
CONSISTENT CORTICAL RESPONSES FROM SUBCORTICALY
DELIVERED ELECTRICAL STIMULI
A Study Oriented to Visual Prostheses
Fivos Panetsos, Elena Diaz-de Cerio, Abel Sanchez-Jimenez, Juan Jose Navarro-Valls
Neurocomputing and Neuro-robotics Research Group, and School of Optics, Complutense University of Madrid
Avda Arcos de Jalon s/n, Madrid, Spain
Jose A. Vega
Department of Anatomy and Embryology, Faculty of Medicine, University of Oviedo, Oviedo, Spain
Idoia Diaz-Guemes
Center of Minimal Invasion Surgery “Jesus Uson”, Caceres, Spain
Keywords: Visual prostheses, neuroprostheses, lateral geniculate nucleus, thalamus and implants.
Abstract: Loss of vision is one of the most important challenges for science nowadays and a large amount of work has
been done in the development and implant of visual neuroprostheses. The applicability of retinal implants is
restricted either because healthy retinal neurons and/or optic nerve are not always available or because of
problems related to the retinal implants themselves. At the present alternatives are restricted to cortical
prostheses which in turn have several physiological and technical limitations. In our communication we
describe a direct proof for the feasibility of subcortical visual prostheses that would solve several of the
limitations of the cortical ones. Our approach consists in stimulating the visual cortex of intact animals by
means of visual stimuli and then to generate similar responses by means of electrical stimulation of the
lateral geniculate nucleus.
1 INTRODUCTION
1.1 General Context
Loss of vision is one of the most important
challenges for science nowadays. Vision impaired
people are among the most vulnerable and
emarginated, normally with low incomes or
unemployed, with poor education, inadequate social
protection, many problems for public or private
transport, perception of the environment, access to
the buildings, etc. In addition to these adversities,
the negative attitudes of the society create a hostile
environment to both, the blind people and their
families.
For all these reasons the development of visual
prostheses represents one of the highest priorities in
the field of Biomedical Engineering, despite vision
is the most complicated of our senses and several
extremely complicated physiological, computational
and engineering problems have to be solved at every
step. Up to now, a large amount of work has been
done in the development and chronic implants of
visual neuroprostheses (for a thorough review see
(Maynard, 2001)) including chronic implants in the
retina, optic nerve and occipital cortex of blind
human subjects (Cohen, 2007; Dobelle, 2000;
Gerding, 2007; Humayun et al., 2003; Javaheri et al.,
2006; Lakhanpal et al., 2003; Margalit et al., 2002;
Shenoy et al., 2006; Thanos et al., 2007; Veraart et
al., 1998; Winter et al., 2007).
Retinal prostheses are very useful for the
impaired people but they need functional retinal
neurons and intact optic nerve, thalamus and cortex.
On the other hand cortical prostheses have to be
directly interfaced and inject visual signals to a high-
level neural structure, the visual cortex, designed by
nature to receive and analyse complex information.
155
Panetsos F., Diaz-de Cerio E., Sanchez-Jimenez A., Navarro-Valls J., Vega J. and Diaz-Guemes I. (2009).
CONSISTENT CORTICAL RESPONSES FROM SUBCORTICALY DELIVERED ELECTRICAL STIMULI - A Study Oriented to Visual Prostheses.
In Proceedings of the International Conference on Health Informatics, pages 155-160
DOI: 10.5220/0001510201550160
Copyright
c
SciTePress
Moreover, such information is previously pre-
processed by the retina and visual thalamus and this
process includes an open loop formed by the
corticothalamic and thalamocortical projections
(Mason et al., 1991) the function of which is not
taken into account in the design of cortical
prostheses.
Most of the problems related to the architecture
of the visual cortex and the input of visual signals
could be solved if visual prostheses would be
implanted to the previous relay station of the visual
system, the lateral geniculate nucleus of the
thalamus; such prostheses would also benefit from
the processing capabilities of the thalamocortical
loop (see Section 1.2). But to our knowledge there is
not available experimental work on visual prostheses
to implant in subcortical structures and in particular
in the visual thalamus, except the work of Pezaris
and Reid (2007) in the generation of visual percepts
after stimulation of the lateral geniculate nucleus.
