ADAPTIVE AURICULAR ELECTRICAL STIMULATION
CONTROLLED BY VITAL BIOSIGNALS
Transition from Fixed to Adaptive and Synchronized Electrical Stimulation
Controlled by Heart Rate Variability and Blood Perfusion
Eugenijus Kaniusas
Institute of Electrical Measurements and Circuit Design, Vienna University of Technology
Gusshausstrasse 27-29/E354, Vienna, Austria
Jozsef Constantin Szeles, Tilo Materna
Department of Surgery, University of Vienna, Vienna, Austria
Giedrius Varoneckas
Sleep Medicine Centre, Klaipeda University Hospital, Klaipeda, Lithuania
Keywords: Electrical stimulation, heart rate variability, physiological sensors, adaptive stimulation, ear.
Abstract: The auricular electrical punctual stimulation is usually applied for pain relief. The common application
involves fixed stimulation parameters, which makes the simulation insensitive to prevailing pain or stress
level and may lead to a disadvantageous over-stimulation. In order to address this issue, the given position
paper presents an experimental background leading to a conceptual design of an adaptive and synchronized
stimulation technique. Here parameters of the heart rate variability are used as stimulation biofeedback,
while the stimulating signal is synchronized with cardiac or respiratory activity to boost stimulation effects.
1 INTRODUCTION
The auricular electrical punctual stimulation (P-
Stim) is an electrical nerve stimulation technique,
newly introduced by Dr. Szeles (Szeles, 2001a). The
P-Stim is usually applied for acute and chronic pain
relief. A reduction of pain perception and pain-
relieving medications is attained (Szeles, 2001b;
Sator-Katzenschlager, 2006; Likar, 2007), even with
an induction of anaesthesia state (Litscher, 2007).
Furthermore, reduction of body mass index (BMI) in
obese patients (Szeles, 2001b), increase of blood
flow velocity and oxygenation (Szeles, 2004) were
reported during the P-Stim application. The
advantages of the electrical stimulation over
conventional (manual) acupuncture with respect to
pain relief, well-being and sleep quality were
documented in (Sator-Katzenschlager, 2004) for
extended periods of time up to 3 months.
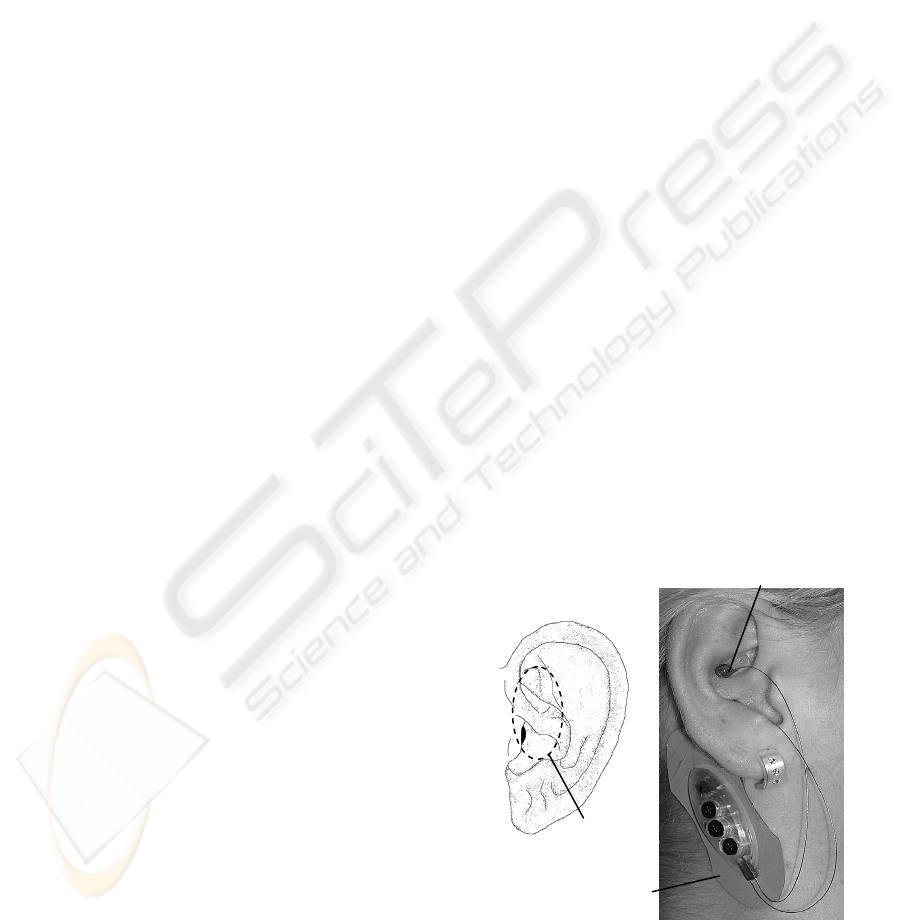
Figure 1: a) Ear with indicated approximate auricula
r
branch of vagus nerve according to (Peuker, 2002; Gao,
2008). b) Electrical punctual stimulation of the auricula
r
vagus nerve (P-Stim).
