
SELECTIVE OSTEOBLASTIC CELL MICRO-ARRAYS ON
DIAMOND FILMS
Bohuslav Rezek, Lenka Michalíková, Egor Ukraintsev, Alexander Kromka
Institute of Physics, Academy of Sciences of the Czech Republic, Cukrovarnicka 10, Prague 6, Czech Republic
Marie Kalbacova
Institute of Inherited Metabolic Disorders, 1st Faculty of Medicine, UK, Ke Karlovu 2, Prague 2, Czech Republic
Keywords: Cell adhesion, Proteins, Diamond, Biotechnology, Biosensors, Osteoblasts, Atomic force microscopy.
Abstract: Unique combination of chemical and biocompatible properties with semiconducting properties makes
diamond an attractive material for merging solid state and biological systems. Microscopic chemical
patterning of diamond films by hydrogen and oxygen surface atoms is used for self-assembly of human
osteoblastic cells into micro-arrays. The cell adhesion and assembly on the diamond is further controlled
and optimized by cell and protein (fetal bovine serum - FBS) concentration. The cells are characterized by
fluorescence microscopy of actin fibers and nuclei. The protein adsorption is studied by atomic force
microscopy (AFM). The cells are arranged into arrays on O-terminated patterns. The best cell selectivity is
achieved for the lowest cell concentrations of 2500 cells/cm
2
. Higher cell concentrations enable to colonize
unfavorable H-terminated regions due to mutual cell communication. Based on AFM, the proteins are
present on both H- and O-terminated surfaces, however, pronounced differences in the thickness, surface
roughness, morphology, and phase images indicate different conformation of the proteins and hence the cell
selectivity. There is no cell selectivity when no protein is supplemented in the medium. These results may
be applicable in tissue engineering, implants, bio-electronics, and biotechnology in general.
1 INTRODUCTION
Diamond is not only a gem but also a promising
technological material. Its properties include high
hardness, fracture toughness, low friction
coefficient, high Young modulus, increased wear
resistance and a variety of substrates onto which it
can be prepared (Potocky, 2007). Although diamond
is considered inert, its surface can be functionalized
by various atoms or molecules (Rezek, 2007a). This
gives rise to striking properties (Nebel, 2003).
For instance, electrical conductivity and electron
affinity of diamond are strongly influenced by the
O- or H-termination of the diamond surface
(Kawarada, 1996; Maier, 2001). The differences are
mainly caused by the surface dipole of C-H and C-O
bonds (Tachiki, 2003). O-terminated diamond is
highly resistive, whereas H-terminated surface
induces p-type surface conductivity even on an
undoped diamond (Maier, 2001). These features can
be applied for field-effect transistor (FET) devices
(Rezek, 2007b; Garrido, 2003).
Furthermore, O-terminated surfaces are
hydrophilic while H-terminated surfaces are
hydrophobic. H-terminated surfaces were thus found
less favorable for osteoblastic cell adhesion,
spreading and viability compared to O-terminated
surfaces (Kalbacova, 2007a). On the other hand, H-
terminated diamond surface is an ideal starting point
for covalent attachment of biomolecules (Yang,
2002). Chemical functionalization can also lead to
bio-passivation or bio-active properties (Bajaj,
2007).
Unique combination of the mechanical,
chemical, and biocompatible properties (Tang, 1995,
Kalbacova, 2007a) with semiconducting properties
makes diamond an attractive material for merging
solid state and biological systems (Yang, 2004;
Rezek, 2007b). Hence the hydrogen and oxygen
surface patterns are highly relevant for bio-
electronics as well as for tissue engineering.
347
Rezek B., Michalíková L., Ukraintsev E., Kromka A. and Kalbacova M. (2009).
SELECTIVE OSTEOBLASTIC CELL MICRO-ARRAYS ON DIAMOND FILMS.
In Proceedings of the International Conference on Biomedical Electronics and Devices, pages 347-354
DOI: 10.5220/0001434203470354
Copyright
c
SciTePress

Characterization of the interaction between cells and
surfaces is essential for cell-based biosensors,
engineered tissue therapies and the optimization of
implant biomaterials. Cells recognize their
environment and consequently start to change it by a
production of appropriate extracellular matrix
(ECM) proteins to form the basis for cell spreading,
increased adhesion and expression of differentiated
phenotypes (Schakenraad, 1989). This complex and
flexible process is dependent on culture conditions,
including the underlying substrate and the pre-
adsorbed protein layer.
Until now, the research on the cell-diamond
interfaces has been focused on overall homogeneous
surface terminations (Yang, 2004; Kalbacova,
2007a; Rezek, 2007c; Song, 2007). In this work we
show selective adhesion and arrangement of
osteoblast-like cells on NCD thin films that are
microscopically patterned with H- and O-terminated
regions (Michalikova, 2008). We control initial cell
density and serum concentration in medium
influencing cellular colonization of patterned
susbtrate. Furthermore, we employ atomic force
microscopy (AFM) to characterize the structural
properties of mediating proteins (fetal bovine serum,
a crucial component for the cell growth) adsorbed
onto the diamond micro-patterns. The data are used
to discuss the selectivity of the cell adsorption on the
patterns, i.e. to what degree the cell adhesion and its
selectivity is driven by serum adsorption and
conformation on H- and O-terminated surfaces or by
a direct effect of diamond surface dipoles on the
cells. We also provide perspectives for potential bio-
electronic applications.