In the present paper we describe our work on
visual prostheses implanted in the lateral geniculate
nucleus of the thalamus and a direct way to
demonstrate the appearance of artificially generated
cortical responses similar to those elicited by natural
visual stimuli.
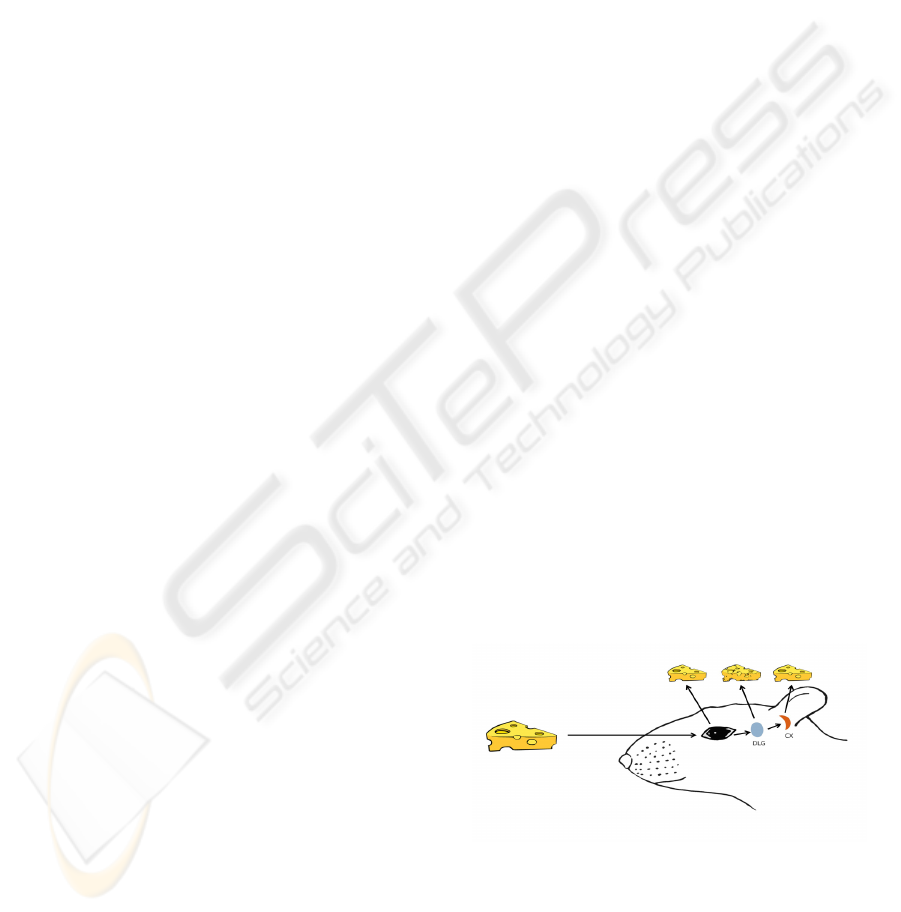
1.2 The Visual System
The visual system processes information in a
hierarchical manner from the retina to the cortex
through increasing the complexity of feature
extractions using a chain of three neurons and two
neural relay stations connected in a massively
parallel fashion. The first information processing
station is the eyeball’s inner lining, the retina, where
a bidimensional sheet of photoreceptors transform
the image of the external world to a multi-
dimensional spatiotemporal and intensity pattern of
electrical signals (Baylor et al., 1979; Saito et al.,
1978) further processed by the bipolar, horizontal,
amacrine and ganglion cells (Kuffler, 1953). Via the
optic nerve, the outputs of the two retinas are
transmitted to the lateral geniculate nucleus of the
thalamus (LGN) where are processed (Kastner et al.,
2006; Derrington and Fuchs, 1979) and integrated
into a binocular representation of the world (Murphy
and Sillito, 1989).
LGN output goes directly to the primary visual
cortex (V1) which gives in turn a massive feedback
to LGN and also sends information to higher visual
cortical regions for further processing (Mason et al.,
1991). The corticothalamic information feedback
plays a major role to information processing of the
visual signal, selecting the most interesting inputs
and imposing processing rules to the thalamus
(Sillito et al., 2006).
