
IMPROVING SURFACE ENERGY AND HYDROPHILIZATION
OF POLY(ETHYLENE TEREPHTHALATE)
BY ENZYMATIC TREATMENTS
Isabel C. Gouveia
1,3*
, Laura C. Antunes
3
and João A. Queiroz
2,3
1
Departamento C.T. Têxteis,
2
Departamento Química,
3
R&D Materiais Têxteis e Papeleiros
Universidade da Beira Interior, 6201-001 Covilhã, Portugal
Keywords: Poly(ethylene terephtalate), Enzymatic treatment, Contact angle, Surface energy, Hydrophilicity, Esterases,
Lipases, Adhesion.
Abstract: In order to increase the hydrophilicity and adhesion of poly (ethylene terephthalate) (PET) fabrics it was
studied the action of three types of enzymes (Texazym PES sp5, Aspergillus niger and Aspergillus oryzae)
applied at different incubation times and concentrations. This processes aims to modify morphologically
and chemically the superficial structure of the polymeric materials (PET), forming new carboxyl, hydroxyl
and other polar groups at the surface, in order to increase adhesion and hydrophilicity. The increase in the
hydrophilicity of the fabric was evaluated by measuring the contact angle being the best results obtained for
the Texazym PES (87.45º), much smaller than the non-treated fabric (122.95º); and by the wicking height,
which revealed an important improvement in the hydrophilicity. The formation of carboxyl and hydroxyl
groups was evaluated by a staining procedure with a cationic and reactive dye, respectively. It was also
confirmed by the increasing in the polar component of the surface energy, determined by the Qwens-Wendt
method. The higher surface energy and thus, the higher adhesion properties, were obtained for the esterase
Texazym, using 0.12U during 90 minutes. The surface morphology of the non-enzymatic-treated and
enzymatic-treated samples was analyzed by scanning electron microscopy (SEM) showing no degradation
of fibers treated under the selected optimum conditions. In contrary, this method showed an important
surface cleaning action by removing some undesirable polyester oligomers.
1 INTRODUCTION
Advances of biotechnology in textile industry have
brought new products and processes for specialty
applications as for instance in biomedical materials.
Polymers and textiles are usually used as films
and foils for packaging, protective coating, material
for biomedical and sealing applications because of
their superior bulk properties, such as transparency,
high resistance, strength, good thermal resistance,
etc. But these excellent characteristics are often
unsuitable for biomedical applications due to their
low surface energies. Therefore, surface treatments
are usually necessary to improve surface wetting and
adhesion properties (Inagaki et al, 2001), (Yang and
Gupta, 2004), (Guebitz and Cavaco-Paulo, 2008),
(Huemann et al, 2006).
The synthetic fibers, in particular, polyester
made from poly(ethylene terephthalate), (PET) have
a reduced number of polar groups (hydroxyl and
carboxyl groups) capable to establish hydrogen
bonds with water, reflecting in its weak capacity to
absorb water also related to its high degree of
cristalinity. This property can be changed appealing
to chemical methods as, for example, the alkaline
treatment. This method can, however, damage not
only to the fibers but can also be harmful to the
environment. In this way, alternative processes,
simultaneously ecological, efficient and safe, have
been studied.
Earlier studies demonstrated that the application
of lipases, cutinases and esterases to synthetic fibers,
help increasing hydrophilicity through the hydrolysis
of ester bonds, under moderate conditions (low
concentration and low reaction time at room
temperature), accompanied by a slight reduction of
the resistance to rupture and weight loss
(Vertommen et al, 2005), (Heumann et al, 2006).
Several studies of enzymatic treatments have
been proposed in order to modify the surface
properties of polymers such as adhesivity,
268
Gouveia I., Antunes L. and Queiroz J. (2009).
IMPROVING SURFACE ENERGY AND HYDROPHILIZATION OF POLY(ETHYLENE TEREPHTHALATE) BY ENZYMATIC TREATMENTS.
In Proceedings of the International Conference on Biomedical Electronics and Devices, pages 268-275
DOI: 10.5220/0001552102680275
Copyright
c
SciTePress
hydrophobicity, oleophobicity, wettability/
hydrophilicity, pilling and static charges, by
hydrolysing polymers without affecting the bulk
properties, having the advantage of being eco-
friendly compared with conventional chemical
treatments (Guebitz and Cavaco-Paulo, 2008),
(Heumann et al, 2006), (Vertommen et al, 2005).
Other authors (Heish and Cram, 1998) confirmed
that the increasing of the hydrophilicity after
modification of polyester with lipases was superior
to the one achieved with conventional chemical
treatments (alkaline treatment: 3N of NaOH during 2
hours).
Our new approach focus on the surface
modification of PET fabrics by enzymatic treatments
using three different enzymes (Texazym PES and
Aspergillus niger, Aspergillus oryzae, respectively),
in order to form new polar groups (carboxyl and
hydroxyl) at the surface, capable to establish
hydrogen bonds with water and capable to improve
surface adhesion. The study was undertaken to
analyze and compare the effect of enzymatic
treatments applied in a textile material (100% PET
fabric), by studying the morphological and chemical
changes at the surface, the mechanical properties
and surface energy, in order to establish whether or
not the material can be functionalized and its surface
adhesion properties can be improved.
2 EXPERIMENTAL
The enzymatic treatment aims to improve the
hydrophilicity without harming the mechanical
properties of the material. For that purpose, it was
investigated a new approach by studying the effect
of the three types of enzymes, esterases and lipases
(Texazym PES sp5, Aspergillus niger and
Aspergillus oryzae), varying the incubation time and
the enzyme concentration.
The chemical modifications were investigated by
measuring the contact angle and the wicking height.
The indirect determination of the formed carboxyl
and hydroxyl groups was measured by staining with
a cationic dye (Methylene Blue) and a reactive dye
(Reactive Black 5), respectively, and by measuring
the surface energy by the Owens-Wendt method.
The surface morphological changes were analyzed
by scanning electron microscopy (SEM).
