
HAND-HELD LUMINOMETER WITH ECL-BASED BIOSENSOR
FOR LACTATE DETERMINATION
A. Martinez-Olmos, A. J. Palma
Department of Electronics and Computer Technology, University of Granada, Granada 18071 Spain
J. Ballesta-Claver, M. C. Valencia-Miron and L. F. Capitan-Vallvey
Department of Analytical Chemistry, University of Granada, Granada 18071, Spain
Keywords: Electrochemiluminescence, Portable instrumentation, Potentiostat, Biosensor, Screen-printed device,
Lactate determination, Saliva.
Abstract: A new hand-held luminometer for electrochemiluminescence (ECL)-based one-shot biosensor for lactate is
described. The lactate recognition system is based on lactate oxidase and the transduction system consists of
luminol, all reagents immobilized in a Methocel membrane. The measurement of ECL from a screen-
printed electrode by a portable instrument designed and developed by the authors makes it possible to
determine lactate concentration. The compositions of the membrane and reaction conditions have been
optimized to obtain adequate sensitivity. The one-shot biosensor responds to lactate rapidly, with the typical
ECL acquisition time being 3 min, with a linearized dependence whose dynamic range was from 9·10
-6
to
2·10
-3
M, a detection limit of 2.4·10
-6
M and a sensor-to-sensor reproducibility (relative standard deviation
RSD) around 10 % at the medium level of the range. The performance of the ECL one-shot biosensor and
portable instrument was tested for the non invasive analysis of lactate in saliva, validating the results against
a reference procedure.
1 INTRODUCTION
L(+)-Lactate is produced in the anaerobic
metabolism of glucose and its determination is of
interest in clinical analysis, sports medicine and food
analysis. The measurement of lactate is routinely
performed with liquid chromatography (Ewaschuk,
2002), spectrophotometry (Benthin, 1991) and
amperometry, mainly with enzymatic electrodes
(Compagnone, 1998). Lactate analysis is needed in
different fields such as food, sports medicine and
health. In foodstuffs, lactate is produced by bacterial
fermentation and is an essential component related
to the manufacture of cheese, yoghurt, milk, etc.,
thus monitoring lactate being an important quality
control parameter.
Rapid evaluation of lactate levels can be
performed with one-shot sensors, that mainly are of
electrochemical type (Klonoff, 2003).
Chemiluminescence measurement could be of
interest for one-shot sensor design due to its good
sensitivity and selectivity, although the use of
electrochemiluminescence (ECL) could offer clear
advantages for controlling the chemical system
(Richter, 2004).
The use of screen printing technologies, with
benefits for low cost and mass production, appears
to be interesting to develop ECL one-shot biosensors
next to portable instrumentation.
The presented lactate biosensor is based on its
enzymatically catalyzed oxidation and back ECL
transduction using luminol (L) according to:
Lactate + O
2
⎯⎯→⎯
LOD
Piruvate + H
2
O
2
L – e → L* (electrode)
H
2
O
2
+ L* → 3-aminophthalate + N
2
+ H
2
+ hν
2 MATERIALS
The one-shot biosensor is formed by a screen printed
electrode where the working electrode contains all
343
Martínez-Olmos A., J. Palma A., Ballesta-Claver J., C. Valencia-Miron M. and F. Capitan-Vallvey L. (2009).
HAND-HELD LUMINOMETER WITH ECL-BASED BIOSENSOR FOR LACTATE DETERMINATION.
In Proceedings of the International Conference on Biomedical Electronics and Devices, pages 343-346
DOI: 10.5220/0001432703430346
Copyright
c
SciTePress
needed reagents immobilized in a Methocel
membrane. The sensing layer was spotted as
solutions of luminol, lactate oxidase (LOD), BSA,
sodium chloride and 8.8 pH phosphate buffer in
aqueous solution of Methocel. The screen-printed
electrode was covered by a thick overlapping plastic
layer with a 40-μl volume hole in the electrode area
to place the sample.