1.3 Visual Prostheses
Blindness results from either an inability of the
visual system to transduce light energy into electric
signals or a failure of the generated electrical signals
to reach the higher relay stations of the visual
pathway. It is classically accepted that electrical
stimulation of V1 can elicit a visual percept of light
denominated phosphene (Tehovnik and Slocum,
2007; Krisch and Hosticka, 2007).
In retinal prostheses arrays of electrodes are
placed either on the retinal surface or in the
subretinal space where they stimulate ganglion cells
(Humayun, 2003). A new approach to retinal
prosthesis is electrical stimulation of the retina with
extraocular electrodes (Chowdhury et al., 2008).
However they need an undamaged retina and an
intact optic nerve (Margalit, 2002).
Cortical prostheses consist of arrays of electrodes
which are placed to or penetrate the cortical surface
and stimulate layer VI of the visual cortex to create
discrete phosphenes (Brindley and Lewin, 1968;
Dobelle, 2000) and have the advantage to be suitable
for almost any kind of blind patients.
However the visual cortex is non-linear and non-
conformal with visual space, not letting us to predict
in a precise way where will phosphenes be elicited
when stimulating with each electrode (Warren et al.,
2001). Moreover, the power of cortical processing is
mainly based to the continuous feedback of the
thalamocortical loop and to the influence V1
exercises to the thalamus, both of them excluded due
to the direct introduction of visual information to
V1.
Figure 1: Schematic representation of the visual pathway.
In the cases in which the retina and/or the optic
nerve are damaged or not functioning, the target
with more advantages and less technical,
experimental or clinical problems for visual
prostheses seems to be the thalamus:
HEALTHINF 2009 - International Conference on Health Informatics
156

1) the receptive fields (RFs) of LGN neurons are
simple, well characterized, and similar to those of
their retinal afferents (Hubel and Wiesel, 1961;
Wiesel and Hubel, 1966)
2) fovea and parafovea are spatially represented
in the LGN facilitating the accessibility of neurons
with central visual fields (Pezaris and Reid, 2007)
3) LGN cells give rise to axons that terminate in
primary visual cortex, in a highly specific manner
making monosynaptic connections with simple cells
predominantly when the pre- and postsynaptic
receptive fields overlap and match in sign, size, and
time course (Alonso et al., 2001), consequently
stimulation of a small number of LGN neurons
should achieve simple, focal percepts (Pezaris and
Reid, 2007)
4) LGN cells receive a massive cortical feedback
that directly control the processing capabilities of
these neurons conditioning and selecting the visual
information from LGN to the cortex (Sillito et al.,
2006). Consequently, LGN implants will use the
plasticity and adaptability of the Central Nervous
System to modify the responses of its own neurons
to recognize the artificial signals through the
influence of the corticothalamic LGN-V1 loop.
Despite the above considerations LGN has never
been taken into account in the development of
prosthetic visual devices, except the mentions in
Pezaris and Reid (2007).
The direct test of the feasibility of visual devices
implanted in the thalamus is just the target of the
present work. The data we present here come out
from our research carried out within the framework
of our projects on visual neuroprostheses. In this
work our objective was to generate perception
sensations similar to the natural ones and assess
them by comparing the responses of the cortical
neurons to electrical stimulation of the thalamus
with those generated by visual stimulation of the eye
(see Section of Materials and Methods).
2 EXPERIMENTS
2.1 General Approach
Data were obtained from 36 urethane- (1.5g/kg i.p.)
or sodium pentobarbital (35mg/Kg i.p.)
anaesthetized Wistar rats of both sexes, weighting
200-240g. Experiments were carried out according
to the national legislation (R.D. 1201/2005) and EU
Directives on this matter (86/609/EC). Rats have
been used due to their simple but complete visual
system. In a previous work we had realised a
mapping of the thalamic and cortical visual areas
and to characterized the responses of their neurons
to simple and complex visual stimuli. The 36
animals were used in a series of combined
experiments in which one group of electrodes was
placed in the LGN and another group in V1.
