
DROPLET MANIPULATION ON HIGH ADHESION
SUPERHYDROPHOBIC SURFACES
Daisuke Ishii, Masatusgu Shimomura
WPI-AIMR, Tohoku University,2-1-1 Katahira, Aoba-ku, Sendai 980-8577, Japan
CREST, Japan Science and Technology Agency, 4-1-8 Hon-cho, Kawaguchi 332-0012, Japan
Hiroshi Yabu
IMRAM, Tohoku University,2-1-1 Katahira, Aoba-ku, Sendai 980-8577, Japan
CREST, Japan Science and Technology Agency, 4-1-8 Hon-cho, Kawaguchi 332-0012, Japan
Keywords: Superhydrophobicity, Microfluidics, Water droplet, Adhesion, Lotus effect.
Abstract: Micro droplet handling is very important for micro and nano fluidic devices and an intelligent bio interface.
Micro droplet transfer via high adhesion superhydrophobic surfaces has been reported in recent years. We
demonstrated water droplet adhesion controllable superhydrophobic metalpolymer surfaces. Moreover we
achieved micro droplet transfer between superhydrophobic surfaces by using different droplet adhesion
properties. Water micro droplets were transferred from a low-adhesive superhydrophobic surface to a
middle-adhesive superhydrophobic surface via a high-adhesive superhydrophobic surface without any mass
loss. After transferred droplet possessed high water contact angle over 150 degrees. These moving processes
were performed repeatedly. Droplet handlings on the adhesion superhydrophobic surfaces will be expected
for fluidic bio devises with energy saving.
1 INTRODUCTION
Droplet manipulations mimicking behaviours on
plant or insect surfaces such as lotus leaf effect are
now interesting because simple surface structures
provide amazing functionalities. Superhydrophobic
surfaces which have the water contact angle lager
than or near 150° are much paid attention, since its
good water repellent property is used various
applications in coating and electronic technologies
(Zhang, 2008). Many researchers have been reported
to obtain strong water repellent surface such as a
hydrophobic fractal surface (Onda, 1996) and a
nanopin array surface (Hosono, 2005). Recently
several reports were published about water droplet
adhesive superhydrophobic surfaces in mimicry of
gecko’s feet (Cho, 2008) and rose’s petals (Feng,
2008). These adhesion properties were caused by
van der Waals’ force on large real surface area
against small apparent surface area. It was difficult
to control the adhesion forces because the adhesion
was caused by the surface structures.
Herein we demonstrated that a superhydrophobic
metal−polymer (MP) surface with different droplet
adhesion properties. The adhesive superhydrophobic
surfaces were composed of hexagonally ordered
polymer pillar arrays made from a self-organized
honeycomb-patterned polystyrene film (Yabu, 2005)
and metal micro domes deposited by nickel
electroless plating (Ishii, 2008). The dome density
was changed by catalyzation process for electroless
plating.
Droplet manipulations such as a transfer were
achieved by using the MP surface possessing
different water adhesion force. Micro droplet
handling by control of wettability is important for
further understanding of superhydrophobic surfaces
and application in microfluidic bio devices.
2 EXPERIMENTAL
2.1 Preparation Method
The superhydrophobic metal−polymer surface (MP
surface) composed of hydrophobic polymer pillar
113
Ishii D., Shimomura M. and Yabu H. (2009).
DROPLET MANIPULATION ON HIGH ADHESION SUPERHYDROPHOBIC SURFACES.