2 MATERIALS AND METHODS
Diamond films are grown on (100) oriented silicon
substrates (13 mm in diameter, 500 µm thickness,
RMS roughness of < 0.6 nm) by microwave plasma
process using total gas pressure 50 mbar, substrate
temperature 800°C, 1% CH
4
in H
2
and total power
2.5 kW. This process results in a growth of
continuous, smooth and high quality nanocrystalline
diamond film (Potocky, 2007; Kromka, 2008). X-ray
photocurrent spectroscopy (XPS) detects that the
films are 95% pure diamond (Zemek, 2006). The
diamond film thickness is 300-400 nm. Average
crystal size is 50 nm, RMS roughness at 1x1 µm
2
area is 15-20 nm as measured by AFM using
standard silicon tips of nominal radius < 10 nm. The
silicon substrates are coated with NCD film on both
sides, silicon is thus hermetically encapsulated in the
diamond.
The diamond films were further chemically
cleaned in acids (97.5% H
2
SO
4
+ 99% powder
KNO
3
) at 200°C for 30 minutes. The surface was
then hydrogenated at 800°C for 10 minutes. NCD
films were lithographically processed to generate
alternating H- and O-terminated patterns of 30 to
200 µm widths. A positive photoresist ma-P 1215
(micro resist technology GmbH, Germany) was
applied. NCD films with lithography mask were
treated in oxygen radio-frequency plasma (300W
power, 3 minutes process time) to oxidize the
surface and hence to generate the hydrophilic
patterns. Then the sample was rinsed in a stripper,
de-ionized water and dried. This process removed
possible surface contamination (Rezek, 2006a). The
H-/O-termination quality was proved by a scanning
electron microscope (SEM; JEOL Superprobe 733).
Electronic measurements detected a surface
conductivity of 10
-5
S/sq on the H-terminated
surfaces (Kozak, 2008). Surfaces with O-termination
were highly resistive. The NCD samples were
sterilized in 70% ethanol for 10 minutes prior to cell
plating. The device concept is schematically shown
in Figure 1.
Figure 1: Schematic picture of silicon substrate
hermetically coated with NCD layer with stripe-like
patterns having hydrogen or oxygen surface termination.
Cell adhesion on the O-terminated region is also
schematically indicated.
SAOS-2 cells (human osteoblast-like cell line)
(DSMZ GmbH), were grown in McCoy’s 5A
medium (BioConcept) supplemented with heat
inactivated fetal bovine serum (FBS; Biowest) of
various concentrations (0-15%), penicillin (20 U/ml)
and streptomycin (20 µg/ml). Note that the SAOS-2
is a standard and well-defined cell line. Thus the
results can be compared between various series of
experiments as well as with reports in the literature.
Cells were plated at the densities of 2,500 and
10,000 cells/cm
2
using a droplet technique: substrate
surface was covered by 100 µl droplet of cell
BIODEVICES 2009 - International Conference on Biomedical Electronics and Devices
348
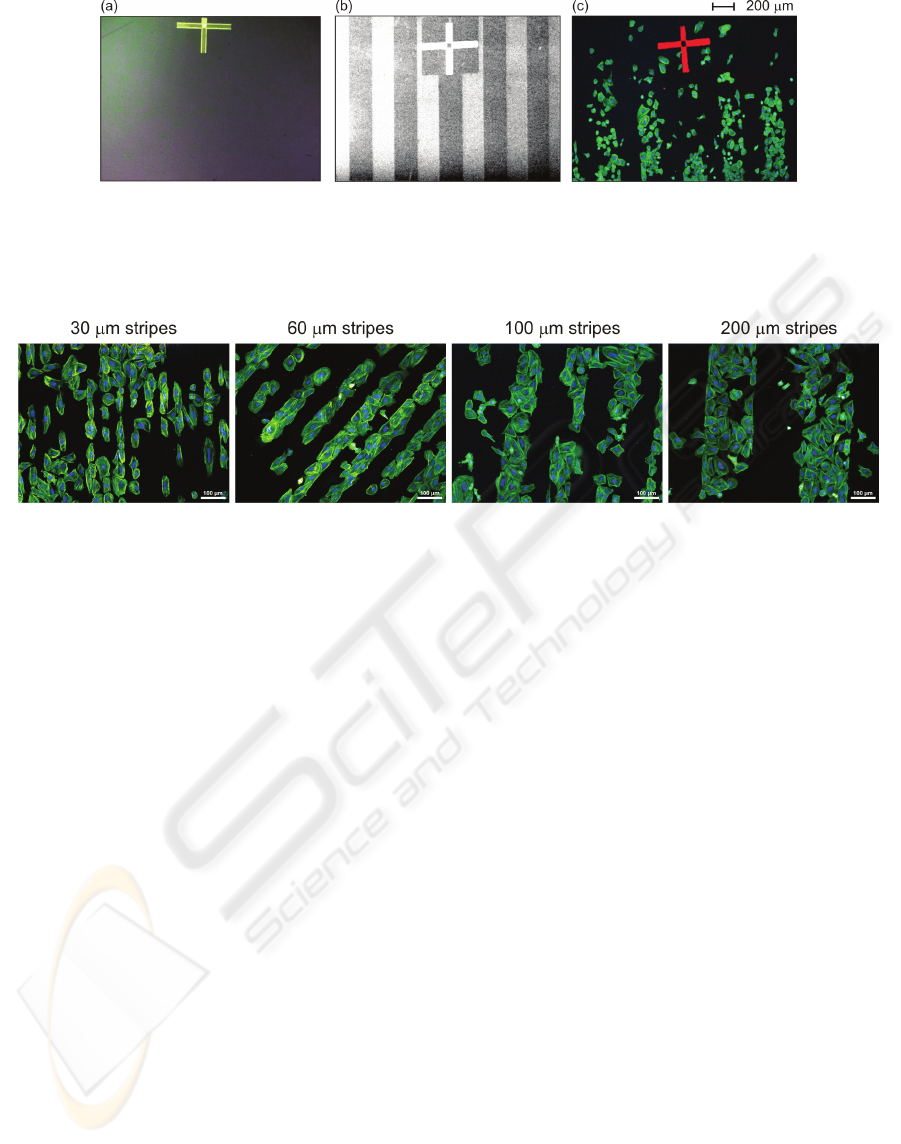
Figure 2: Nanocrystalline diamond film with 200μm wide H-/O-terminated patterns: (a) optical (bright field) image prior to
cell plating showing optically transparent and featureless surface, (b) scanning electron microscopy image prior to cell
plating where bright stripes correspond H-termination and dark stripes O-termination of the diamond surface due to their
opposite electron affinity, (c) fluorescent microscopy image of osteoblastic cells cultivated on the substrate. The alignment
cross is used for correlation of the surface termination micro-patterns with the cells.