2.1 Materials
The substrate (textile material) used in this work was
a 100% poly (ethylene terephthalate)
fabric, Batavia
Twill, with the characteristics indicated in Table 1.
The substrate was pre-washed with 1 g/L Plurafac
LF 400, at 50ºC during 60 minutes, with mild
mechanical agitation (25 rpm). Subsequently, the
substrate was rinsed and washed under running
water, followed by a thermofixation at 170ºC during
15 minutes.
2.2 The Enzymes
The enzymes selected for this study, were an
esterase (Texazym PES sp 5 from inoTEX Ltd.) and
two lipases (Aspergillus niger and Aspergillus
oryzae from Sigma). These enzymes were applied
according to the literature reviewed under the
conditions of pH and temperature indicated by the
manufacturer. In Table 2 are presented the principal
characteristics of the enzymes used.
It was studied the action of Texazym PES,
Aspergillus oryzae and Aspergillus niger, applied at
different concentrations (0.06, 0.09, 0.12, 0.15 and
0.18 U) and different incubation times (30, 60, 90
minutes and 24 hours) with a liquor ratio of 1:25.
The enzymatic treatments with Texazym were
performed at 30ºC, using 50 mM of sodium acetate
buffer solution (pH 5.5). The enzymatic treatments
with Aspergillus niger and Aspergillus oryzae were
performed by incubating 2 g of polyester fabric at
45ºC and 40ºC, using 50 mM phosphate buffer (pH
7.0).
Immediately before and after treatments all
samples were placed in a standard atmosphere
(
20 ± 2º C, 65% HR) during 24±2 hours.
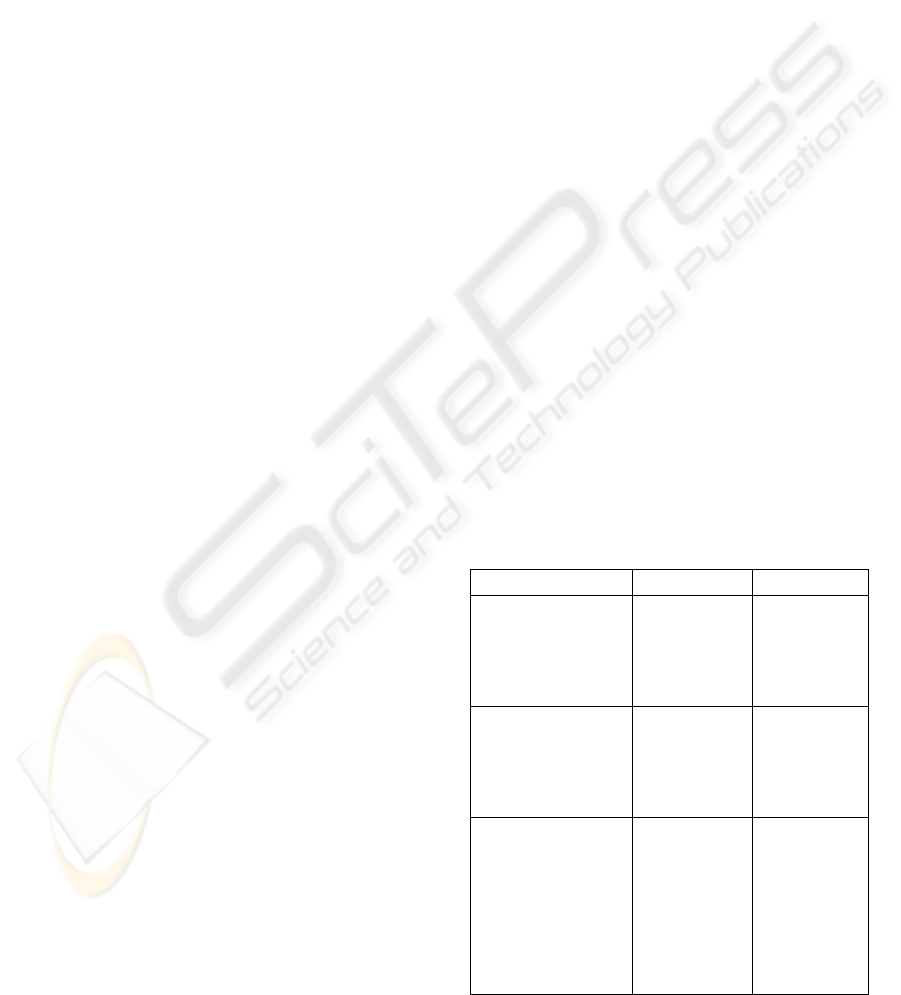
Table 1: Fabric characterization.
Characterization Test Method Value
Warp direction
Linear Mass (Tex)
Density
(Yarns/cm)
Diameter (Den)
NP – 4105
NP – EN
1049-2
NP – 3160
48.47
21.4
3.3
Weft direction
Linear Mass (Tex)
Density
(Yarns/cm)
Diameter (Den)
NP – 4105
NP – EN
1049-2
NP – 3160
36.67
32
3.3
Weaving
construction
Mass per area
(g/m
2
)
Composition
NP EN 1700
NP EN 1701
NP EN 1808.
2247 and
2248
Batavia twill
211.65
100%
Polyester
IMPROVING SURFACE ENERGY AND HYDROPHILIZATION OF POLY(ETHYLENE TEREPHTHALATE) BY
ENZYMATIC TREATMENTS
269
2.3 The Dyes
The dyes were selected to fulfill the objectives
focalized on assessing the number of hydroxyl and
carboxyl groups.
Therefore, a reactive dye Remazol Black B (C.I.
Reactive black 5, from DyStar) and a cationic dye
(Methylene blue, from Sigma-Aldrivh) were
selected. The reason for this choice is essentially the
ability to establish bonds with hydroxyl and
carboxyl end groups in the polyester fabric,
respectively.