In Proceedings of the International Conference on Biomedical Electronics and Devices, pages 113-116
DOI: 10.5220/0001553901130116
Copyright
c
SciTePress
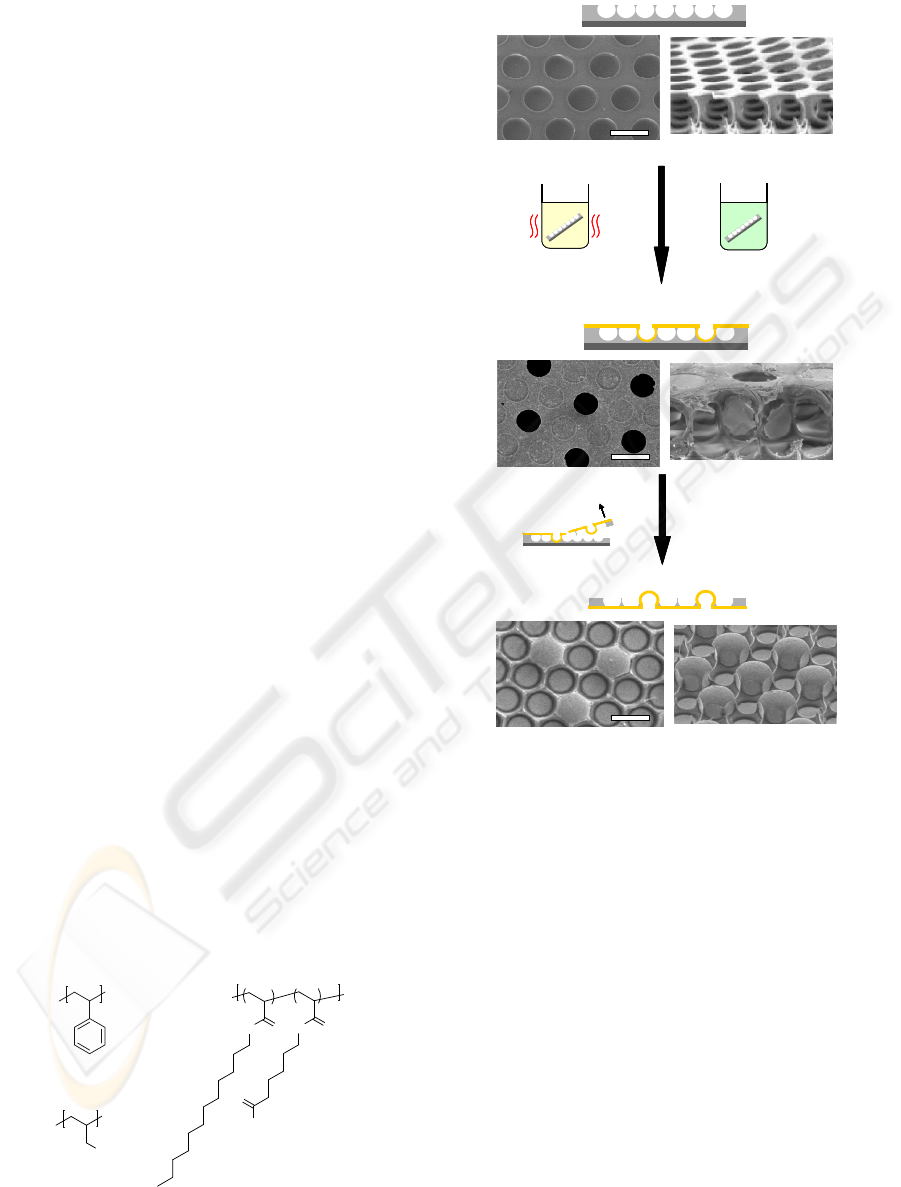
arrays and metal micro domes was fabricated by
electroless plating for honeycomb-patterned polymer
films and peeling process (See Figure 2).
According to our previous report (Karthaus,
2000), the honeycomb films were prepared by
casting a chloroform solution of 10:1 mixture of
polystyrene (PS; M
w
= 280 000 g mol
-1
) and
synthesized amphiphilic copolymer (CAP; M
w
=
270 000 g mol
-1
) on a glass substrate with
hexagonally condensed water droplet arrays. The
honeycomb film cut to 1 × 1 cm
2
was soaked in a
catalytic mixture solution of 6.0 ml containing 0.010
mol dm
-3
poly(allylamine hydrochloride) (PAH; M
w
= 14 000 g mol
-1
) and 0.010 mol dm
-3
PdCl
2
at 25°C.
The catalytic solution was gradually heated to 30°C,
45°C, and 60°C, respectively, and kept for 10 min
under horizontal shaking at 10 rpm. Treated
honeycomb films were immersed in a nickel plating
bath (Ishii, 2006) at 25°C containing 0.10 mol dm
-3
Ni(H
2
PO
2
)
2
⋅6H
2
O, 0.19 mol dm
-3
H
3
BO
3
, 0.030 mol
dm
-3
CH
3
COONa and 0.0098 mol dm
-3
(NH
4
)
2
SO
4
without any rinse and drying. Then the plating bath
including the treated honeycomb film was heated to
70°C and kept for 2h with no stirring. After rinsing
and drying, a nickel layer was covered on the
honeycomb film. After electroless plating, metallic
faces of the nickel-covered honeycomb films were
adhered on an acryl substrate by an epoxy resin.