Our approach consisted in: 1) presenting a series
of visual stimuli to one eye and record the responses
of the contra lateral LGN and V1, R
Th
and R
V1
respectively, by means of the implanted
multielectrodes, 2) inject to LGN an electrical
pattern R
Th
*
similar to the previously recorded R
Th
during the presentation of the visual stimuli; at the
same time record the cortical responses R
V1
*
to this
electrical stimulation of LGN and 3) modify the
parameters of R
Th
*
looking for the best matching
between R
V1
and R
V1
*
, that means between the
response to natural and the response to the electrical
stimuli.
The ability to elicit R
V1
*
responses similar to R
V1
for a large number of natural visual stimuli would be
a proof of the feasibility of visual prostheses
implanted to the thalamus.
In total, 216 complete cycles of experiments
(visual stimulation – LGN & V1 recordings,
electrical stimulation – V1 recordings were
performed.
2.2 Stimulation and Recordings
Animals were placed in a stereotaxic device that
enables the conduct of visual experiments. An
incision was performed and two holes were made in
the skull to allow access to the rat brain in the
appropriate coordinates. Recording and stimulating
multielectrodes were developed and tested in our lab
following the methodology described by Neuralynx
(http://www.neuralynx.com) and then introduced in
the brain. Anaesthesia level was controlled by the
amplitude of the EEG waves. A frontal hole was
made to record the electroencephalogram (EEG).
EEG recordings were performed through an
insulated (except in the tip) 1mm diameter Cr-Ni
macroelectrode introduced in the frontal cortex at
1.0mm from the surface. The EEG was continuously
monitored in the oscilloscope. In case of reduction
of the amplitude of the waves supplementary doses
of anaesthesia were administered.
2.3 Data Acquisition and Analysis
Single channel recordings were performed using
tungsten microelectrodes (2.0MΩ) and Micro1401
hardware by Cambridge Electronic Design with
CONSISTENT CORTICAL RESPONSES FROM SUBCORTICALY DELIVERED ELECTRICAL STIMULI - A Study
Oriented to Visual Prostheses
157
accompanying software Spike2. Multichannel
recordings were performed using the above
described multielectrodes and neural activity was
acquired using a PCI-6071E E Series data
acquisition card from National Instruments, with
accompanying Recorder software amplified and
displayed on a Plexon Inc PCI device, stored and
then imported to a Spike2 software and analyzed
using MATLAB (©MathWork corporation) and
Spike2 software. Data were sampled and digitalized
at 20 KHz, stored in personal computers and then
processed off-line. We first performed single
channel recordings both in LGN and V1 with
tungsten electrodes in order localize a region with
response to visual stimulation.
Once the most suitable region was identified the
tungsten electrode was substituted by a 4x4
multielectrode array. The neural tissue was then let
to recover its normal activity for 20 minutes and
then we started with the standard
recording/stimulation procedure. The exact location
of the recording electrode was also confirmed on
subsequent histological preparations.
Spikes were threshold-isolated offline using
Spike2 software (Cambridge Electronic Design)
taking as threshold a value equal at least three times
the level of the noise, and converted into discrete
processes. To determine basic features of neuronal
response and behaviour we performed peristimulus
histograms, interspike interval histograms, auto- and
cross-correlation histograms.
2.4 Histology
To ascertain the localization of the electrodes, the
histology of the brain was analyzed 1 mm rostral and
dorsal to the electrodes placed in the LGN and the
occipital cortex, respectively.
Briefly, after recording, and because the
electrodes are too thick to easily identify the cerebral
structured where they are placed into, a 2mA electric
current was passed though them for 10s in order to
electro coagulate the recorded structures. Then the
animals were sacrificed with an intracardiac
injection of NaCl hypertonic solution (3ml) and the
brain was quickly removed and whole fixed in
Bouin’s fixative for 24 hours, then dehydrated
through increased concentrations of ethanol (from
70º to absolute) and xylene to remove the picric
acid, and embedded in paraffin.
The blocks were cut serially to obtain coronal
sections 10μm thick, deparaffinized, rehydrated and
stained with methylene blue-eosin. The sections
were then washed in tap-water, dehydrated and
mounted with Entellan®, and studied in a light
photomicroscope. The electrode implanted into the
lateral geniculate body has a trajectory perpendicular
to the brain surface. The structural techniques used
to identify the lesioned zones were routinely
haematoxylin & eosin and alzian blue &
haematoxylin. Both techniques consented identify
the structure of the brain and the electrocoagulation-
induced lesion.