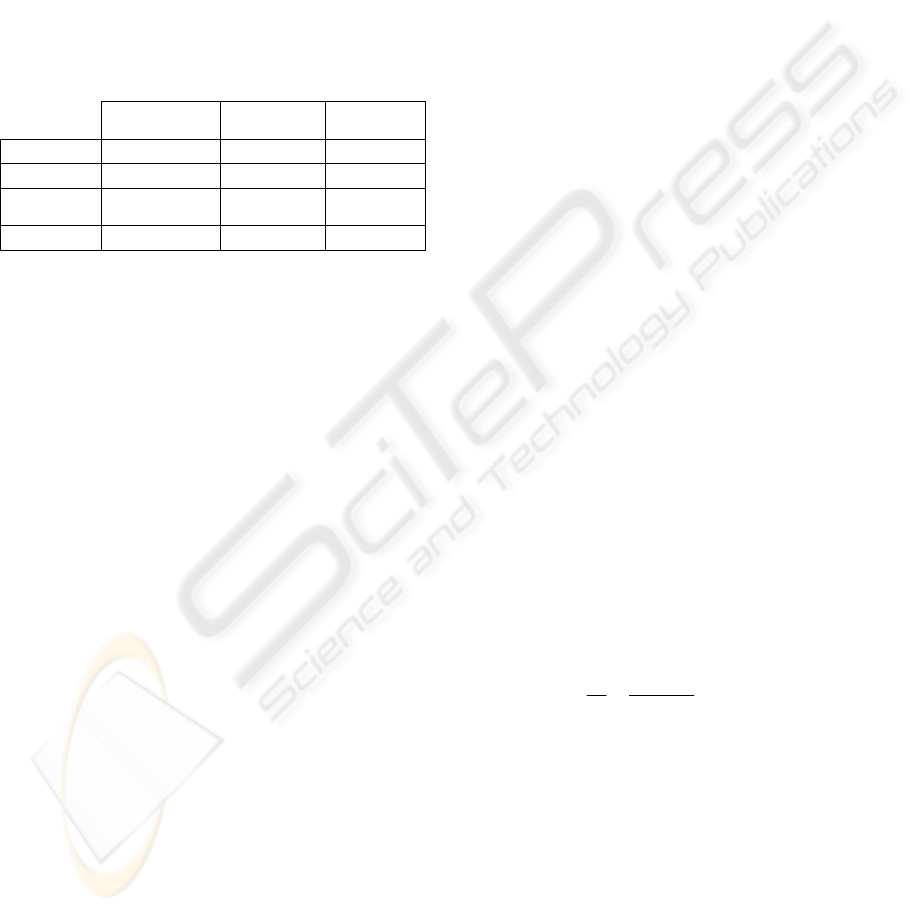
Table 2: Characterization of the enzymes.
Texazym PES
sp 5
Aspergillus
o.
Aspergillus
n.
pH Optimal 5.5 7.0 7.0
Temp. (º) 30 40 45
Origin Thermobifida
fusca
Aspergillus
oryzae
Aspergillus
niger
Activity 0.6 U/ml 50 U/mg 4 U/g
2.4 Evaluation of the Effectiveness
of the Enzymatic Treatments
2.4.1 Determination of the Contact Angle
and Surface Energy Estimation
The Dataphysics is composed by a camera, a
computer and a monitor which are used to measure
the contact angle on the samples. Liquid drops were
dispersed on each fabric sample using a micrometer
pipette. The image of each drop was captured by the
camera connected to a computer, and the captured
images were viewed at the monitor. The standard
testing methods were according to Tappi T 558 pm-
95. The liquid used in the experiment was glycerol
and a drop of 5 µl was deposited on the fabric
surface and the contact angles were measured. The
measurements were performed, after one week since
enzymatic application, were repeated eight times and
the average was calculated. Afterwards, surface
energies were determined according to the Owens-
Wendt approach (Owens and Wendt. 1969). This
method takes into account the dispersive and polar
components of the surface energy. Using different
test liquids, in this case water and glycerol, it is
possible to calculate the solid surface energy as the
sum of the polar and dispersive contributions.
Constant values for the test liquids used for contact
angle measurements are as follows:
Water: γ = 72.8 mJ/m
2
; γ
s
d
= 21.8 mJ/m
2
; γ
s
p
= 51.0
mJ/m
2
;
Glycerol: γ = 64.0 mJ/m
2
; γ
s
d
= 34.0 mJ/m
2
; γ
s
p
=
30.0 mJ/m
2
.
2.4.2 Wicking Rate
The determination of the wicking rate by measuring
the rising height was according to DIN 53924
vertical wicking tests and performed after one week
since the enzymatic application. Samples of 3cm ×
10 cm were prepared and were suspended in a
standard atmosphere (20 ± 2º C. 65% HR) for 24±2
hours. The samples were then placed in a solution
0.05 % w.o.f. of dye (Methylene Blue), and
immersed at a height of 1 cm. After 10 minutes the
samples were removed and the rinsing height was
measured up. It was used a solution of dye instead of
water to facilitate the reading and the measurement
of the rinsing height.
2.4.3 Determination of Carboxyl End
Groups
The carboxyl end groups were determinate after
dyeing the samples at 50º C with a cationic dye
(Methylene Blue. 0.5 % (w.o.f.)) during 20 minutes.
Subsequently, the samples were washed in hot and
cold water and then dried in an oven at 40º C during
24 hours. The dyed samples were analyzed in a
reflectance measuring apparatus (Spectraflash 300
Datacolor, LAV/Spec. Incl., d/8. D65/10º). This
procedure aims to evaluate the increase or decrease
in the intensity of color (by determining the value of
K/S), in order to evaluate the formation of carboxyl
groups. The relative color strength (K/S values)
were established according to the Kubelka-Munk
equation (1), where K and S stands for the
absorption and scattering coefficients and R stands
for the reflectance value, respectively (Shah and
Gandhi, 1990; Pandiyaraj and Selvaranjan, 2008):
(
)
R
R
S
K
2
1
2
−
=
(1)
An increase in the value of K/S, when compared
to the non-treated sample, indicates an increase of
carboxyl groups known to react with this type of
dye.