After heating at 70°C for 2h, a lower half layer of
the nickel-covered honeycomb film was peeled off
from the acryl substrate.
2.2 Physical Measurements
Surface structures of the MP surfaces were observed
by a scanning electron microscope (SEM; Hitachi S-
5200, Japan). A water contact angle (WCA) to 3 mg
water droplet on a surface was measured by contact
angle meter (Kyowa Interface Science DW-300,
Japan). A sliding angle (SA) was measured to tilt the
surfaces with a micro-droplet of 5 mg. Density of
the metal dome which is defined by division of the
*
*
0.8
0.2
N
N
O
O
OH
O
n
*
*
n
*
*
n
NH
2
HCl
PS
CAP
PAH
Figure 1: Chemical structures used in this report.
70°C
(a) Honeycomb film
30°C, 45°C, 60°C
1) Catalyzation
2) Electroless plating
3) Rinse and drying
4) Peel off top layer
(b) Nickel-covered honeycomb film
(c) Metal polymer co-existing surfaces
70°C
(a) Honeycomb film
30°C, 45°C, 60°C
1) Catalyzation
2) Electroless plating
3) Rinse and drying
4) Peel off top layer
(b) Nickel-covered honeycomb film
(c) Metal polymer co-existing surfaces
Figure 2: Schematic illustrations of a preparation method
of MP surfaces. SEM images of top (left) and tilt (right)
views of (a) a honeycomb film, (b) a nickel-covered
honeycomb film fabricated by immersion in the catalytic
mixture solution at 45°C, and (c) a MP surface fabricated
by peeling off a top layer of the nickel-covered
honeycomb film shown in Figure 2b. (Scale bar: 10 μm).
number of metal domes by the number of
honeycomb holes was calculated by means of low-
magnified SEM images.
2.3 Droplet Manipulations
Droplet manipulations such as droplet transfer were
demonstrated by using the MP surfaces with
deferent adhesion properties. Figure 3 shows a
schematic illustration of water droplet transfer. The
5-mg water droplet was prepared on the MP surface
fabricated by using the catalytic mixture solution at
30°C. Then the MP surface fabricated by using the
catalytic mixture solution at 60°C was closed to the
BIODEVICES 2009 - International Conference on Biomedical Electronics and Devices
114
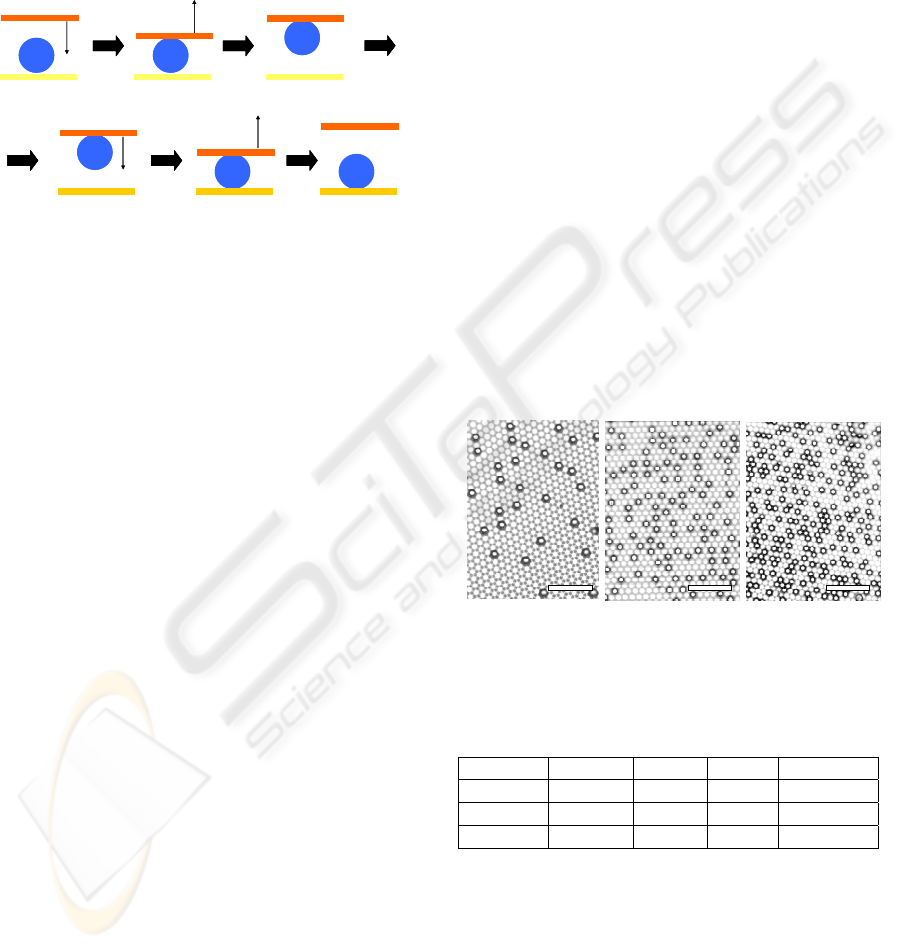
water droplet from above and touched a little bit.