Figure 3: Fluorescent microscopy images of osteoblastic cells (SAOS-2) cultivated in McCoy's medium supplemented with
15% FBS for 2 days on H-/O-terminated stripes of different widths (30μm, 60μm, 100μm and 200μm) on diamond films.
Initial cell concentration was 2,500 cells/cm
2
. The fluorescence shows actin stress fibers (green) and nuclei (blue). Scale bar
is 100μm.
suspension in the appropriately supplemented
medium, let to incubate for 2 h (adhesion time), and
then 1.4 ml of the medium was added. In case of 0%
FBS, the cells were plated and incubated for 2 h in
the medium without the serum. Then the 15% FBS-
supplemented medium was added to facilitate
further cell cultivation. After plating, the cells were
cultivated for 48 hours in 5% CO
2
at 37°C.
An advantage of the applied droplet technique is
a precise control of the applied number of cells on
the sample. A disadvantage is slightly non-
homogenous distribution of cells over the sample
with lower concentration on the edge and higher
concentration in the middle of the sample.
Therefore, the microscopic images were taken from
comparable areas on the samples.
Adhesion and morphology of SAOS-2 cells were
characterized by fluorescent staining of actin stress
fibers (phalloidin-Alexa 488 - 1:100, Molecular
Probes) and nuclei (DAPI - 1:1000, Sigma)
according to the protocol in Ref. (Kalbacova,
2007b). The staining was visualized using the E-400
epifluorescence microscope (Nikon); digital images
were acquired with a DS-5M-U1 Color Digital
Camera (Nikon).
As the adhesion and growth of osteoblastic cells
is mediated by proteins, the adsorption, adhesion,
and conformation of FBS itself on the H- and O-
terminated diamond was also investigated. Polished
IIa (100) mono-crystalline diamonds were used as
substrates to minimize the contribution from surface
morphology of NCD films. The mono-crystalline
diamond surface was H- or O-terminated using the
same procedures as for NCD films. A droplet of
15% FBS in the McCoy’s 5A medium was applied
on the diamond substrates for 10 min. Then the
whole samples were immersed in the fluid cell
containing the same FBS/McCoy’s medium and
characterized by AFM (Ntegra, NTMDT).
AFM measurements were performed in the
medium using doped silicon cantilevers
(BSMulti75Al) with the typical force constant of 3
N/m, resonance frequency 75 kHz in air (30 kHz in
the medium), and nominal tip radius <10 nm.