2.5 Stimulations and Responses
In a first phase, visual stimuli of increasing
complexity were used to determine the response
patterns evoked in both LGN and V1. Two types of
visual stimuli were used, flashes (Grass model PS33,
20-40 stimuli at 1Hz) and persistent geometric black
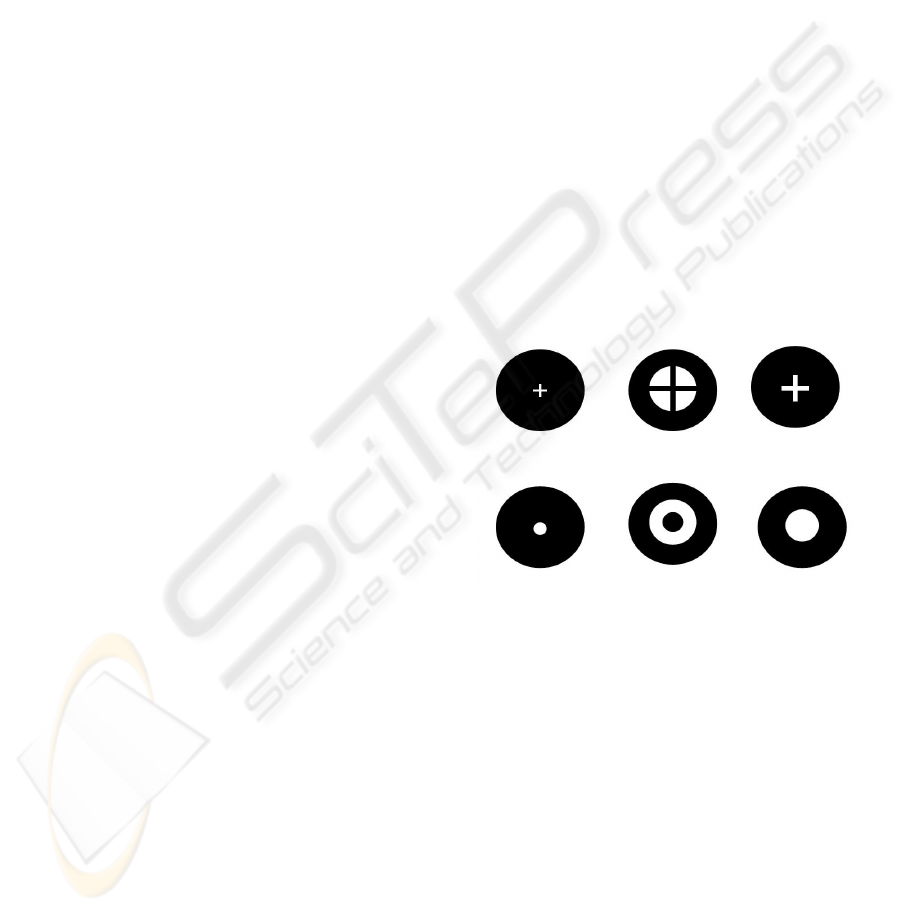
and white figures (see figure 2): horizontal stripes,
circle, ring of light, cross and black cross on a white
background. Persistent stimuli were generated on the
screen of a PC (324 X 244 mm and 1024 x 768
pixels resolution) applied for 3 seconds at 0.3Hz.
Every stimulus had a TTL synchronization signal
toward the data acquisition system.
Figure 2: Geometric stimuli used in the experiments.
Responses were analyzed, correlated and patterns
of electrical stimuli were generated and applied to
the LGN according to the procedure described in
Section 2.2. Then it has been possible to extract
basic characteristics of the electrical stimulation that
evoked cortical responses comparable to those of the
visual stimuli.
After that, each animal was applied a battery of
visual stimuli, thalamic and cortical activity were
recorded, online analyzed, and sets of electrical
stimulation patterns were generated. Such
stimulation patterns were then applied to the LGN,
and cortical responses were recorded and compared
to those generated by the visual stimuli (see figure
3).