The upper MP surface was pulled up slowly from
the lower MP surface. Then the upper MP surface
catching the water droplet was closed and touched to
the other MP surface fabricated by using the
catalytic mixture solution at 45°C. Finally the upper
MP surface was pulled up again.
Touch
High-adhesion
Low-adhesion
Middle-adhesion
Pull up
Pull upTouch
Transfer
Touch
High-adhesion
Low-adhesion
Middle-adhesion
Pull up
Pull upTouch
Transfer
Figure 3: Schematic model of a micro-droplet transfer by
using the MP surfaces with different adhesion properties.
3 RESULTS AND DISCUSSION
3.1 Superhydrophobic Metal−polymer
Surface
SEM images of a honeycomb film, a nickel covered
honeycomb film fabricated by immersion in the
catalytic solution at 45°C, and a MP surface
fabricated by immersion in the catalytic solution at
45°C are inserted in Figure 2. An average diameter
of a honeycomb hole was about 7 μm. A nickel-
covered honeycomb film possessing some pores,
which were distributed in the honeycomb holes,
were obtained after electroless plating including
immersion in the catalytic mixture solution. A tilted
SEM image shown in a right column of Figure 2b
clears that the pores were openings of micro mono
vessels. When the temperature of the catalytic
mixture solution was changed low (30°C) and high
(60°C), the number of the vessels was decreasing
and increasing, respectively. In general, wettability
of all surfaces including the PS honeycomb film is
influenced by a solution temperature, because the
surface tension of all solutions is represented by
function of the solution temperature. This result
indicates that the number of the vessels was
dependent on wettability of the catalytic mixture
solution to the honeycomb film. In the case of
immersion in the catalytic solution at low
temperature, wettability of the honeycomb film was
low, so that the number of the vessels in the nickel-
covered honeycomb film was a few. On the other
hand, in the case of immersion in the one at high
temperature, the number of the vessels was much
because of good wettability to the honeycomb film.
The number of the vessels of the nickel-covered
honeycomb film was easily changed by the catalytic
mixture solution temperature.
The MP surfaces after peeling off the top half of
the nickel-covered honeycomb film were composed
of superhydrophobic PS pillar arrays and
hydrophilic nickel micro-domes as shown in Figure
2c. The nickel micro-dome was reverse side of the
micro mono vessel in the nickel-covered honeycomb
film. This result anticipates that density of the nickel
dome to the honeycomb hole is controlled indirectly
by temperature of the catalytic mixture solution.
Figure 4 shows SEM images of the MP surfaces
having different nickel dome density. The nickel
dome density of the MP surface prepared by
immersion in the catalytic mixture solution at 30°C,
45°C, and 60°C was about 3%, 15%, and 25%,
respectively. The surface properties such as surface
wettability and droplet adhesion properties were
controlled easily, because hydrophilic-hydrophobic
balance was varied by difference of the nickel dome
density (See Table 1).
(a) 30°C(b)45°C(c)60°C(a) 30°C(b)45°C(c)60°C(a) 30°C(b)45°C(c)60°C
Figure 4: SEM images of the superhydrophobic
metal−polymer surfaces fabricated by using the catalytic
mixture solution at (a) 30°C, (b) 45°C, and (c) 60°C. The
black dots indicate the nickel domes. (Scale bar: 100 μm).