Surface morphologies were investigated in
oscillating-mode AFM (OM-AFM), where the tip-
surface interaction is controlled by adjusting the
AFM amplitude set-point ratio. Free oscillation
amplitude of 60 nm and the set-point ratio of 50%
were typically used. The parameters were optimized
not to influence the soft FBS layer yet to provide
optimal resolution and contrast. A nanoshaving
procedure (Rezek, 2006b; Rezek, 2007a) was
applied to evaluate the protein layer thickness. First,
SELECTIVE OSTEOBLASTIC CELL MICRO-ARRAYS ON DIAMOND FILMS
349
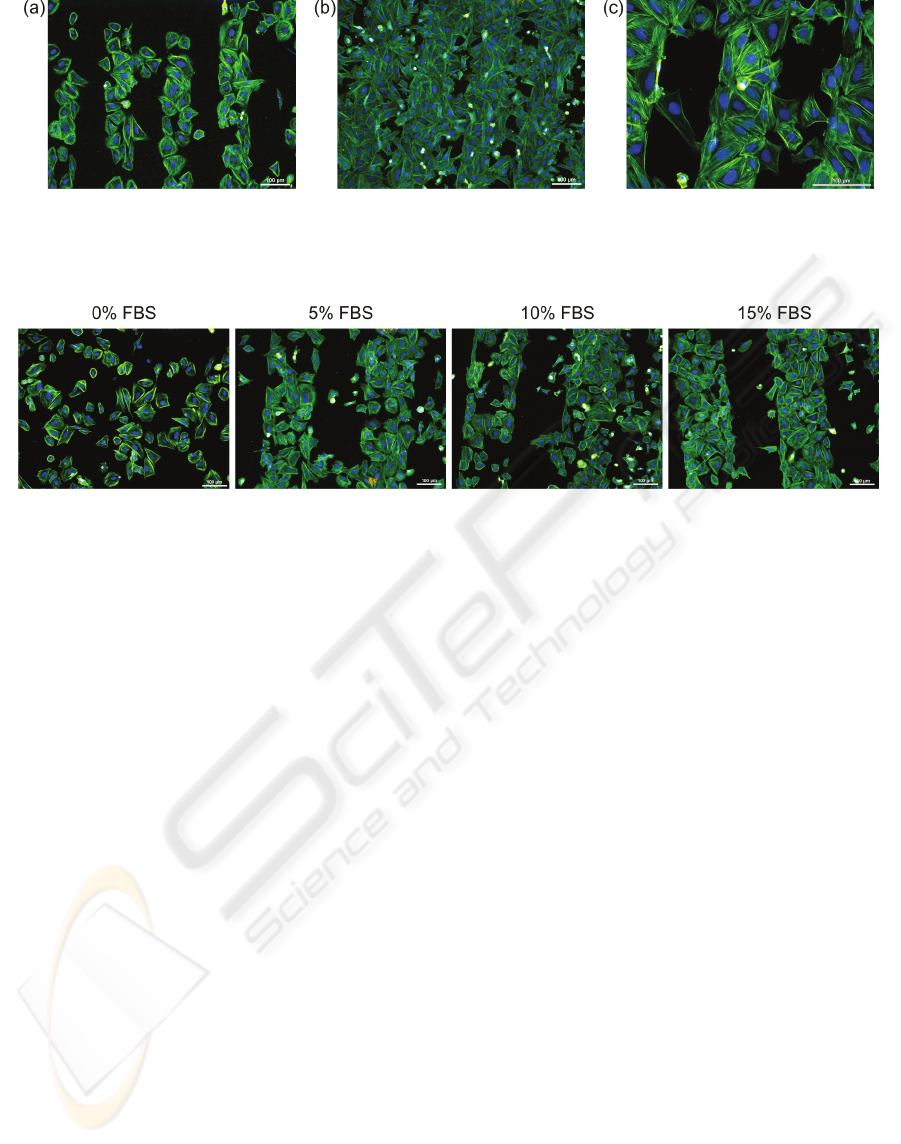
Figure 4: Fluorescent microscopy images of osteoblastic cells (SAOS-2) cultivated for 2 days on 100μm H-/O-terminated
stripes on diamond films: (a) low initial cell seeding concentration (2,500 cells/cm
2
), (b) high initial cell seeding
concentration (10,000 cells/cm
2
), and (c) cells bridging of unfavorable H-terminated regions. The fluorescence shows actin
stress fibers (green) and nuclei (blue). Scale bar is 100μm.
Figure 5: Fluorescent microscopy images of osteoblastic cells (SAOS-2) cultivated for 2 days on 200μm H-/O-terminated
stripes on diamond films in McCoy's medium supplemented with different fetal bovine serum (FBS) concentrations (0, 5,
10, and 15%). Scale bar is 100μm.
a region of 2x2 μm
2
was scanned in contact AFM
(C-AFM) and then re-measured across somewhat
larger area by OM-AFM. The force applied during
C-AFM was approx. 200 nN. The interaction forces
in OM-AFM are orders of magnitude lower. The
FBS layer thickness was then determined as the
difference between average height values across 1
um
2
of the FBS layer surface and 1 μm
2
of the
nanoshaved area where FBS was removed. Several
regions were probed on each sample to determine
the error bar from root-mean-square (RMS)
roughness values and statistical errors.
Autocorrelation function of the images was
calculated to determine typical lateral feature size
(Lx).
3 RESULTS
Correlation of oxygen- and hydrogen-terminated
micro-patterns on the diamond films with patterns of
cell adhesion on such structures is illustrated in
Figure 2. Figure 2(a) shows a bright field image of
the micro-structured sample in optical microscope
before cell seeding. The surface is featureless as the
patterns are optically invisible. Figure 2(b) presents
SEM image of the sample, where H- and O-
terminated patterns (width of 200 µm) are clearly
identified due to their different electronic properties.
The bright stripes correspond to the H-terminated
NCD surface, having negative electron affinity
(Maier, 2001). The dark stripes represent the O-
terminated NCD surface. Fluorescently stained
human osteoblasts adherent on 200 µm wide
patterned surface are presented in Figure 2(c). By
correlating a position of the alignment mark in SEM
and fluorescent microscopy pictures, it is evident
that the osteoblastic cells preferentially colonize the
O-terminated (hydrophilic) patterns.
Figure 3 shows that the cells adhere
preferentially onto O-terminated stripes
independently of the stripe width in the range of 30-
200µm. Two types of cell adhesion patterns are
detectable. Cells on the narrow stripes (30µm –
smaller than the cell size) are elongated and form
cell-by-cell arrays. On wider stripes (60, 100, and
200µm – bigger than the cell size) the cells spread
and fill the entire width of the stripe. At the micro-
pattern borders they form a sharp boundary.
Osteoblast adhesion onto the NCD surface is
affected by the initial cell seeding concentration.