Stimulating device
(= reference electrode)
Vagal stimulating point
Vagal nerve
branch
a) b)
304
Kaniusas E., Szeles J., Materna T. and Varoneckas G. (2009).
ADAPTIVE AURICULAR ELECTRICAL STIMULATION CONTROLLED BY VITAL BIOSIGNALS - Transition from Fixed to Adaptive and Synchronized
Electrical Stimulation Controlled by Heart Rate Variability and Blood Perf.
In Proceedings of the International Conference on Biomedical Electronics and Devices, pages 304-309
DOI: 10.5220/0001779703040309
Copyright
c
SciTePress
The particular beneficial effects of the P-Stim are
still under discussion, whereas a number of the
following mechanisms seem to be involved. The
electrical stimulation of the afferent nerve receptors
may influence gate mechanisms in the central
nervous system (CNS), preventing pain-related
action impulses from reaching the CNS and avoiding
the person’s perception of pain. Furthermore, an
indirect stimulation of pain receptors and activation
of inhibitory pain control systems may be involved,
as well as a stimulated release of neurotransmitters,
e.g., endorphins and other endogenous opioids.
Though the efficiency of the P-Stim was subject-
ively proved in many cases and the P-Stim is already
in clinical use, only recently some objective and
statistical evidence was established on the
stimulation effects. Given that an auricular branch of
vagal nerve (Fig. 1a) is electrically stimulated by the
P-Stim device (Fig. 1b), effects on the heart rate
variability (HRV) were assessed in the time and
spectral domain (Kaniusas, 2008; Gbaoui, 2008a)
and in the state space (Gbaoui, 2008b) by our group.
In addition, blood perfusion (BP) changes during
stimulation were investigated (Kaniusas, 2008). In
the latter studies optical plethysmography (OPG)
served as biofeedback to derive the HRV and BP.
Here the suitability of the HRV and BP analysis
is given by the fact that the stimulated afferent vagal
nerve goes to the nucleus solitarius in the CNS,
whereas the sinus node of the heart is controlled by
the efferent vagus nerve from the nucleus ambiguous
in the CNS. The node initiates heart contractions
with particular rate dynamic and ejection strength,
thus the HRV and BP being the appropriate
parameters to register the stimulation effects.
The given position paper is intended to introduce
a novel technology for an adaptive and synchronous
P-Stim controlled by the HRV and BP. As a starting
point, technical data and new experimental results
concerning parasympathetic/sympathetic power in
the HRV from the standard P-Stim are presented,
which yield a substantial basis and arguments for the
introduction of the adaptive stimulation.
2 ESTABLISHED STIMULATION
2.1 Methodology
The P-Stim was applied in supine position of three
healthy volunteers: two men aged 41/29 with BMI
25/23 kg/m
2
and one female aged 19 with BMI of
20 kg/m
2
. A precise positioning of the needle in the
vicinity of the vagal nerve (Fig. 1) was facilitated by
local conductivity measurements, for the local
conductivity increases in the region of the nerve and
its supporting blood vessels.