Table 1: Surface properties of the MP surfaces.
Sample Density WCA SA Adhesion
30°C
3%
155° <5°
Low
45°C
15%
150° 30°
Middle
60°C
25%
145°
N/A High
3.2 Micro-droplet Transfer
The water droplet adhesion properties were
measured by water contact angles (WCAs) and
sliding angles (SAs). The MP surface with nickel
dome density of 3% possessed a WCA of 155° and a
SA of less than 5°, and was abbreviated as a low-
adhesion MP surface. The MP surface with dome
DROPLET MANIPULATION ON HIGH ADHESION SUPERHYDROPHOBIC SURFACES
115
density of 15% possessed a WCA of 150° and a SA
of 30° (a middle-adhesion MP surface). The MP
surface with dome density of 25% possessed a WCA
of 145° and a not measured SA because the droplet
adhered the surface when turned upside down (a
high-adhesion MP surface). As the dome density
was increasing, the WCA was decreasing and the SA
was increasing, which means that the hydrophilic
nickel domes gave the adhesion behaviors. This
result made clear that the adhesion property was
controlled by the quantity of the nickel dome easily
changed by the catalytic mixture solution
temperature for electroless plating.
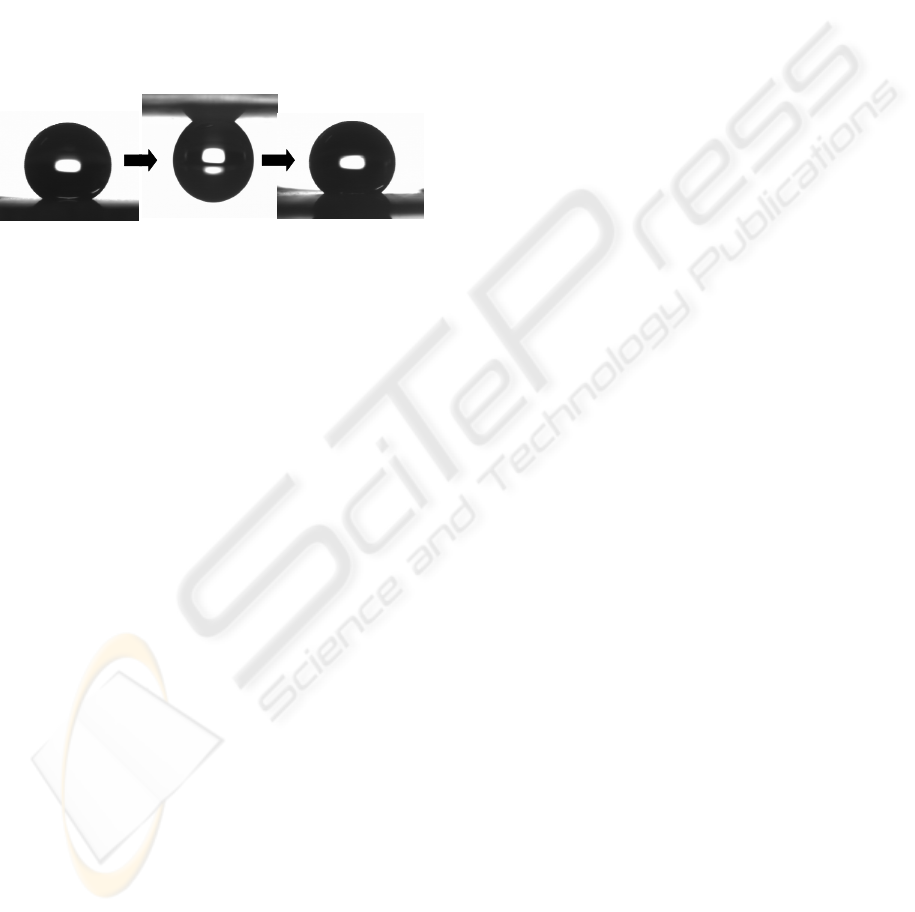
155° 150°
Low-adhesion surface
HIgh-adhesion surface
Middle-adhesion surface
155° 150°
Low-adhesion surface
HIgh-adhesion surface
Middle-adhesion surface
Figure 5: Droplet transfer of 5.0-mg water droplet from
the low-adhesion MP surface to the middle-adhesion MP
surface via the high-adhesion MP surface.