Figure 4 illustrates higher selectivity for cell
adhesion on the O-terminated surface at lower initial
cell seeding density (2,500 cells/cm
2
). There is still
some free space for cell spreading and expansion
within the hydrophilic region. On the other hand,
cells plated at the higher density (10,000 cells/cm
2
)
BIODEVICES 2009 - International Conference on Biomedical Electronics and Devices
350
colonize not only hydrophilic areas but also
unfavorable hydrophobic regions (Figure 4(b)).
Figure 4(c) presents an abnormally long single cell
(left image side) as well as clusters of several cells
(right image side) that can bridge and colonize the
hydrophobic area.
Figure 5 demonstrates the influence of different
initial FBS concentrations (0, 5, 10, and 15%) in the
culture medium on the cell attachment onto the H-
/O-patterned surface. The range of serum
concentrations 5-15% does not significantly affect
the cell adhesion pattern. The cells follow the H-/O-
terminated micro-patterns in the same way as shown
in the previous figures. In a sharp contrast, cells
plated in FBS-free medium colonize the surface
independently of the micro-patterns. The cell
selectivity is obviously determined by the FBS
proteins.
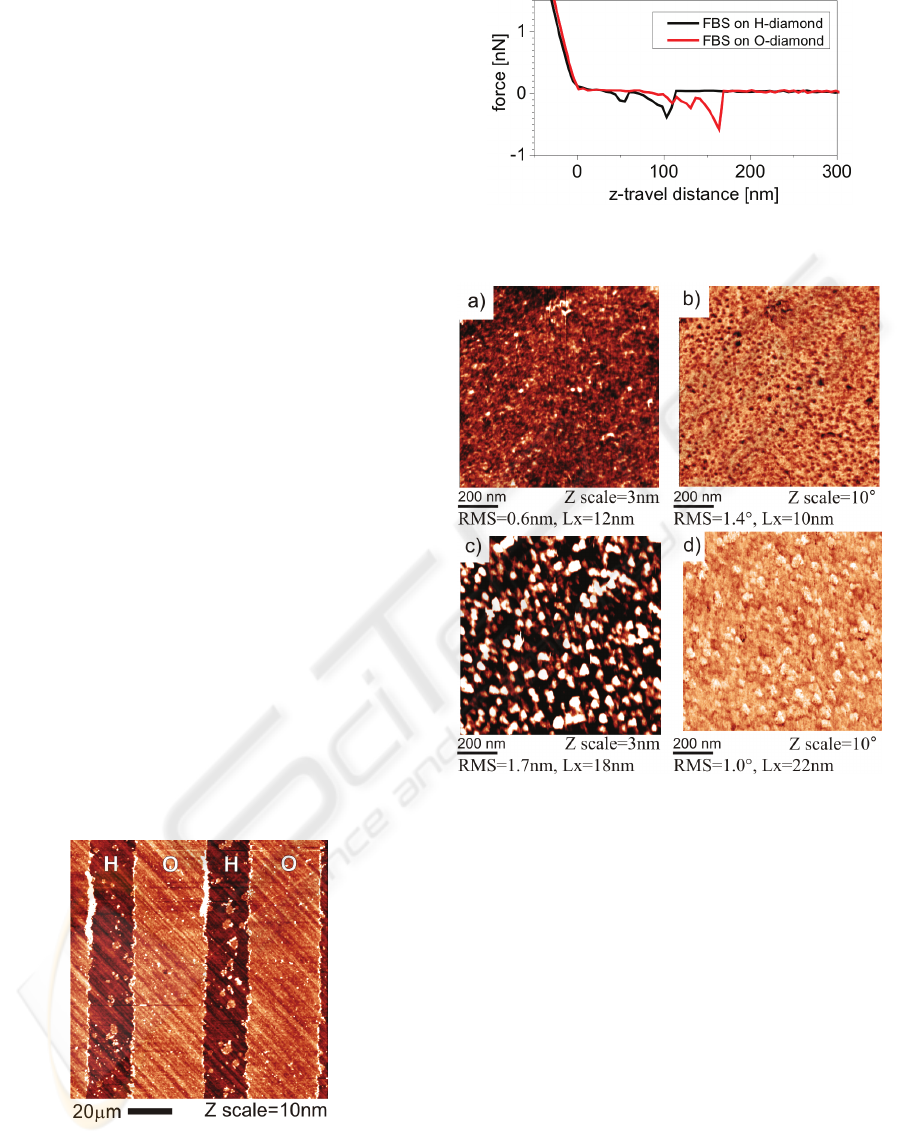
Figure 6 shows OM-AFM topography image of
the FBS layer on diamond with stripe-like patterns
of hydrogen and oxygen surface terminations. The
diagonal lines in the background are due to polishing
of the diamond substrate. The roughness of diamond
substrate is about 0.6 nm. On this background one
can see clear stripes on the O-terminated surface.
There are also some small scattered islands of
similar thickness on the H-terminated stripes, most
likely due to certain degree of non-specific
adsorption. When the height of stripes is probed by
the nanoshaving method, we find that the layer
thickness of the layer adsorbed on O-terminated
diamond is 4 ± 2 nm. Even on H-terminated surface
(outside of the islands) there is a thin layer of 1.5 ± 2
nm. Hence the FBS layer is present on both types of
diamond surfaces, although in the different
thickness.
Figure 6: AFM topography image of a fetal bovine serum
(FBS) layer on the diamond with stripe-like patterns of H
and O surface terminations.
