaneous F and Ag ion
implantation-deposition by PBII-D process.
The results have shown that F and Ag were
detected on the surface of the F+Ag implanted-
deposited PMMA by XPS analysis (Figure 1). The
existence of carbon-fluoride complexes, such as p-
(CF
2
=CF
2
), and Ag were also detected on the F+Ag
implanted-deposited PMMA surface. Moreover, F
and Ag were deposited on the surface and implanted
Group
Contact angle θ (degree)
Surface free
energy
(mJ/m
2
)
θ
W
θ
Di
θ
EG
Control 64.4° 36.3° 50.8° 44.05
F deposited 100.0° 52.2° 62.7° 33.77
F+Ag
implanted/
deposited
123.9° 82.3° 95.2° 20.65
SURFACE MODIFICATION OF DENTAL DEVICES - Surface Analysis of Plasma-based Fluorine and Silver Ion
Implanted & Deposited Acrylic Resin
159

to about 90 nm depth (Figure 2). All these suggested
that both F and Ag ion implantation-deposition
method by PBII-D was applicable to PMMA. In ion
implantation-deposition of insulating materials such
as PMMA, electric charge-up could be a serious
problem, which damages the specimens. In this
study, it was thought that Ag mesh was used not
only for providing Ag ions but also for decreasing
the electric charge-up on the specimens by providing
electrons.
It was reported that some properties are specific
to the inert surface, such as the surface free energy,
surface charge, hydrophobic property, surface
roughness and surface chemistry (Perini, 2006). One
approach in the attempt to reduce the bacterial
colonization is to modify the surface free energy and
chemistry. The contact angle is characteristic of the
surface energy of a solid surface, and has been used
for determining the wettability and hydrophobic
property of various solid materials. Bacterial
adhesion is energetically unfavourable, if the solid
surface free energy is less than 50 mJ/m
2
(Busscher,
1984). In this study, the surface free energy of the
F+Ag implanted-deposited PMMA was 20.65 mJ/m
2
(< 50mJ/m
2
), which may imply potential inhibition
of bacterial adhesion by hydrophobic mechanism. In
addition, the negative relationship between the
contact angle and the bacterial adhesion property
was reported (Zhao, 2007, and Nurhaerani, 2007). In
the present study, the contact angle of the F+Ag
implanted-deposited PMMA after 60,000 brushing
strokes was significantly higher than that of the
control PMMA. Kanter et al. (1982) estimated that
20,000 brushing strokes were the equivalent to
approximately 5 years of brushing. The present
study confirmed that the high contact angle of the
F+Ag implanted- deposited PMMA could remain
after the equivalent of 15 years of brushing with a
toothbrush. These results suggested that dual F and
Ag implantation-deposition could possibly inhibit
the bacterial adhesion to the PMMA devices by
increasing the contact angle and decreasing the
surface free energy.
These results suggested that both F and Ag ion
implantation-deposition by PBII-D process was the
superior surface modification method for acrylic
materials to inhibit bacterial adhesion.
5 CONCLUSIONS
In this study, PMMA plates were simultaneously
implanted-deposited with both F and Ag ions by a
hybrid process of PBII-D. The F+Ag implanted-
deposited PMMA surface has obtained low surface
free energy (20.65 mJ/m
2
) and the presence of
carbon-fluoride complexes and Ag on the surface
was indicated. Moreover, due to the presence of both
F and Ag ions, the hydrophobic properties remained
after brushing with a toothbrush. These results
suggested that both F and Ag ion implantation-
deposition by PBII-D has the potential to give the
medical and dental devices antibacterial qualities.
ACKNOWLEDGEMENTS
This study was supported by a Grant-in-Aid for
Scientific Research (C) (No. 20592300) from Japan
Society for the Promotion of Science (JSPS).
REFERENCES
Briggs, D., Seah, MP., 1990. Practical surface analysis 2
nd
edition., Volume 1 Auger and X-ray Photoelectron
spectroscopy, 595-634.
Busscher, HJ., Weerkamp, AH., Mei, HC., Pelt, AWJ.,
Jong, HP., 1984. Appl. Environ. Microbiol. 980-983.
Conrad, JR., Radtke, JL., Worzala, FJ., 1987. J. Appl.
Phys. 62, 4591-4596.
Grastina, A. G., 1987. Science.237, 1588-1595.
Kanter, J., Koski, RE., Martin, D., 1982. J. Prosthet. Dent.,
47, 505-513.
Kuze, E., Teramoto, T., Yukimura, K., Maruyama, T.,
2002. Surf. Coat. Technol, 577-581.
Liu, Y., Zhao, Q., 2005. Biophys. Chem. 39-45.
Nurhaerani., Arita, K., Shinonaga, Y., Nishino, M., 2007.
Dent.Mater. J, 684-692.
Perini, CI., Zhao, Q., Liu, Y., Abel, E., 2006. Colloids.
Surf. B: Biointerfaces., 143-147.
Sumi, Y., Miura, H., Michiwaki, Y., Nagaosa, S., Nagaya,
M., 2007.. Arch. Gerontol. Geriatr. 44, 119-124.
Zhao, Q., Liu, Y., Wang, C., Wang, S., Peng, N., Jeynes,
C., 2007. Appl. Surf. Sci, 8674-8681.
BIODEVICES 2009 - International Conference on Biomedical Electronics and Devices
160