The characterization of the screen-printed
electrodes was investigated through cyclic
voltammetry. The ECL measurements were
performed measuring the light intensity emitted
while triggering the ECL reaction.
3 INSTRUMENTATION
After describing the biosensor, a portable instrument
based in ECL detection and designed and fabricated
for this sensor will be detailed. The prototype has
been applied to lactate concentration determination
in saliva.
The system is based on a solid-state photodiode
detector, which generates an electric current
proportional to the ECL being measured. In Figure 1
the general scheme of the system is presented.
Figure 1: Block diagram of the instrument.
The light resulting from the ECL reaction on the
sensor excites the photodiode detector (PD) (S1227-
66BR, Hamamatsu Photonics) which generates an
electric current in response. The analog circuit for
measuring this current is shown in Figure 3. ECL
is
produced when a voltage difference of 0.5V is
applied between the reference and the working
electrodes in the biosensor. This polarization of the
sensor is carried out using a programmable built-in
potentiostat, which is designed to apply variable
voltage steps between the sensor electrodes.
A detailed schematic of the potentiostat is
presented in Figure 2. In this circuit, a serial digital-
to-analog converter (DAC) (DAC8574, from Texas
Instruments) generates an analog voltage from a 16-
bit digital word sent by the microcontroller, which is
the input value to the potentiostat. If the
electrochemical cell is full of a conductive liquid,
the operational amplifiers A1 and A2 form a non-
inverting amplifier stage with gain 2. This
establishes a voltage at the working electrode that is
double than the input voltage value. The voltage at
the reference electrode is forced to virtual ground
because of the negative feedback of the operational
amplifier A3. Thus, the voltage difference between
the working and the reference electrodes is simply
twice the analog value generated by the DAC.
Figure 2: Potentiostat circuit.
The voltage at the working electrode is
monitored directly by the microcontroller, since its
function is to detect when the test drop is deposited
on the biosensor. This event causes the start of a
time count, thus allowing a precise determination of
the time elapsed between the drop deposition and the
beginning of the measurements. Therefore, a perfect
timing control of the measurement procedure can be
achieved.
Figure 3: Measurement circuit.
The current is converted into voltage trough the
current-to-voltage converter formed by the
operational amplifier A1 (TLC277, from Texas
Instruments). This device has a feedback network
BIODEVICES 2009 - International Conference on Biomedical Electronics and Devices
344
compounded by the resistors R
f
, R
1
and R
2
, which
results in a high gain conversion, being the output
voltage of A1:
(
)
12 12 2
2
() ( )
pd f f
o
VR R I RR R RR
V
R
+
+− ++
=
(1)
where V
+
is the voltage at the non-inverting input of
the operational amplifier A1, and I
pd
is the current
generated by the photodiode. The voltage V
+
takes a
value of few mV and can be changed through the
variable resistor R
var
. The effect of this parameter is
to compensate the input offset voltage of the
operational amplifier, which can have a high
influence on the output because of the great gain of
this stage.
Under ideal conditions (V
+
=0) and assuming
R
f
>> R
2
, Equation (1) can be rewritten as:
1
2
1
opdf
R
VIR
R
⎛⎞
=+
⎜⎟
⎝⎠
(2)
Selecting a high value for R
f
and making R
1
>R
2
,
a gain factor of 10
11
– 10
13
V/A can be achieved.
The output voltage of A1 is conditioned using
two parallel stages , formed by the operational
amplifiers A2 and A3. In each stage, the signal is
firstly filtered through a RC low-pass filter. The
operational amplifier A2 acts as a buffer, whereas
A3 amplifies the output voltage of the I/V converter
before sending it to the microcontroller. In this way,
the μC receives two signals, one corresponding to
the filtered output of the first stage, and another that
is an amplification of this last one. The purpose of
having two different channels for measuring the
same signal is to expand the range of lactate that can
be analysed.