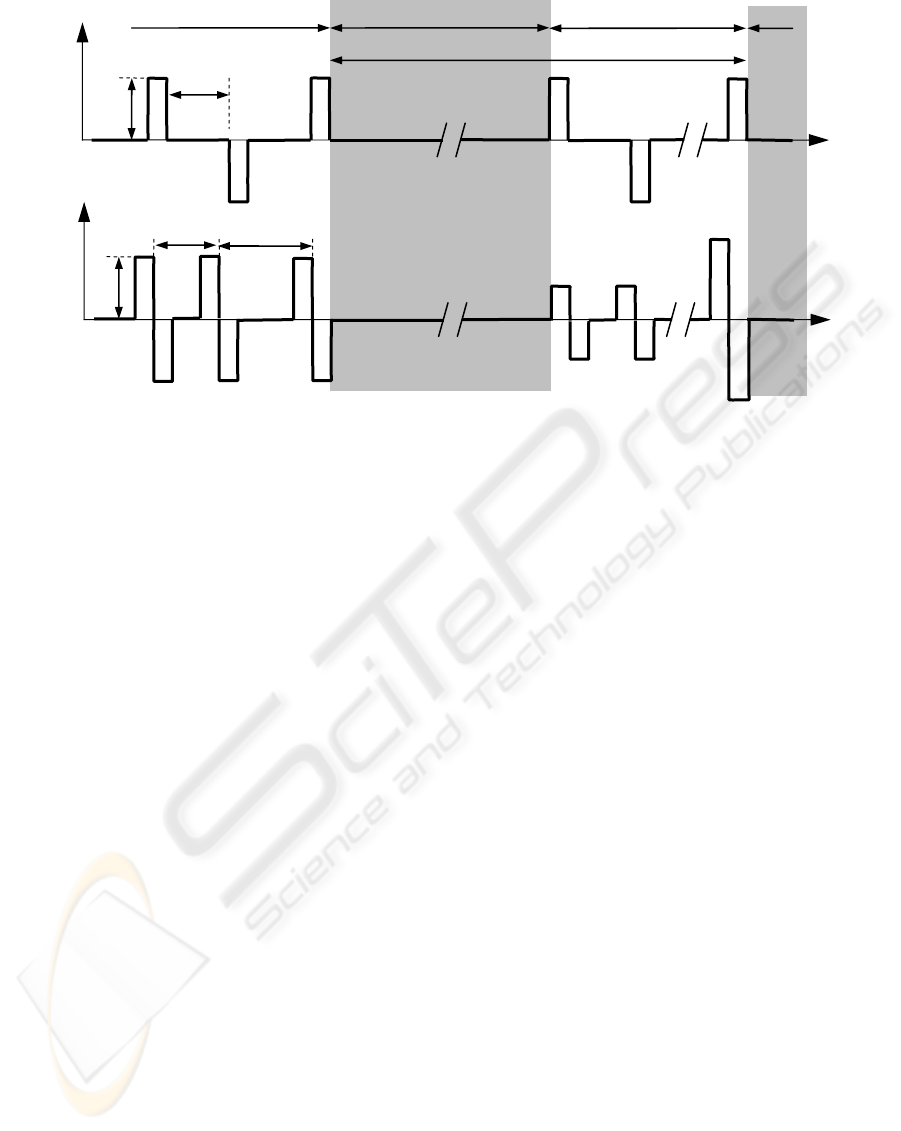
As demonstrated in Fig. 2a, the voltage U of the
electrical stimulation comprises monophasic
impulses with changing polarity, stimulation
(=repetition) rate f
S
of 1 Hz, amplitude A of 4 V and
impulse duration of about 1 ms.
The duration of the recordings was about 15 min
before, during, and after the stimulation,
respectively. At least two recordings were performed
per volunteer with a time-lag in-between of more
Figure 2: Stimulation waveforms of a fixed (a) and (b) adaptive electrical punctual stimulation.
a)
b)
U
t
A
1 /f
S
Pause Stimulation Stimulation
1 /f
A
U, I
t
A
1 /f
S
1
Pause
1 /f
S
2
(≠ 1 /f
S
1
)
ADAPTIVE AURICULAR ELECTRICAL STIMULATION CONTROLLED BY VITAL BIOSIGNALS - Transition from
Fixed to Adaptive and Synchronized Electrical Stimulation Controlled by Heart Rate Variability and Blood Perfusion
305
than 10 days. It should be noted that the needles for
stimulation were inserted about 5 min before the
recording to avoid needle’s positioning effects, i.e.,
to avoid temporal effects of manual acupuncture.