2.4.4 Determination of Hydroxyl End
Groups
The hydroxyl end groups in the non-treated and
treated samples were determinate by a dyeing
procedure performed at 60º C with a reactive dye
(Remazol Black B) during 90 minutes. The dye bath
contains 2% w.o.f. of dye, 20 mg.ml
-1
of Na
2
CO
3
BIODEVICES 2009 - International Conference on Biomedical Electronics and Devices
270
and 60 mg.ml
-1
of Na
2
SO
4
, pH 11, with a liquor ratio
1:100 and a mechanical agitation of 40 rpm.
Subsequently, the samples were washed in hot and
cold water and then dried in an oven at 40 ºC during
24 hours. After dyeing, the samples were analyzed
in the reflectance measuring apparatus above
described to evaluate the increase or decrease in the
intensity of color, in order to evaluate the formation
of hydroxyl groups. An increase in the K/S values
indicates an increase in the formation of hydroxyl
groups which react covalently with this type of dye.
2.5 Quality Control Test
2.5.1 SEM Analysis
The surface morphology of the treated polyester
fabric was observed using a scanning electron
microscope. SEM analysis was performed in all
samples after one week since the application of the
enzymatic treatments, using a HITACHI S2700
Electron Microscope and an EMITECH-K550 gold
evaporator.
2.5.2 Determination of the Resistance to
Abrasion
The Martindale evaluation system was used for
measuring the resistance to abrasion (mechanical
properties) of the non-treated and treated samples.
The standard testing method was according to IWS
TM 112. In this method, the samples are tested
under a weight of 9 KPa and run until the rupture of
two yarns.
3 RESULTS AND DISCUSSION
3.1 Evaluation of the Effectiveness of
the Enzymatic Treatments
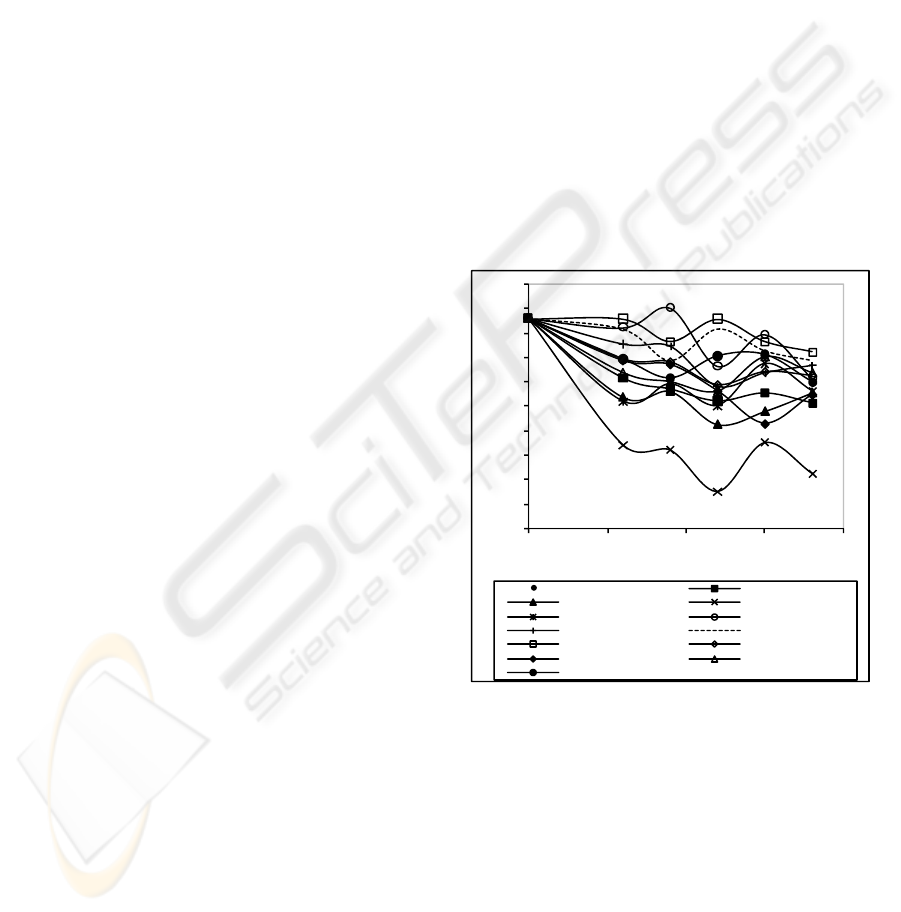
The results for the effect of the enzymatic
treatments, over the contact angle and the
hydrophilicity are indicated in Figures 1 and 2. The
results for the contact angle (Figure 1) showed a
slight decrease for all enzyme concentration, but the
most significant decrease was observed with
Texazym PES, when compared to the non-enzymatic
treated sample (Control). For the others enzymes
(Aspergillus niger and Aspergillus oryzae) it can be
also observed a slight decrease. However, for higher
concentrations of enzyme and incubation times a
significant decrease of contact angle in relation to
the control can be observed, being in accordance
with the results obtained by other authors (Hsieh e
Cram. 1998).
After a comparative analysis, we can conclude
that either the use of Aspergillus oryzae or an
Aspergillus niger made possible to achieve a lower
contact angle, hence greater hydrophilicity for
polyester fabric. However the Texazym PES led to
the better results. Definitely, by applying the
Texazym PES with a concentration of 0.12 U during
90 minutes it can be achieved a contact angle of
87.45º; by applying the Aspergillus niger with a
concentration of 0.15 U during 60 minutes a contact
angle of 101.50º, is obtained.
The use of Aspergillus oryzae, (0.12 U during 60
min) also allows to achieve lower values for the
contact angle (109.03º). The more favourable
conditions to decrease the contact angle seens to be
using the Texazym PES with a concentration of 0.12
U during 90 minutes. For lower incubation times (30
to 60 minutes) there are no important changes in the
contact angle values.