The outputs of A2 and A3 are connected directly
to the microcontroller (μC) (PIC18F2550,from
Microchip Inc.), which uses an internal 10-bit
analog-to-digital converter to alternatively sample
these signals at high frequency. A serial EEPROM
module (24LC512, from Microchip Inc.) of 512kbit
is used to store the sampled data. Finally, once the
calibration function (see next section) programmed
in the microcontroller is applied, results are sent to
the LCD display (Figure 1). All electronic circuitry
is included in an enclosure with optical, magnetic
and electrical shielding.
Moreover, control software written in Visual
Basic allows the user to optionally communicate the
instrument with a computer via an USB port to
receive the data for further analysis.
Main advantages of our design lie on portability,
low cost because of the use of a photodiode instead
of a costly or bulky photomultiplier, and the use of
non invasive samples. Most commercial portable
lactate meters use blood or serum for lactate analysis
(www.lactate.com, Poscia, 2005).
4 BIOSENSOR COMPOSITION
AND MEASUREMENT
CONDITIONS
Composition of sensing membrane was optimized in
terms of type and concentration of membrane
polymer, supporting electrolyte, pH and buffer,
luminol concentration, enzyme units, and BSA
concentration.
0
200
400
600
0 5 10 15 20
t / s
ECL relative sign
a
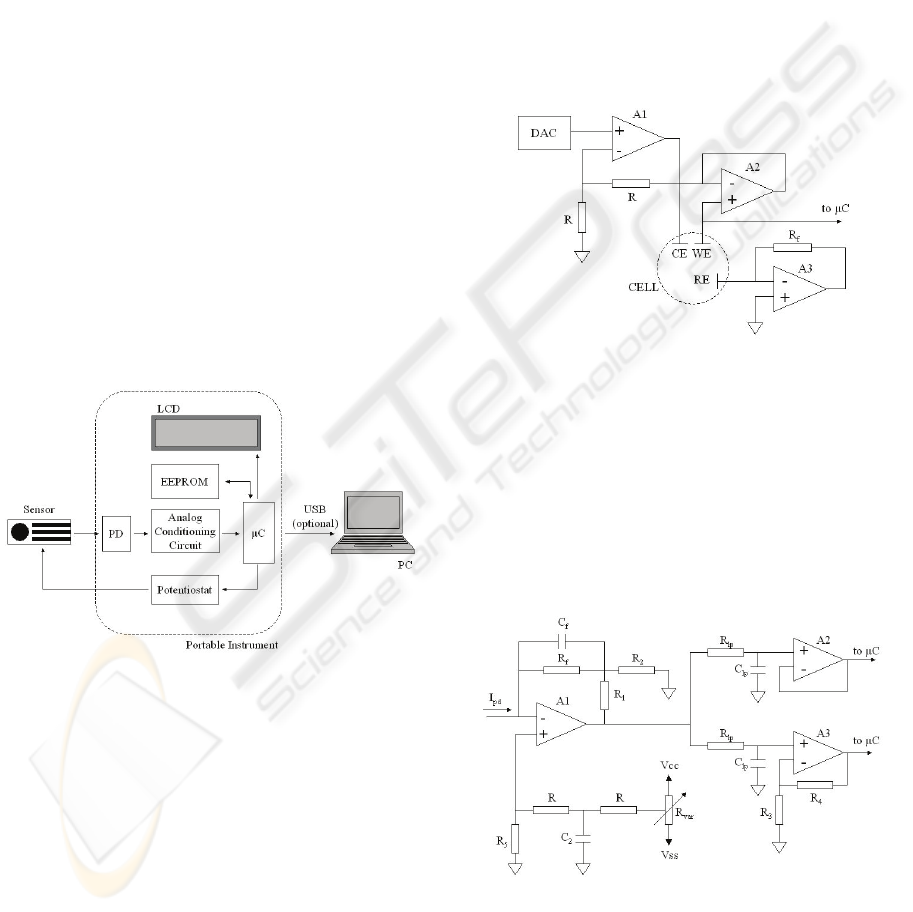
Figure 4: Relative ECL lactate signals.