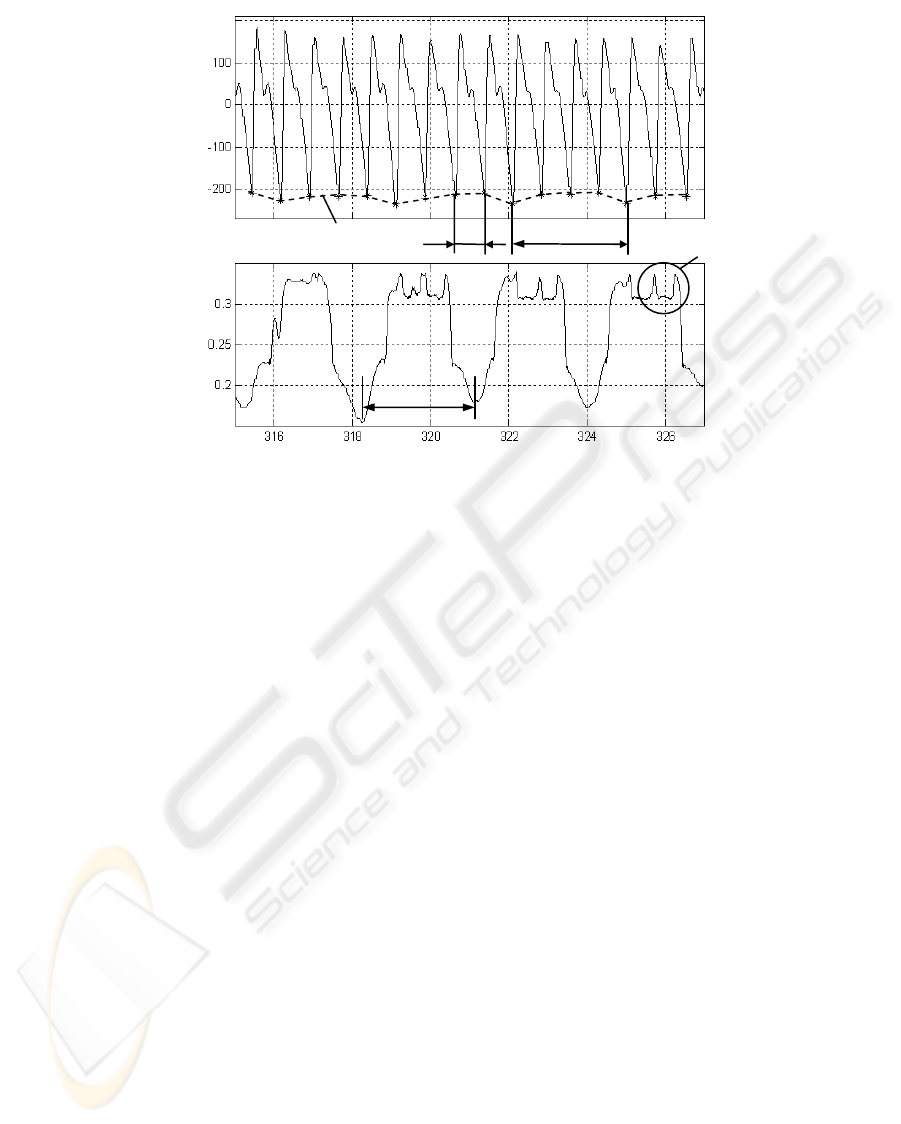
In parallel, the OPG signal s
OPG
from the finger
was assessed as biofeedback. Here the relatively
high sampling rate of 2 kHz is needed for an
accurate HRV analysis (Guidelines, 1996). A typical
course of s
OPG
is depicted in Fig. 3a.
The instantaneous heart rate f
C
for the HRV
analysis was estimated from s
OPG
, as demonstrated
in Fig. 3a, with artefacts and noisy segments being
manually removed. The prominent minima in s
OPG
,
which correspond to the onset of the systole or blood
ejection, were detected as fiducial points for the
calculation of the instantaneous f
C
.
The investigation of the resulting f
C
sequence in
the spectral domain comprised power in the
established frequency ranges (Guidelines, 1996):
low frequency range 0.04-0.15 Hz corresponding to
sympathetic power P
SYM
and high frequency range
0.15-0.4 Hz corresponding to parasympathetic
power P
PAR
. Both P
SYM
and P
PAR
were estimated for
sequence windows of 300 s with 50 % overlap. It
should be noted that there are controversial
indications that P
PAR
is also present in the low
frequency range.
The BP is given by the course of s
OPG
(Fig. 3a).
In particular, the amplitude deflection of s
OPG
within
a single heart cycle corresponds approximately to
both amount of blood ejected (=left ventricular
stroke volume) and vesicular compliance.
The respiration reference s
R
(Fig. 3b) was
established by a skin curvature sensor on the chest,
as described in (Pfützner, 2006; Kaniusas, 2004).
2.2 Results
2.2.1 Heart Rate Variability
Fig. 4b and Fig. 5b demonstrate a temporal increase
of P
PAR
during stimulation, which temporal
activation is given in Fig. 4a and Fig. 5a. The
relative increase of P
PAR
among volunteers was
about 20 %, which was observed in all sessions but
one, probably because of a relatively high initial
value of P
PAR
. A temporal dip of P
PAR
was often
observed during the stimulation.
No unique tendencies were registered in the
behaviour of P
SYM
, as demonstrated in Fig. 4c and
Fig. 5c. However, stress relaxation effects could be
observed in some cases even in healthy volunteers.
In Fig. 4b,c and Fig. 5b,c dashed ellipses mark the
corresponding time intervals, where P
PAR
increases
and P
SYM
concurrently decreases. In general, such
changes of P
PAR
and P
SYM
tend to indicate ongoing
restorative effects.