Water micro droplet transfer was attempted by
using the MP surfaces with different adhesion
properties as shown in Figure 5. A water droplet of 5
mg on the low-adhesion MP surface was carried
with the high-adhesion MP surface by means of
pulling off after slight contact from above. However,
in the case of the middle-adhesion MP surface, a
water droplet did not remove from the low-adhesion
MP surface. On the other hand, the high-adhesion
MP surface was not caught a water droplet on the
middle-adhesion MP surface from above. These
results suggest that the adhesion force of the high-
adhesion MP surface was stronger than that of the
low-adhesion MP surface plus gravity on the water
droplet, and weaker than that of the middle-adhesion
MP surface plus gravity on the water droplet. By
using this difference, the water droplet was
transferred from the low-adhesion MP surface to the
middle-adhesion MP surface via the high-adhesion
MP surface. After transfer, the water droplet on the
middle-adhesion MP surface was sliding when the
surface was tilted at about 30°. A droplet transfer
reported in the past is from a superhydrophobic
surface to a hydrophilic surface via an adhesion
superhydrophobic surface (Cho, 2008). Therefore,
after transfer, the water droplet is spreading, and is
unable to be handled. The novel transfer method in
this report remains a droplet shape after transfer, so
that the droplet was handily manipulated again.
These behaviors were useful to microfluidic devices,
bio interfaces, and micro-reactors.
4 CONCLUSIONS
We could fabricate water repellency and adhesion
properties of superhydrophobic metal-polymer
surfaces by electroless plating for self-organized
honeycomb films including immersion in a catalytic
Pd salt and a cationic polymer mixture solution. It
was found that a water contact angle and a water
droplet adhesion property were changed by metal
dome density which was easily controlled by the
temperature of the catalytic mixture solution.
Droplet transfer between superhydrophobic surfaces
was demonstrated by means of using the metal-
polymer surfaces with different adhesion properties.
REFERENCES
Cho, W. K., & Choi, I. S. (2008). Fabrication of hairy
polymeric films inspired by geckos: wetting and high
adhesion properties. Advanced Functional Materials,
18, 1089−1096.
Feng, L., Zhang, Y., Xi, J., Zhu, Y., Wang, N., Xia, F., &
Jiang, L. (2008). Petal effect: a superhydrophobic state
with high adhesive force. Langmuir, 24, 4114−4119.
Hosono, E.,Fujihara, S., Honma, I., & Zhou, H. (2005).
Superhydrophobic perpendicular nanopin film by the
bottom-up process. Journal of the American Chemical
Society, 127, 13458−13459.
Ishii, D., Yabu, H., Shimomura, M. (2008). Selective
metal deposition in hydrophobic porous cavities of
self-organized honeycomb-patterned polymer films by
all-wet electroless plating. Colloid and Surface; A
313–314, 590–594.
Ishii, D., Udatsu, M., Iyoda, T., Nagashima, T., Yamada
M., & Nakagawa, M. (2006). Electroless deposition
mechanisms on fibrous hydrogen-bonded molecular
aggregate to fabricate Ni-P hollow microfibers.
Chemistry of Materials. 18, 21522158.
Karthaus, O., Maruyama, N., Cieren, X., Shimomura, M.,
Hasegawa, H., & Hashimoto, T. (2000). Water-
assisted formation of micrometer-size honeycomb
patterns of polymers. Langmuir, 16, 6071−6076.
Onda, T., Shibuichi, S., Satoh, N., & Tsujii, K. (1996).
Super-water-repellent fractal surface. Langmuir, 12,
2125−2127.
Yabu, H., Takebayashi, M., Tanaka, M., & Shimomura,
M. (2005). Superhydrophobic and Lipophobic
Properties of Self-Organized Honeycomb and
Pincushion Structures. Langmuir, 21, 3235−3237.
Zhang, X., Shi, F., Niu, J., Jiang, Y., & Wang, Z. (2008).
Superhydrophobic surfaces: from structural control to
functional application. Journal of Materials Chemistry,
18, 621−633.
BIODEVICES 2009 - International Conference on Biomedical Electronics and Devices
116