80
85
90
95
100
105
110
115
120
125
130
0,00 0,05 0,10 0,15 0,20
Contact Angle (º)
Enzyme concentration (U)
Contro
l
Texazyme - 30 min
Texazym - 60 min Texazym - 90 min
Texazym - 1440 min Oryzae - 30 min
Oryzae - 60 min Oryzae - 90 min
Oryzae - 1440 min Niger - 30 min
Niger - 60 min Niger - 90 min
Niger 1440 min
Figure 1: Values of contact angle for all enzymes.
The results of hydrophilicity obtained by the
effect of capillary are illustrated in Figure 2. Their
analysis indicates that the best results are achieved
using the Texazym PES, followed by the use of the
Aspergillus niger. In those circumstances it’s
obtained a wicking height of 5.40 cm and 4.60 cm,
respectively. Other studies revealed that the
application of other enzymes promoted similar
results, however with a lower wicking height (≈ 4
cm) (Alisch-Mark et al, 2006). In agreement with
the results of the contact angle and hydrophilicity,
the use of Aspergillus oryzae leads to lower values
IMPROVING SURFACE ENERGY AND HYDROPHILIZATION OF POLY(ETHYLENE TEREPHTHALATE) BY
ENZYMATIC TREATMENTS
271
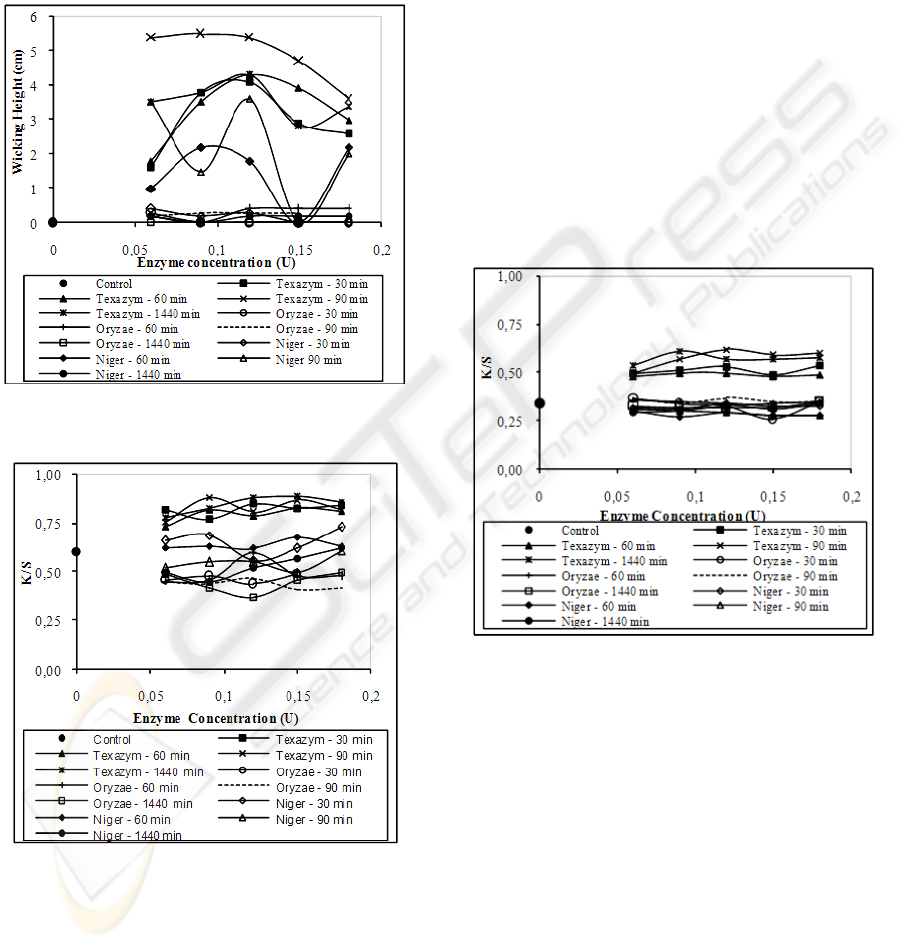
in the capacity of absorption of water by capillarity
effect (Figure 2).
The results obtained by the analysis of the
carboxyl end groups (Figure 3), formed by the
application of Tezaxym PES for any concentration
and incubation time, showed a significant increase
detected by the reaction with the cationic dye, when
compared to the other enzymes and the control.
Figure 2: Values of hydrophilicity (expressed by the
wicking height).
Figure 3: Values of K/S (620 nm), after dyeing with
cationic dye.
The more favourable conditions to the grafting of
these groups are by applying a concentration of 0.12
to 0.15 U for any incubation time. These results are
in accordance with the results obtained before
(contact angle and hydrophilicity), showing that the
presence of carboxyl groups favours the
establishment of hydrogen bonds with water. An
application with Aspergillus oryzae seems to be less
desirable with regards with the formation of these
functional groups, reflecting up in the lower capacity
of the treated fabrics to absorb water.
The formation or not of the hydroxyl groups,
using different enzymes, can be indirectly measured
by the higher or lower intensity of color that results
from the reaction of a reactive dye with the hydroxyl
groups (-OH) by the formation of covalent bonds.
After analyzing the results presented in Figure 4, it
can be concluded that a higher formation of the
hydroxyl groups is achieved by using the Texazym
PES, whichever the incubation time, comparing with
the others enzymes and with the control. This feature
is extremely important with respect to future
applications of this enzyme in the textile industry,
where the incubation time is a cruel factor in textile
treatments.
Figure 4: Values of K/S (600 nm), after dyeing with
reactive dye.
To better elucidate which were the best
conditions for each enzyme, considering the best
results of the contact angle and wicking height, the
selected optimum conditions for each enzyme are
presented in Table 3. The best results regarding the
lower contact angle and higher wicking height are
obtained for the Texazym PES us1ing a
concentration of 0.12 U during 90 minutes, followed
by the Aspergillus niger using 0.15 U, 60 minutes
and finally, by the Aspergillus oryzae using 0.12U
during 60 minutes. In those conditions, an
application with Texazym PES yielded a rinsing
height of 5.40 cm, which is a very good value when
compared with the ones obtained by other authors
(Alisch-Mark et al, 2006). In this study they
BIODEVICES 2009 - International Conference on Biomedical Electronics and Devices
272
achieved a rinsing height of 4.2 cm (approximately)
after 48 hours of incubation with T.fusca (16 U). Our
results revealed an important achievement since
much lower incubation time and enzyme
concentration was used.