The stimulation effects on P
PAR
were discussed
in a wider context in (Kaniusas, 2008; Gbaoui,
2008a), considering additionally parameters in the
Figure 3: a) Optical plethysmography signal s
OPG
with an estimated cardiac rate f
C
from indicated systolic onset points (*)
and an estimated respiratory rate f
R
from the envelope. b) The corresponding respiration signal s
R
from the chest ski
n
curvature sensor.
a)
b)
s
OPG
(rel.units)
s
R
(rel.units)
t (s)
Cardiac components
Envelope
1/ f
R
1/ f
R
1/ f
C
BIODEVICES 2009 - International Conference on Biomedical Electronics and Devices
306
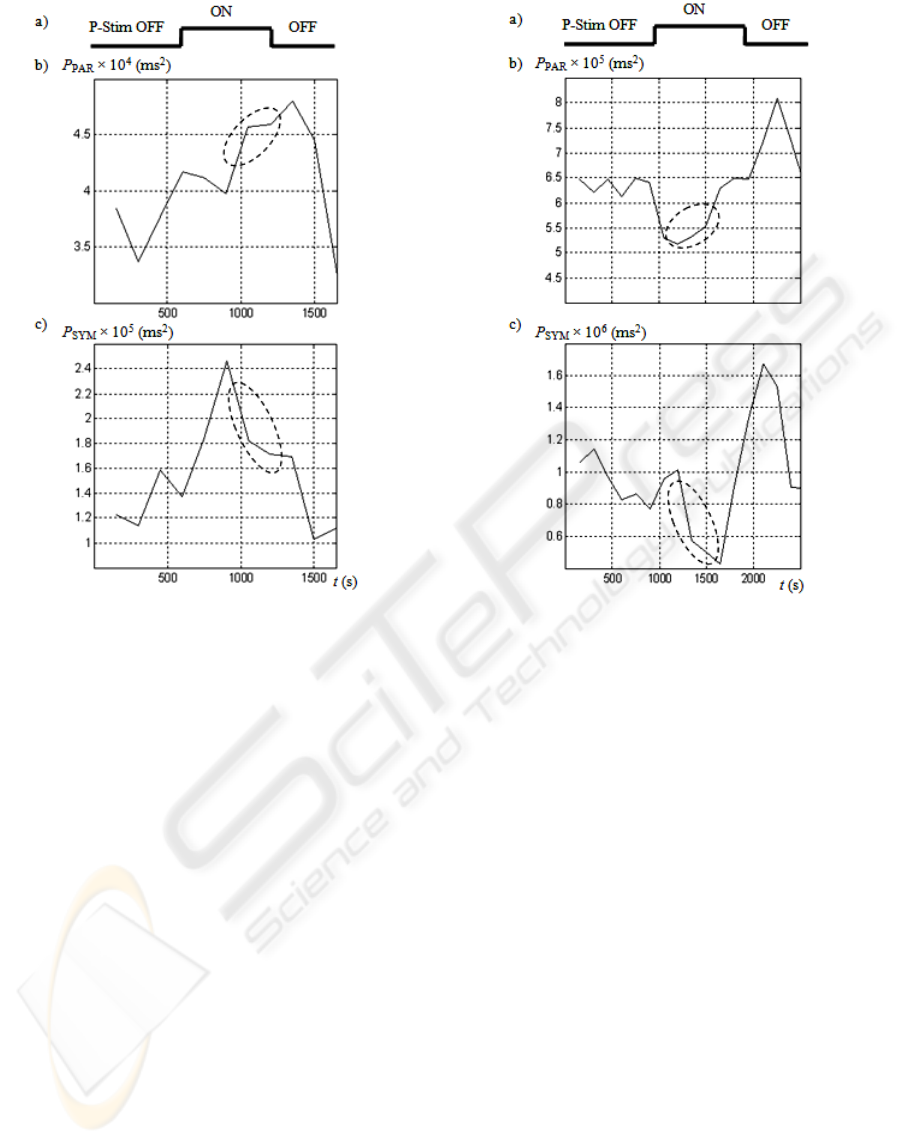
Figure 4: Effects on heart rate variability in the female
subject. a) Temporal activation of the electrical
stimulation (P-Stim OFF or P-Stim ON). b) The
corresponding parasympathetic power P
PAR
. c) The
corresponding sympathetic power P
SYM
.
time domain and state space. Aforementioned
tendencies of P
PAR
were also found in (Haker, 2000),
even during non-electrical auricular stimulation by
acupuncture needle.