Figure 7: AFM force curves on H- and O-terminated
diamond with the adsorbed FBS layer.
Figure 8: AFM measurements in FBS/McCoy’s medium
on hydrogen- and oxygen-terminated diamond surfaces
with adsorbed FBS layers: topography and phase image on
(a-b) FBS/H-terminated diamond (c-d) FBS/O-terminated
diamond.
Figure 7 shows force curves obtained by force
spectroscopy on H- and O-terminated diamond. The
force curves exhibit 500 ± 100 pN interaction
between tip and surface on both H- and O-
terminated diamond. Similar forces and shapes were
found between cantilevers functionalized by bovine
serum albumin and glass surfaces after deposition of
proteins (Popov, 2007). Hence also the protein
molecules from FBS are present on both H- and O-
terminated diamond.
Figure 8 shows the detailed topography and
phase images on both types of surfaces. Values of
RMS roughness and lateral feature size (Lx) are also
given. The roughness of FBS layer on O-terminated
diamond (1.7 nm) is about two times higher
SELECTIVE OSTEOBLASTIC CELL MICRO-ARRAYS ON DIAMOND FILMS
351

compared to H-terminated diamond (0.6 nm). The
topographic features are different, with a kind of
ridge-like shapes around valleys on H-terminated
diamond and hillock-like shape on O-terminated
diamond. Correspondingly, the feature size is also
different, about 10 nm on H-terminated diamond and
20 nm on O-terminated diamond. A pronounced
difference is detected also in the AFM phase images.
AFM phase image of the adsorbed layer on H-
terminated diamond is dominated by dark dots
correlated with protrusions in morphology. On O-
terminated diamond, brighter spots having darker
boundaries are correlated with the hillocks.
4 DISCUSSION
In correlation with previous reports on homogeneous
surface termination of diamond (Kalbacova, 2007a,
Bacakova, 2007) we find that in case of H/O micro-
patterns the cells colonize preferentially hydrophilic
(O-terminated) stripes forming confluent arrays with
sharp edges separating O- and H- terminated
regions. The cells generally did not show any
decreased viability, however some of them
(preferentially on hydrophobic region) remain
rounded for an extended period of time exhibiting
poor cell-substratum-compatibility (Liu, 2007;
Michalikova, 2008). Evolution of cell morphology
on hydrophobic surfaces is slower, but otherwise not
remarkably different than that observed for human
osteoblasts (hFOB) (Liu, 2007) or SAOS-2 on more
hydrophilic surfaces – it is an example of the time-
cell-substratum-compatibility-superposition
principle.
Also noteworthy is bridging of unfavorable H-
terminated regions as illustrated in Figure 4(c). This
is obviously enabled by connection to the cells on
the O-terminated regions because solitaire cells on
the H-terminated regions exhibit bad adhesion and
reduced metabolic activity (Kalbacova, 2007a;
Kalbacova, 2008). To reach the optimal status on
unfitting surface, cells will communicate with each
other, exchanging growth factors and various stimuli
as well as produce extracellular matrix (ECM) and
thus modify the surface with proteins and
proteoglycans underneath to overcome the
inhospitable environment. It is known that proteins
adsorbed onto the substrate surface do not become
permanently immobilized. They will be
enzymatically degraded, denatured, they undergo
conformational and configuration changes and will
even be replaced by other proteins (Zeng, 1999).
However, when more cells are able to gently attach
to hydrophobic surface in a specific pattern (forming
a bridge between two hydrophilic stripes) then these
cells may form ECM faster due to support from their
proliferating neighbors, thus masking unsuitable
properties of the surface. This may be very useful
mechanism for bio-electronic applications as it
enables to overgrow electrically conductive H-
terminated surface when it is surrounded by O-
terminated regions at small enough dimensions.
As the cell adsorption is protein mediated, a
question arises whether the specific cell adsorption
is due to direct effect of diamond surface dipoles on
the cells or due to differences in protein adsorptions
on the micro-patterns. Figure 5 clearly demonstrates
that cells plated without protein (FBS-free medium)
do not sense any chemical micro-patterning, whereas
cells plated in FBS-supplemented medium clearly
follow the hydrophilic patterns. It proves that the
cell selectivity is driven by the FBS protein
adsorption. Since protein adsorption is much more
rapid than the transport of cells to the surface, it is
expected that the interaction of host cells with the
material is determined by the nature of this adsorbed
protein layer.
AFM study of the protein layers revealed that
FBS adsorbs on both types of diamond surfaces.
This is in agreement with previous reports that
albumin adsorbs on both hydrophilic and
hydrophobic surfaces (Browne, 2004). Here, the
adsorbed thickness differs by few nm. It should be
noted that FBS layer is a soft matter so there is some
uncertainty in determining its thickness by AFM
because even in OM-AFM the height may be
underestimated (Rezek, 2007a; Rezek, 2007b).
Another influence on the observed step in the height
across the nanoshaved region may be wear of the
substrate material. As the flat bulk diamond is very
hard compared to proteins and its wear is extremely
low, only the FBS layer was penetrated and removed
by the nanoshaving forces applied here.
The cell selectivity is thus not determined merely
by FBS layer presence. More subtle differences must
be considered for explaining the selective
adsorption, such as protein denaturation on
hydrophobic surfaces (Ukraintsev, 2007; Zeng,
1999). Detailed studies of surface morphology
revealed clear differences in surface roughness,
morphological features and phase images between
the protein layers on H- and O-terminated diamond.