However, the other results regarding the quality
control parameters are important to better define the
optimal conditions overall.
Table 3: Optimal conditions for different operating
parameters.
Contact
Angle
(glycerol)
(º)
Rising
Height
(cm)
Cationic
Dye
K/S
Reactive
Dye
K/S
Control 122.95 2.7 0.60 0.34
Texazym
PES
(0.12 U,
90min)
87.45 5.40 0.81 0.62
Asperg.
niger
(0.15 U,
60 min)
101.50 4.60 0.50 0.28
Asperg.
oryzae
(0.12U,
60 min)
109.03 0.40 0.60 0.34
The total surface energy, the dispersion
component and polar component of the fabrics were
calculated according to the Owens-Wendt approach
(γ
s
p
. γ
s
d
. γ
s
are the polar component, the dispersion
component and the total surface energy, of fabric,
respectively) and are indicated in Tables 4 to 6.
It is clear that the total surface energy increases
with incubation time and enzyme concentration. The
values of surface energy obtained for the control was
0.60 mJ/m
2
for the polar component, 3.28 mJ/m
2
for
the dispersion component and 3.88 mJ/m
2
for the
total surface energy. Analyzing the results, an
important increase of the polar component for the
Texazym PES is verified when compared with the
other enzymes. The higher value (187.31 mJ/m
2
)
was obtained to the incubation time of 1440 minutes
with a concentration of 0.15U. However, good
values for the polar component can be obtained for
lower incubation times (183.85 mJ/m
2
, 153.52
mJ/m
2
), when a concentration of enzyme between
0.12U to 0.18 U is used during an incubation time of
90 minutes. Considering the other enzymes, much
lower values for the polar component are obtained:
30.36 mJ/m
2
(using 0.18 U during 60 min for the
Aspergillus niger), and 11.47 mJ/m
2
and 10.04
mJ/m
2
using 0.12 U during 30 minutes and 0.15 U
during 60 minutes, respectively, for the Aspergillus
oryzae.
Table 4: Surface energy determination for different
operating parameters of Texazym PES.
Time
(min)
Concentration of
enzyme (U)
γ
s
p
(mJ/m
2
)
γ
s
d
(mJ/m
2
)
γ
s
(mJ/m
2
)
30
0.06 22.49 1.9 29.4
0.09 67.88 15.9 83.78
0.12 99.57 29.15 128.72
0.15 97.6 29.76 127.36
0.18 82.13 20.29 102.41
60
0.06 31.02 1.78 32.8
0.09 81.07 21.81 102.88
0.12 98.54 24.12 122.66
0.15 110.76 32.74 143.5
0.18 102.32 32.31 134.62
90
0.06 61.00 6.32 67.32
0.09 122.07 29.78 151.85
0.12 183.85 63.43 247.38
0.15 153.52 39.21 192.73
0.18 141.68 29.69 171.36
1440
0.06 45.98 5.70 51.68
0.09 117.12 42.4 159.53
0.12 147.85 54.21 202.06
0.15 187.31 91.66 278.97
0.18 145.94 57.2 203.14
Table 5: Surface energy determination for different
operating parameters of Aspergillus niger.
Time
(min)
Concentration of
enzyme (U)
γ
s
p
(mJ/m
2
)
γ
s
d
(mJ/m
2
)
γ
s
(mJ/m
2
)
30
0.06 2.67 2.76 5.43
0.09 3.9 1.94 5.84
0.12 0.1 11.34 11.43
0.15 0.00 11.55 11.55
0.18 14.95 0.02 14.97
60
0.06 0.52 6.49 7.01
0.09 0.88 5.84 6.71
0.12 0.04 15.78 15.82
0.15 0.45 25.27 25.72
0.18 30.36 1.77 32.13
90
0.06 2.44 2.94 5.37
0.09 3.11 3.82 6.93
0.12 0.28 10.81 11.09
0.15 1.79 3.62 5.41
0.18 5.82 1.39 7.21
1440
0.06 5.31 1.01 6.38
0.09 9.47 0.42 9.89
0.12 21.49 1.47 22.97
0.15 3.03 2.19 5.22
0.18 0.42 8.99 9.42
It is mainly due to the incorporation of polar
groups like carboxyl and hydroxyl on the fabric
IMPROVING SURFACE ENERGY AND HYDROPHILIZATION OF POLY(ETHYLENE TEREPHTHALATE) BY
ENZYMATIC TREATMENTS
273
surface, verified by other authors (Pandiyaraj and
Selvarajan, 2008) and in our present study.
Table 6: Surface energy determination for different
operating parameters of Aspergillus oryzae.
Time
(min)
Concentration
of enzyme (U)
γ
s
p
(mJ/m
2
)
γ
s
d
(mJ/m
2
)
γ
s
(mJ/m
2
)
30
0.06 18.48 2.11 20.59
0.09 4.85 0.07 4.92
0.12 3.24 2.66 5.09
0.15 11.47 0.17 11.64
0.18 0.92 6.98 7.89
60
0.06 2.66 1.97 4.63
0.09 6.74 0.29 7.03
0.12 0.05 12.22 12.27
0.15 10.04 0.22 10.26
0.18 1.91 4.07 5.98
90
0.06 6.68 0.06 6.74
0.09 0.00 10.31 10.31
0.12 8.77 0.01 8.78
0.15 5.49 0.72 6.21
0.18 0.95 5.31 6.26
1440
0.06 1.18 2.21 3.39
0.09 3.16 1.48 4.64
0.12 5.69 0.07 5.76
0.15 2.05 2.39 4.43
0.18 1.54 3.61 5.15
The change in the polar component verified for
all enzymatic treatments, when applying different
enzyme concentrations and incubation times, affects
the total surface energy as a function of enzymatic
operating parameters. These results show that the
main contribution to the increase in surface energy is
due to the polar components which can incorporate
with moisture through hydrogen bonds. Thus, good
wettability is obtained when the values of polar
component is high (Pandiyaraj and Selvarajan,
2008), being the most favorable conditions the one’s
for the enzymatic treatment with Texazym PES.