In contrast to P
PAR
, none of the mentioned
studies indicate clear tendencies of P
SYM
. This is
likely to be attributed to the study enrolment of only
healthy unstressed pain-free individuals in resting
state, where potential changes or improvements of
P
SYM
are strongly restricted.
2.2.2 Blood Perfusion
The BP is given by the course of s
OPG
, as show in
Fig. 3a. It is important to observe that not only the
instantaneous cardiac activity but also the respiration
can be derived from s
OPG
.
In particular, the systolic onset points, as marked
by asterisks in Figure 3a, give a useful reference to
heart excitation. These points are delayed by about
200 ms from the actual excitation of the heart
ventricles (= R peaks in electrocardiography (ECG))
with the delay being nearly constant.
Figure 5: Effects on heart rate variability in a male subject.
a) Temporal activation of the electrical stimulation (P-
Stim OFF or P-Stim ON). b) The corresponding
parasympathetic power P
PAR
. c) The corresponding
sympathetic power P
SYM
.
The respiratory cycle can be derived from s
OPG
,
as indicated by the envelope in Fig. 3a. Here the
amplitude modulation of s
OPG
results from the
respiratory induced modulation of the left ventricu-
lar stroke volume which temporally increases during
expiration. The simultaneously recorded respiration
reference s
R
(Fig. 3b) proves the respiratory related
modulation of the s
OPG
deflection.
3 PROPOSED STIMULATION
3.1 Rationale
Since the spectral HRV parameters are specifically
influenced by the standard P-Stim application and
the instant cardio-respiratory data can be derived
from the BP, as shown above, a novel adaptive and
synchronized P-Stim could be established.
A targeted control of the stimulation waveform
(compare Fig. 2) is highly reasonable for avoiding
over-stimulation and realising stimulation on-
ADAPTIVE AURICULAR ELECTRICAL STIMULATION CONTROLLED BY VITAL BIOSIGNALS - Transition from
Fixed to Adaptive and Synchronized Electrical Stimulation Controlled by Heart Rate Variability and Blood Perfusion
307
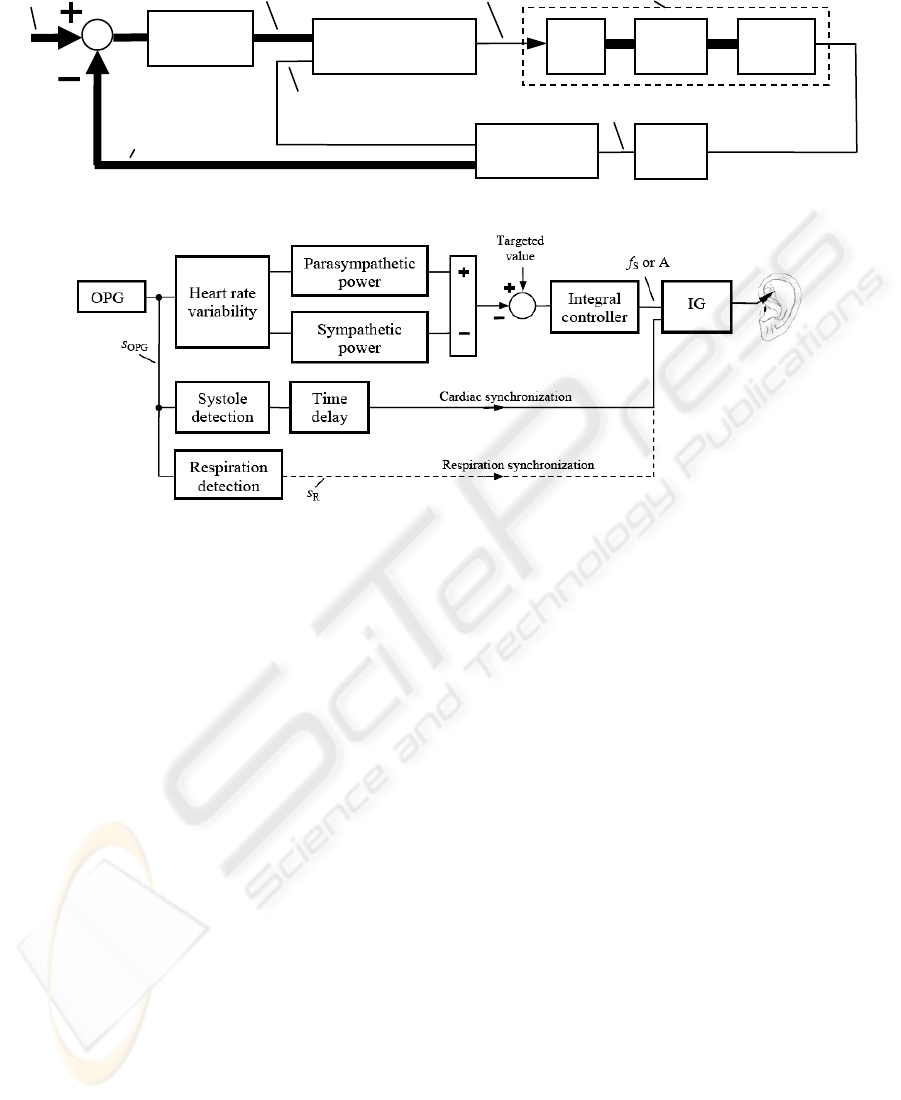
Figure 7: Establishment of biofeedback for controlling and synchronization purposes with IG as the impulse generator.