HEALTHINF 2009 - International Conference on Health Informatics
158
Current was applied across the four electrodes in
the geniculate nucleus using SIUs (World Precision
Instruments, A365 and A360 Stimulus Isolator Unit)
controlled by the Spike software with the Micro1401
mkII data acquisition unit (Cambridge Electronic
Design). We used intensities between 100μA and
600μA and applied different patterns of stimulation
differing in the number of stimuli trains, the interval
between trains, the number of stimuli per train and
their duration and the number of electrodes and the
temporal relation between the current applied by
each electrode.
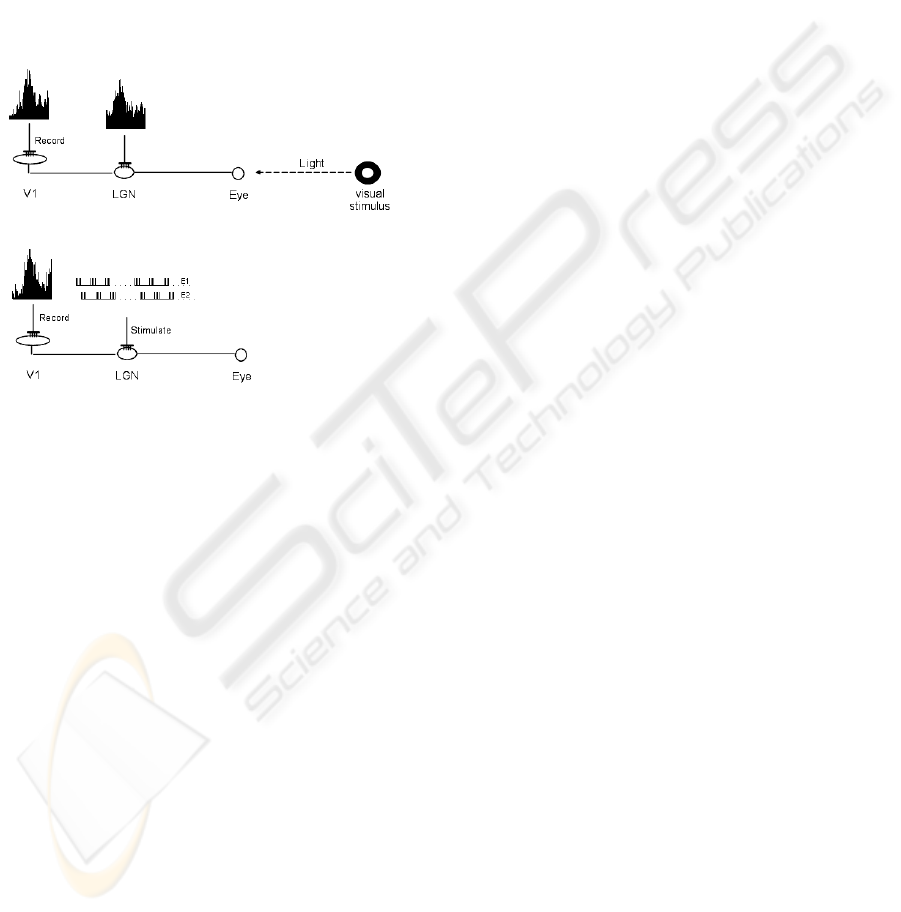
Figure 3: A visual stimulus is presented to the eye and
neural responses are recorded from the lateral geniculate
nucleus in the thalamus and the primary visual cortex (up).
The characteristics of the thalamic electric activity are
extracted and an artificial electrical stimulus is generated
and delivered to the thalamic neurons in absence of
external (natural) visual stimulus (down). A fine
adjustment of the spatiotemporal and intensity
characteristics of the artificial stimuli delivered through
the multiple electrodes implanted to the thalamus allow us
to obtain responses from the cortical neurons that are very
similar to those induced by the natural stimulation using
geometric stimuli. Similar results were obtained from the
entire set of visual stimuli (geometric forms) and the
corresponding electrical stimuli.