3.2 Quality Control Tests
With the test of resistance to abrasion was intended
to assess whether the mechanical properties of
polyester fabrics would have suffered any significant
change after the treatment with enzymes (Texazym
PES, Aspergillus niger and Aspergillus oryzae).
These results were referenced in Table 7.
After their evaluation it can be seen that there
weren´t verify important losses in the resistances to
abrasion using any enzymatic treatment when
compared with the control. The loss of around 4% in
the resistance is perfectively safe for the textile.
Table 7: Values of the resistance to the abrasion in the
optimal conditions for each enzymatic treatment.
Resistance to
abrasion (cycles)
Control 24000
Texazym PES (0.12 U. 90min) 23000
Aspergillus niger (0.15 U. 60 min) 23000
Aspergillus oryzae (0.12U. 60 min) 23000
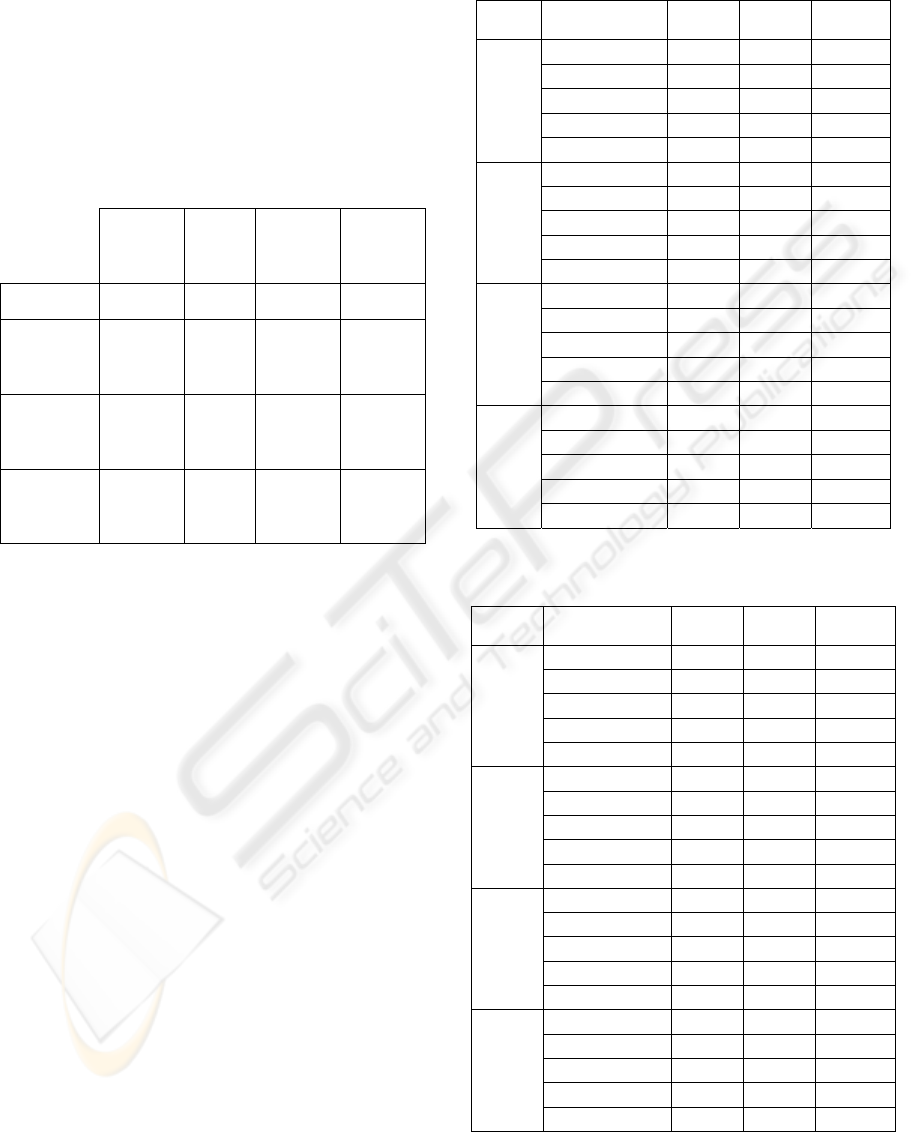
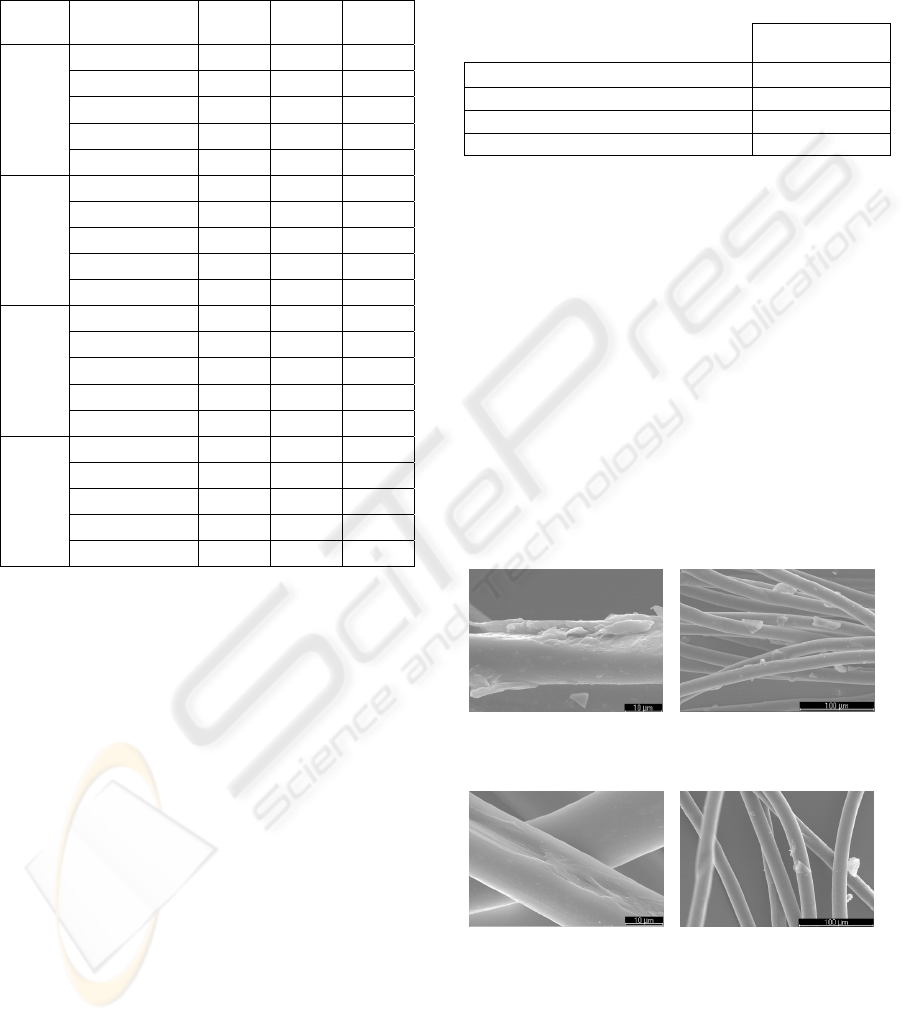
The SEM analysis aims to observe any surface
changes on fibers after the enzymatic treatment. The
images presented are referred to the control and to
the optimum conditions of application of the
Texazym PES. The others enzymes didn’t revealed
any changes when compared to the control. Looking
at images of Figures 5 and 6 it can be seen that there
is no degradation of the surface of the fibers under
study. In contrary, the use of the Texazym PES
applied under the optimum conditions seems to be
the cause of a greater effect of cleaning since it can
be seen the presence of a smaller number of particles
deposited on the fibers (oligomers). This effect of
total or partial elimination of them is of utmost
importance, especially in what concerns to adhesion
and dyeing properties.
A B
Figure 5: SEM micrographs of Control samples (A-
magnification: 2500x, B-magnification: 500x).
A B
Figure 6: SEM micrographs (Texazym PES, using 0.12 U
during 90 min).
4 CONCLUSIONS
The effect of the different enzymatic treatments,
incubation time and enzyme concentration on the
BIODEVICES 2009 - International Conference on Biomedical Electronics and Devices
274

hydrophilization of PET fabrics and surface energy
increasing was analyzed. Depending on the
enzymatic process used, the wettability of PET
fabrics can be significantly improved. It was found
the formation of carboxyl and hydroxyl polar groups
by the Texazym PES action. The enhancement of
polar groups on the fabric surface was confirmed