demand controlled by HRV parameters. In other
words, if pain perception is already reduced, as
detected by e.g., reduced stress and diminished
P
SYM
, then A, f
S
(Fig. 2b) could be reduced as well.
In addition, efficient energy use in the stimulation
would be facilitated.
The synchronization of the stimulation waveform
with the cardio-respiratory activity would allow a
constructive interference of the stimulated pain-
relieving effects and the residual body attempts. In
particular, the cardiac synchronization would allow a
timely activation of the gate mechanisms in the CNS
or a timely and indirect stimulation of receptors
(e.g., blood pressure), regulating vital body
functions. The respiratory synchronization would
help to interfere with body phenomena like
respiratory sinus arrhythmia, yielding a forced
increase of P
PAR
in the expiration phase.
3.2 Realization
The proposed set-up is shown in Fig. 6. The input
parameters A, f
S
and the activation rate f
A
of the
impulse generator are adaptively adjusted according
to the HRV parameters via a control loop. The
cardio-respiratory synchronization signal for the
impulse generator is also derived from s
OPG
.
In particular, Fig. 7 suggests the difference P
PAR
- P
SYM
as a possible realization of the stimulation
feedback, while the targeted value could be the pain
intensity to be reduced. That is, the higher P
PAR
and
the lower P
SYM
get in the course of the stimulation,
the more strongly the pain has already been reduced.
Similar behaviour of P
PAR
and P
SYM
during
stimulation was already observed in Fig. 4 and Fig.
5. Obviously the ratio P
PAR
/ P
SYM
could be used
instead of the difference.
According to Fig. 7 an adaptive control of A and
f
S
is established, assuming that these parameters are
directly interrelated with the stimulation strength. In
an analogous way, a composition of bursts by
controlling of f
A
could be attained (compare Fig. 2).
Here a proportional-integral controller or integral
controller could be applied, for the human (Fig. 6)
can be roughly approximated as a proportional
control process with a single time constant (compare
Fig. 4b). The time delay in Fig. 7 may be needed for
synchronizing the stimulation pulses with a
particular time instant in the heart cycle.
Fig. 2b exemplifies a possible adaptive control-
ling of the stimulation curve, while more efficient
biphasic impulses are used (compare Fig. 2a). In
addition, constant current stimulation would be
preferred over voltage application, for the skin
impedance is relatively low with electrode needles
inserted and thus the risk of local tissue damage
Figure 6: Control loop of the adaptive auricular stimulation with OPG as the optical plethysmography.
Impulse generator Ear Body
Stimulation electrode
OPG
Human (= control process)
Parameters
of OPG
Stimulation
parameters: A, f
S
, f
A
Synchronization
(heart or respiration)
Controller
Targeted
values
Feedback (= actual
HRV parameters)
s
OPG
Finger
BIODEVICES 2009 - International Conference on Biomedical Electronics and Devices
308

though locally increased current density is low.
4 DISCUSSION
It is worth to note that the HRV is usually derived
from the ECG (Guidelines, 1996). However, the P-
Stim induced very strong artefacts in the ECG since
the stimulation and the ECG have the same electrical
origin. In contrast, the OPG with optical origin
serves as a reliable biosignal, being independent of
the P-Stim activation. However, the OPG conveys
mechanical information on the systole-diastole cycle
rather than electrical on the heart excitation (= origin
for the HRV). In addition, the OPG exhibits
relatively slow changes if compared to the ECG, for
the pulse waves are much more inert than electrical
heart excitation. The use of the OPG may have
reduced an effective time resolution of f
C
.
The time delay of about 200 ms between the
systolic onset in the OPG and the R peak in the ECG
depends on the speed of the heart excitation and
mechanical vessel properties. Nevertheless, the
delay can be assumed to be constant, if the respirato-
ry induced blood pressure changes and thus arterial
distension and stiffness changes can be neglected.