By comparison with the literature (Browne, 2004) ,
where similar difference in topography on
polystyrene substrates were shown, the most
important factor for the cell growth on diamond
seems to be the wetting property of the surface
BIODEVICES 2009 - International Conference on Biomedical Electronics and Devices
352

rather than any other specific property of the
diamond films.
One has to critically consider that the actual
composition of the adsorbed layers may be different
on H- and O-terminated diamond because various
proteins (albumin, fibronectin, vitronectin, etc.) from
FBS may influence the cell adhesion in different
ways. Further experiments are needed to elucidate
these details.
5 CONCLUSIONS
Chemical patterning of diamond films by hydrogen
and oxygen surface atoms enables self-assembly of
human osteoblastic cell micro-arrays. The cell
adhesion and assembly on diamond can be further
controlled and optimized by biochemical factors.
The cells strongly prefer O-terminated patterns. The
best selectivity is achieved for lower initial cell
concentrations (2,500 cells/cm
2
), regardless of
surface geometry and commonly used protein (FBS)
concentrations (5 to 15%). Widths of the patterns
affect the shape of adhered cells in the following
way: i) good cell spreading with a sharp boundary
was observed on broader stripes and ii) elongated
cell chains were observed on stripes which were
narrower than the cell size. Higher initial
concentration of cells enables colonization of less
favorable H-terminated surface regions, which are
electrically conductive and can be employed in
electronic devices. A non-preferential cell adhesion
is found when the initial cell adhesion occurs
without the serum presence. Hence the cell
selectivity is driven by the FBS properties on H- and
O-terminated surfaces. AFM detected presence of
the FBS layer on both types of surfaces. However,
the layer thickness and microscopic morphology are
rather different. This may be the reason for the cell
selectivity. Further experiments are needed to
elucidate details of the selectivity, such as particular
composition of the adsorbed layers and so on.
Nevertheless, the presented data may already
provide valuable information for application of
diamond films in tissue engineering, implants, bio-
electronics, and biotechnology in general. We
speculate that similar cell behavior will occur also
when using other cell lines.
ACKNOWLEDGEMENTS
This work was supported by the Academy of
Sciences of the Czech Republic contracts
KAN400100701, AV0Z10100521, and Czech
Ministry of Education, Youth and Sport projects LC-
510 and MSM0021620806. The authors would like
to express their thanks to Ing. Vlastimil Jurka, Zdena
Poláčková, Dr. Zdeněk Potměšil (all Inst. Phys.
ASCR) for a kind assistance with photolithography,
and surface treatments, Jana Sovová (Inst. Inh. Met.
Disorders, 1st Fac. Med., Charles Uni.) for technical
assistance and Mgr. Veronika Barešová (Inst. Inh.
Met. Disorders, 1st Fac. Med., Charles Uni.) for a
kind assistance with fluorescent microscopy.
REFERENCES
Bacakova, L., Grausova, L., Vacik, J., Franczek A.,
Blazewicz, S., Kromka, A., Vanecek, M. & Svorcik,
V. (2007). Improved adhesion and growth of human
osteoblast-like MG 63 cells on biomaterials modified
with carbon nanoparticles. Diamond and Related
Materials, 16, 2133–2140.
Bajaj, P., Akin, D., Gupta, A., Sherman, D., Shi, B.,
Auciello, O. & Bashir, R. (2007). Ultrananocrystalline
diamond film as an optimal cell interface for
biomedical applications. Biomedical Microdevices, 9,
787-794.
Browne, M. M., Lubarsky, G. V., Davidson, M. R. &
Bradley, R. H. (2004). Protein adsorption onto
polystyrene surfaces studied by XPS and AFM.
Surface Science, 553, 155–167.
Garrido, A.J., Nebel, C. E., Todt, R., Roesel, G., Amann,
M. –C. & Stutzmann, M. (2003). Fabrication of in-
plane gate transistors on hydrogenated diamond
surfaces. Applied Physics Letters, 82, 988-990.
Kalbacova, M., Kalbac, M., Dunsch, L., Kromka, A.,
Vanecek, M., Rezek, B., Hempel, U. & Kmoch, S.
(2007a). The effect of SWCNT and nano-diamond
films on human osteoblast cells. Physica status solidi
(b), 244, 4356–4359.
Kalbacova, M., Roessler, S., Hempel, U., Tsaryk, R.,
Peters, K., Scharnweber, D., Kirkpatrick, C. J., &
Dieter, P. (2007b). The effect of electrochemically
simulated titanium cathodic corrosion products on
ROS production and metabolic activity of osteoblasts
and monocytes/macrophages. Biomaterials, 28, 3263–
3272.
Kalbacova, M., Michalikova, L., Baresova, V., Kromka,
A., Rezek, B. & Kmoch, S. (2008). Adhesion of
osteoblasts on chemically patterned nanocrystalline
diamonds. In press in Physica status solidi (b).
Kozak, H., Kromka, A., & Rezek, B. (2008). Enhancing
nanocrystalline diamond surface conductivity by
deposition temperature and chemical post-processing.
Submitted in Physica status solidi (a).
SELECTIVE OSTEOBLASTIC CELL MICRO-ARRAYS ON DIAMOND FILMS
353

Kromka, A., Rezek, B., Remes, Z., Michalka, M.,
Ledinsky, M., Zemek, J., Potmesil, J. & Vanecek, M.