Different types of ECL analytical signals were
studied using the instrument described in the
previous section in order to obtain an analytical
parameter for lactate concentration. The intensity of
the collected light, resulting from the reaction on the
sensor, did not show a direct relationship with the
lactate concentration, as can be seen in Figure 4,
where three steps at fixed potential were applied to
the same problem drop. The intensity of the light is
increased with successive potential steps. From these
current pulses, a kinetic signal derived from the
relative increase of the signal was chosen for the
measurement of the lactate concentration, since it
remains stable for different excitation pulses. The
measurement conditions studied were: a) applied
potential (0.5 V); b) waiting time before the first
pulse (3 min); c) time between pulses, being 10 s for
better sensitivity; d) pulse time with 1 s as best for
sensitivity and time of analysis.
The sample volume in the screen-printed device
was spotted with a micropipette. From the influence
of sample volume, studied between 20 and 40 µL.
Low volumes have high ECL signals but poor
repeatability. The signal and the standard deviation
decrease when the volume increases. The reason of
this behaviour is that low volumes don’t cover the
HAND-HELD LUMINOMETER WITH ECL-BASED BIOSENSOR FOR LACTATE DETERMINATION
345
three electrodes totally, specially the reference
electrode, making then oscillating potentials.
Therefore a volume of 35 uL for the test drop has
been selected, wich provides a good precision (5-8
% RSD).
5 ANALYTICAL
CHARACTERIZATION
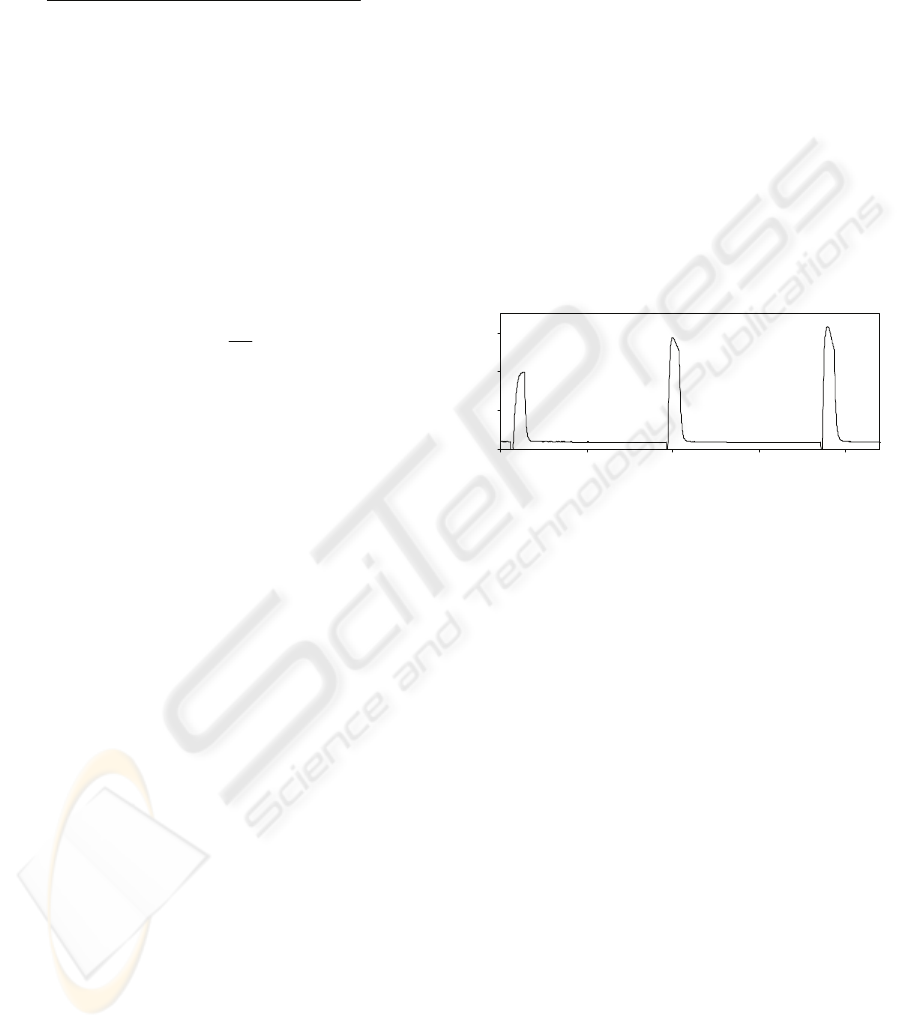
The dependence of ECL signal with lactate
concentration was studied between 10
-7
and 10
-3
M
obtaining a linear relationship between 10
-6
M and
2·10
-4
M (Figure 6).