with a cationic dye (Methylene blue) and a reactive
dye (Remazol black B). Thus, improvement in
adhesion properties can be expected.
Our results revealed an important achievement
since much lower incubation time and enzyme
concentration was used, comparing to previous
investigations.
The increasing in the hydrophilicity and surface
energy of PET fabrics are also known to have
importance in the increasing in adhesion of cells and
tissues, which is a very important property for
permanent biomedical implants.
REFERENCES
Alisch-Mark, M., Herrmann, A., Zimmermenn, W., 2006.
Increase of the hidrophilicity of polyethylene
terephthalate fibres by hidrolases from T.fusca and
F.solani f. sp. pisi. Biotechnology Letters, 28, 681-685.
Guebitz, G. M., Cavaco- Paulo, A., 2008. Enzymes go big:
surface hydrolysis and functionalisation of synthetic
polymers. Trends in Biotechnology, 26 (1), 32-38.
Heumann, S., Eberl, A., Pobeheim, H., Liebminger, S.,
Fischer-Colbrie, G., Almansa, E., Cavaco-Paulo, A.,
Guebitz, G.M., 2006. New model substrates for
enzymes hydrolysing polyethyleneterephthalate and
polyamide fibres. J. Biochem. Biophys. Methods, 39,
89-99.
Hseih, Y. and Cram, L. A., 1998. Enzymatic Hydrolysis to
Improve Wetting and Absorbency of Polyester
Fabrics. Textile Research Journal, 68 (5), 311-319
Inagaki, N., Tasaka, S., Narushima, K., Kobayashi, H.,
2001. Surface Modification of PET Films by Pulse
Argon Plasma. Journal of Applied Polymer Science,
85 (14), 2845-2852.
Owens, D.K., Wendt, R.C., 1969. Estimation of the
surface free energy of polymers. J. Appl. Polym. Sci.,
13, 174.
Pandiyaraj, K.N., Selvarajan, V., 2008. Non-Thermal
plasma treatment for hydrophilicity improvement of
grey cotton fabrics. Journal of Materials Processing
Technology, 199,130-139.
Shah, H.S., Gandhi, R.S., 1990. Instrumental Colour
Measurements and Computer aided Colour matching
for Textile, (Eds) Mahajan Book Distributors.
Vertommen, M.A.M.E., Nierstrasz*, V.A., van der Veer,
M., Warmoeskerken, M.M.C.G., 2005. Enzymatic
surface modification of poly(ethylene terephthalate).
Journal of Biotechnology, 120, 376-386.
Yang, S., Gupta, M.C., 2004. Surface modification of
polyethyleneterephthalate by an atmospheric-pressure
plasma surce. Surface & Coatings Technology, 187
(2/3), 172-176.
IMPROVING SURFACE ENERGY AND HYDROPHILIZATION OF POLY(ETHYLENE TEREPHTHALATE) BY
ENZYMATIC TREATMENTS
275