3 CONCLUSIONS
Due to the architectonic organisation of the lateral
geniculate nucleus and the lower complexity of the
processing of visual information it performs (if
compared to the visual cortex) LGN is the best
candidate for visual implants when the retina and/or
optic nerve are not functional. Moreover, the direct
action of the visual cortex to the thalamic neurons
through the thalamocortical loop allows a better
adaptation of the Central Nervous System to the
artificial input to the thalamus than to the cortex.
In the present paper we prove that electrical
stimulation of the lateral geniculate nucleus can
generate neural responses in the visual cortex that
resemble those elicited by natural visual stimuli.
Such responses can be achieved after a sampling of
the thalamic responses to the natural stimuli by
means of multielectrode recordings, the extraction of
their basic spatiotemporal characteristics and a
subsequent fine tuning of the electrical stimuli
delivered through the same electrodes implanted into
the thalamus.
Our results are important for the development of
visual prostheses implanted in the subcortical
structures of the brain of blind people although an
extensive work has to be done: in addition to
anatomophysiological problems related to the
implants of the electrode, damages to the brain due
to the chronic stimulation, etc., research on coding
complex visual stimuli, images in movement,
reduction of the complexity of the visual image. So
future steps to solve these problem will be recordand
stimulate with more channels (up to 100), applied
visual stimuli in movement, implant multielectrodes
chronically, mathematical study of the interactions
between recording/stimulating channels to develop
more precise microstimulation, etc.
ACKNOWLEDGEMENTS
Supported by a grant of MAPFRE-Medicine
Foundation (“Connections between Central Nervous
System and electronic devices”, 2004), a grant of
MAPFRE Foundation (“Visual Prostheses”, 2005)
and a grant of the Spanish National Organisation of
Blinds O.N.C.E. (VISNE project: “VISual
neuroprostheses based on adaptive NEuron-silicon
interfaces”, 2006-2009).
REFERENCES
Alonso, J.M., Usrey, W.M., & Reid, R.C. (2001). Rules of
connectivity between geniculate cells and simple cells
in cat primary visual cortex. Journal of Neuroscience,
21, 4002-4015.
Baylor, D.A., Lamb, T.D., & Yau, K.W. (1979).
Responses of retinal rods to single photons. J.Physiol,
288, 613-634.
Brindley, G.S. & Lewin, W.S. (1968). The sensations
produced by electrical stimulation of the visual cortex.
J.Physiol, 196, 479-493.
CONSISTENT CORTICAL RESPONSES FROM SUBCORTICALY DELIVERED ELECTRICAL STIMULI - A Study
Oriented to Visual Prostheses
159

Chowdhury, V., Morley, J.W. & Coroneo, M.T. (2008).
Development of an extraocular retinal prosthesis:
Evaluation of stimulation parameters in the cat.
J.Clin.Neurosci. 15, 900-906.
Cohen, E.D. (2007). Prosthetic interfaces with the visual
system: biological issues. J.Neural Eng, 4, R14-R31.
Derrington, A.M. & Fuchs, A.F. (1979). Spatial and
temporal properties of X and Y cells in the cat lateral
geniculate nucleus. J.Physiol, 293, 347-364.
Dobelle, W.H. (2000). Artificial vision for the blind by
connecting a television camera to the visual cortex.
ASAIO Journal, 46, 3-9.
Gerding, H. (2007). A new approach towards a minimal
invasive retina implant. J.Neural Eng, 4, S30-S37.
Hubel, D.H. & Wiesel, T.N. (1961). Integrative action in
the cat's lateral geniculate body. J.Physiol, 155, 385-
398.
Humayun, M.S., Weiland, J.D., Fujii, G.Y., Greenberg, R.,
Williamson, R., Little, J., Mech, B., Cimmarusti, V.,
Van, B.G., Dagnelie, G., & de, J.E. (2003). Visual
perception in a blind subject with a chronic
microelectronic retinal prosthesis. Vision Research,
43, 2573-2581.
Javaheri, M., Hahn, D.S., Lakhanpal, R.R., Weiland, J.D.,
& Humayun, M.S. (2006). Retinal prostheses for the
blind. Annals of the Academy of Medicine, Singapore,
35, 137-144.
Kastner, S., Schneider, K.A. & Wunderlich, K. (2006).