(2008). Formation of continuous nanocrystalline
diamond layer on glass and silicon at low
temperatures. In press in Chemical Vapor Deposition.
Liu, X., Lim, J. Y. F., Donahue, H. J., Dhurjati, R.,
Mastro, A. M. & Vogler, E. A. (2007). Influence of
substratum surface chemistry/energy and topography
on the human fetal osteoblastic cell line hFOB 1.19:
Phenotypic and genotypic responses observed in vitro.
Biomaterials, 28, 4535–4550.
Maier, F., Ristein, J. & Ley, L. (2001). Electron affinity of
plasma-hydrogenated and chemically oxidized
diamond (100) surfaces. Physical Review B, 64,
165411.
Michalikova, L., Rezek, B., Kromka, A., Kozak, H.,
Grausova, L., Bacakova, L., Vanecek, M., Kocka, J. &
Kalbacova, M. (2008). Selective Adhesion and
Arrangement of osteoblast-like cells on hydrophilic
and hydrophobic nanocrystalline diamond micro-
patterns. Submitted to Thin Solid Films.
Nebel, C. E. (2003). From gemstone to semiconductor.
Nature Materials, 2, 431-432.
Popov, C., Kulisch, W., Reithmaier, J., Dostalova, T.,
Jelinek, M., Anspach, N. & Hammann, C. (2007).
Bioproperties of nanocrystalline diamond/amorphous
carbon composite films. Diamond and Related
Materials, 16, 735-739.
Potocky, S, Kromka, A., Potmesil, J., Remes, Z., Vorlicek,
V., Vanecek, M., & Michalka, M. (2007).
Investigation of nanocrystalline diamond films grown
on silicon and glass at substrate temperature below
400°C. Diamond and Related. Materials, 16, 744-747.
Rezek, B., & Nebel, C. E. (2006a). Electronic properties
of plasma hydrogenated diamond surfaces: A
microscopic study. Diamond and Related Materials,
15, 1374–1377.
Rezek, B., Shin, D., Nakamura, T. & Nebel, C. E. (2006b).
Geometric properties of covalently bonded DNA on
single-crystalline diamond. Journal of American
Chemical Society, 128, 3884-3885.
Rezek, B., Shin, D., Uetsuka, H. & Nebel, C. E. (2007a)
Microscopic diagnostics of DNA molecules on mono-
crystalline diamond. Physica status solidi (a), 204,
2888-2897.
Rezek, B., Shin, D.& Nebel, C. E. (2007b). Properties of
hybridized DNA arrays on single-crystalline undoped
and boron-doped (100) diamonds studied by atomic
force microscopy in electrolytes. Langmuir, 23, 7626-
7633.
Rezek, B., Shin, D., Watanabe, H. & Nebel, C. E. (2007c).
Intrinsic hydrogen-terminated diamond as ion-
sensitive field effect transistor. Sensors and Actuators
B, 122, 596-599.
Shakenraad, J. M., & Busscher, H. J. (1989). Cell-polymer
interactions:the influence of protein adsorption.
Colloids and Surfaces, 42, 331–343.
Song, K. S., Hiraki, T., Umezawa, H. & Kawarada, H.
(2007). Miniaturized diamond field-effect transistors
for application in biosensors in electrolyte solution.
Applied Physics Letters, 90, 063901.
Tachiki, M., Kaibara, Y., Sumikawa, Y., Shigeno, M.,
Banno, T., Song K. S., Umezava, H. & Kawarada, H.
(2003). Diamond nanofabrication and characterization
for biosensing application. Physica status solidi (a),
199, 39-43.
Tang, L., Tsai, C., Gerberich, W. W., Kruckeberg, L. &
Kania, D. R. (1995). Biocompatibility of chemical-
vapour-deposited diamond. Biomaterials, 16, 483-488.
Ukraintsev, E. V., Kiselev, G. A., Kudrinskii, A. A.,
Lisichkin, G. V. & Yaminskii, I. V. (2007). Formation
of lysoyzme fibrils on a solid support. Polymer
Science, 49, 6-9.
Yang, W., Auciello, O., Butler, J. E., Cai, W., Carlisle, J.
A., Gerbi, J. E:, Gruen. D. M., Knickerbocker, T.,
Lasseter, T. L., Russell, J. N. Jr., Smith, L. M. &
Hamers, R. J. (2002). DNA-modified nanocrystalline
diamond thin-films as stable, biologically active
substrates. Nature Materials, 1, 253-257.
Yang, W., Butler, J. E., Russell, J. N. & Hamers, R. J.
(2004). Interfacial electrical properties of DNA-
modified diamond thin films: Intrinsic response and
hybridization-induced field effects. Langmuir, 20,
6778-6787.
Zemek, J., Houdkova, J. Lesiak, B., Jablonski, A.,
Potmesil, J., & Vanecek, M. (2006). Electron
spectroscopy of nanocrystalline diamond surfaces.
Journal of Optoelectronics and Advanced Materials,
8, 2133–2138.
Zeng, H., Chittur, K. K. & Lacefield, W. R. (1999).
Analysis of bovine serum albumin adsorption on
calcium phosphate and titanium surfaces.
Biomaterials, 20, 377–384.
BIODEVICES 2009 - International Conference on Biomedical Electronics and Devices
354