0
200
400
600
800
1000
0.00 0.50 1.00 1.50 2.00 2.50
lactate·10
4
M
CL relative signal
Figure 6: Linear calibration of lactate biosensor.
Table 1 shows some analytical parameters of
biosensor for lactate.
Table 1: Analytical characteristics.
Parameter Value
Linear ran
g
e (M) 9·10
-
6
–
2·10
-
4
Inte
r
cep
t
1.87
Slope 786150
r
2
0.9956
Detection limit (M) 2.4·10
-
6
RSD blank (%) 8.2 %
RSD lactate (%) 8·10
-
4
M 10.3 %
This biosensor was applied to lactate
determination in saliva obtaining good preliminary
results.
6 CONCLUSIONS
A new hand-held luminometer for
electrochemiluminescence (ECL)-based one-shot
biosensor for lactate is described. Exciting the
sample volume with consecutive steps of 0.5V and
measuring the light resulting from the reaction on
the sensor provides a method for the evaluation of
the lactate concentration. A good linear calibration
in the range of 9·10
-6
to 2·10
-4
M has been achieved,
what indicates that lactate in saliva, rather than
lactate in blood can be measured. This fact results in
a better behaviour of the prototype than the existing
commercial instruments, because of its minimal
invasive requirements for the measurement of lactate
in humans. The use of a solid-state photodiode as
optical detector, instead of a photomultiplier, which
is the usual technique in available commercial ECL
systems, as well as the integration of the potentiostat
and the measurement electronics in the same design
has allowed a low cost and compact instrument.
ACKNOWLEDGEMENTS
This work is supported by the Ministerio de
Educación y Ciencia, Dirección General de
Investigación (Spain), under projects CTQ2005-
09060-C02-01 and CTQ2005-09060-C02-02, and by
the Junta de Andalucía under project P06-FQM-
01467.
REFERENCES
J. B. Ewaschuk, G. A. Zello, J. M. Naylor, and D. R.
Brocks, J. Chromatog. B, 781 (2002) 39-56.
S. Benthin, J. Nielsen, and J. Villadsen, Anal. Chim. Acta,
247 (1991) 45-50.
D. Compagnone, D. Moscone, and G. Palleschi, Recent
Res. Devel. in Pure & Applied Anal. Chem., 1 (1998)
73-86.
D. C. Klonoff, Diabetes Tech. Therapeut., 5 (2003) 929-
931.
M. M. Richter, Chemical Rev., 104 (2004) 3003-3036.
www.lactate.com
A. Poscia, D. Messeri, D. Moscone, F. Ricci, and F.
Valgimigli, Biosensors and Bioelectronics 20 (2005)
2244-2250
BIODEVICES 2009 - International Conference on Biomedical Electronics and Devices
346