Beyond a relay nucleus: neuroimaging views on the
human LGN. Prog.Brain.Res 155, 125-143.
Krisch, I. & Hosticka, B.J. (2007). Restoring visual
perception using microsystem technologies:
engineering and manufacturing perspectives. Acta
Neurochir.Suppl. 97, 473-480.
Kuffler, S.W. (1953). Discharge patterns and functional
organization of mammalian retina. Journal of
Neurophysiology, 16, 37-68.
Lakhanpal, R.R., Yanai, D., Weiland, J.D., Fujii, G.Y.,
Caffey, S., Greenberg, R.J., de Juan E Jr, & Humayun,
M.S. (2003). Advances in the development of visual
prostheses. Current Opinion in Ophthalmology, 14,
122-127.
Margalit, E., Maia, M., Weiland, J.D., Greenberg, R.J.,
Fujii, G.Y., Torres, G., Piyathaisere, D.V., O'Hearn,
T.M., Liu, W., Lazzi, G., Dagnelie, G., Scribner, D.A.,
de Juan E Jr, & Humayun, M.S. (2002). Retinal
prosthesis for the blind. Survey of Ophthalmology, 47,
335-356.
Mason, A., Nicoll, A., & Stratford, K. (1991). Synaptic
transmission between individual pyramidal neurons of
the rat visual cortex in vitro. Journal of Neuroscience,
11, 72-84.
Maynard, E.M. (2001). Visual prostheses.
Annu.Rev.Biomed.Eng, 3, 145-168.
Murphy, P.C. & Sillito, A.M. (1989). The binocular input
to cells in the feline dorsal lateral geniculate nucleus
(dLGN). J.Physiol, 415, 393-408.
Pezaris, J.S. & Reid, R.C. (2007). Demonstration of
artificial visual percepts generated through thalamic
microstimulation. Proc.Natl.Acad.Sci.U.S.A, 104,
7670-7675.
Saito, T., Kondo, H., & Toyoda, J. (1978). Rod and cone
signals in the on-center bipolar cell: their different
ionic mechanisms. Vision Research, 18, 591-595.
Shenoy, K.V., Santhanam, G., Ryu, S.I., Afshar, A., Yu,
B.M., Gilja, V., Linderman, M.D., Kalmar, R.S.,
Cunningham, J.P., Kemere, C.T., Batista, A.P.,
Churchland, M.M., & Meng, T.H. (2006). Increasing
the performance of cortically-controlled prostheses.
Conf.Proc.IEEE Eng Med.Biol.Soc., Suppl, 6652-
6656.
Sillito, A.M., Cudeiro, J., & Jones, H.E. (2006). Always
returning: feedback and sensory processing in visual
cortex and thalamus. Trends in Neurosciences, 29,
307-316.
Tehovnik, E.J. & Slocum, W.M. (2007). Phosphene
induction by microstimulation of macaque V1. Brain
Res.Rev. 53, 337-343.
Thanos, S., Heiduschka, P., & Stupp, T. (2007).
Implantable visual prostheses. Acta Neurochir.Suppl,
97, 465-472.
Veraart, C., Raftopoulos, C., Mortimer, J.T., Delbeke, J.,
Pins, D., Michaux, G., Vanlierde, A., Parrini, S., &
Wanet-Defalque, M.C. (1998). Visual sensations
produced by optic nerve stimulation using an
implanted self-sizing spiral cuff electrode. Brain
Research, 813, 181-186.
Warren, D.J., Fernandez, E., & Normann, R.A. (2001).
High-resolution two-dimensional spatial mapping of
cat striate cortex using a 100-microelectrode array.
Neuroscience, 105, 19-31.
Wiesel, T.N. & Hubel, D.H. (1966). Spatial and chromatic
interactions in the lateral geniculate body of the rhesus
monkey. Journal of Neurophysiology, 29, 1115-1156.
Winter, J.O., Cogan, S.F., & Rizzo, J.F., III (2007).
Retinal prostheses: current challenges and future
outlook. J.Biomater.Sci.Polym.Ed, 18, 1031-1055.
HEALTHINF 2009 - International Conference on Health Informatics
160