Lastly, the limitations of the presented
experimental results should be mentioned. The
observed effects, especially concerning P
SYM
, are
restricted by the fact that all volunteers were young
pain-free healthy persons. Furthermore, the
stimulation duration was relatively short: 15 min
versus 4 hours (with 4 hours pause in-between) over
at least seven days, as clinically applied and
subjectively verified for being effective. The initial
state of the volunteers, as their possible excitation at
the beginning of the recording, and their mental
activity changes during the investigation - both
influencing the HRV - may have limited the range of
potential changes or improvements of HRV
parameters during the stimulation.
However, the provided experimental background
leads to a comprehensible design of an adaptive and
synchronized stimulation technique. This would
allow a pain sensitive adjustment of the stimulating
parameters avoiding over-stimulation and
comforting the patients.
REFERENCES
Gao, X.Y., Zhang S.P., Zhu, B., Zhang H.Q., 2008.
Investigation of specificity of auricular acupuncture
points in regulation of autonomic function in
anesthetized rats. Autonomic Neuroscience: Basic and
Clinical, 138, 50-56.
Gbaoui, L., Kaniusas, E., Szeles, J.C., Materna, T.,
Varoneckas, G., 2008a. Heart rate variability during
electrostimulation on ear: spectral domain versus state
space (in german). Proceedings of XXII Symposium on
Measuring Technique, 230-238.
Gbaoui, L., Kaniusas, E., Szeles, J.C., Materna, T.,
Varoneckas, G., 2008b. Effects of the auricular
electrical stimulation on heart rate variability assessed
in phase space: pilot study. Accepted for IEEE Sensors
2008 in Lecce, Italy.
Haker, E., Egekvist, H., Bjerring, P., 2000. Effect of
sensory stimulation (acupuncture) on sympathetic and
parasympathetic activities in healthy subjects. Journal
of the Autonomic Nervous System, 79, 52-59.
Kaniusas, E., Gbaoui, L., Szeles, J.C., Materna, T.,
Varoneckas, G., 2008. Validation of auricular
electrostimulation by heart rate variability and blood
perfusion: possibilities and restrictions. Proceedings of
Microelectronics Conference 2008, 180-184.
Kaniusas, E., Pfützner, H. et al., 2004. Magnetoelastic skin
curvature sensor for biomedical applications.
Proceedings of IEEE Sensors, 1484-1487.
Likar, R., Jabarzadeh, H. et al., 2007. Auricular electrical
punctual stimulation (P-STIM): a randomized, double-
blind, controlled pilot study in laparoscopic
nephrectomy (in german). Schmerz, 21(2), 154-159.
Litscher, G., Wang, L., Gaischek, I., 2007. Electroence-
phalographic responses to laserneedle and punctual
stimulation quantified by bispectral (BIS) monitoring:
a pilot study to evaluate methods and instrumentation.
Internet Journal of Laserneedle Medicine, 1(1).
Peuker, E.T., Filler, T.J., 2002. The nerve supply of the
human auricle. Clinical Anatomy, 15, 35-37.
Pfützner, H., Kaniusas, E. et al., 2006. Magnetostrictive
bilayers for multi-functional sensor families. Sensors
and Actuators A: Physical, 129, 154-158.
Sator-Katzenschlager, S.M., Scharbert, G. et al., 2004. The
short and long term benefit in chronic low back pain
through adjuvant electrical versus manual auricular
acupuncture. Anesthesia & Analgesia, 98, 1359-1364.
Sator-Katzenschlager, S.M., Wölfler, M.M. et al., 2006.
Auricular electro-acupuncture as an additional
perioperative analgesic method during oocyte
aspiration in IVF treatment. Human reproduction,
21(8), 2114-2120.
Szeles, J.C., 2001a. Therapy appliance for punctual
stimulation. Patents WO 01/35897, US7336993.
Szeles, J.C., Hoda, M.R., Polterauer, P, 2001b. Appli-
cation of electrostimulation acupuncture (P-Stim) in
clinical practice. Schmerznachrichten from Austrian
Pain Association, 1.
Szeles, J.C., Litscher, G., 2004. Objectivation of cerebral
effects with a new continuous electrical auricular
stimulation technique for pain management.
Neurological Research, 26(7), 797-800.
Guidelines, 1996. Heart rate variability: standards of
measurement, physiological interpretation, and clinical
use. Circulation, 93(5), 1043-1065.
ADAPTIVE AURICULAR ELECTRICAL STIMULATION CONTROLLED BY VITAL BIOSIGNALS - Transition from
Fixed to Adaptive and Synchronized Electrical Stimulation Controlled by Heart Rate Variability and Blood Perfusion
309
