
DEVELOPMENT OF A BIODIAGNOSTIC DEVICE ASSAY FOR
COAGULATION MONITORING
Magdalena M. Dudek and Anthony J. Killard
Biomedical Diagnostics Institute, National Centre for Sensor Research, Dublin City University, Dublin, Ireland
Keywords: Bioassay, Polymer, Warfarin.
Abstract: There is an urgent need for the development of reliable point-of-care devices capable of anticoagulant dose
monitoring due to the increasing number of patients being treated with clotting control therapy. Millions of
patients suffering from cardiovascular-related disorders rely on the anticoagulant therapy. One of the
commonly administered drugs is warfarin. It is effective for primary and secondary prevention of
venous
thromboembolism, for prevention of cardioembolic events
in patients with atrial fibrillation or prosthetic
heart valves,
for prevention of stroke, recurrent infarction or mortality in patients with acute myocardial
infarction and for
the primary prevention of acute myocardial infarction in high-risk
men. Regular
monitoring of warfarin effect is of paramount importance and therefore affords are made to develop novel,
reliable point-of-care devices for drug level determination. Miniaturized microfluidic systems made of
polymers have gained great interest of the diagnostics industry in recent years. Due to the low cost of
manufacturing and processing, they have been employed in the development of several disposable
diagnostic systems. Among the wide selection of different synthetic polymers, thermoplastics have gained
significant popularity. Cyclic polyolefins (COPs) are a relatively new class of thermoplastics with an
excellent combination of optical and electronic properties and are dimensionally stable while being subject
to a range of operational temperatures and pressures. One such COP is marketed by Zeon Corp. under the
brand name Zeonor®. This material has been used as the base for the developed assay. The technology
developed by Åmic B.V. (Sweden) allowed the formation of an ordered array of micropillars which
introduce controlled and highly reproducible capillary filling forces when liquid samples are introduced to
the substrate. Capillary forces play an important role in most of these systems. Assays based on the flow of
a fluid in a device with some form of immobilized reagents are considered as the most commonly used tool
in many detection systems, including diagnostics. Herein, the concept of monitoring blood clotting
properties by measuring a sample distance traveled in a lateral flow system was shown. Substances known
to be strong coagulation activators were employed in the monitoring system. All necessary components
were incorporated into a test strip, so that no pre-treatment steps were required. These were capable of
inducing rapid clot formation and thus arrest of sample flow. The device was shown to be a viable tool for
measuring the clotting status of samples containing different quantities of an anticoagulant. This idea of a
simple assay device could be employed in a point-of-care determination of a drug level.
1 INTRODUCTION
Close to six million people in the world take oral
anticoagulants on a permanent basis, including
patients with artificial heart valves or those affected
by atrial fibrillation or thrombotic disease. There are
also patients who have to rely on them for periods of
several weeks or several months (www.roche.com,
www.argatroban.com). The number of patients
under vitamin K antagonist anticoagulant therapy is
expected to reach 10 million globally by 2010
(www.roche.com). Coagulation monitoring and drug
dosage adjustment are required to maintain the INR
(International Normalized Ratio) within the
therapeutic range (Ansell et al., 2004). INR is a
system that was established by the World Health
Organization (WHO) and the International
Committee on Thrombosis and Hemostasis for
reporting the results of blood coagulation tests. The
WHO procedure is standardized – thromboplastin
reagents are calibrated against a standard by means
of the International Sensitivity Index (ISI). The INR
can be calculated as follows (www.who.int/en):
183
Dudek M. and J. Killard A. (2010).
DEVELOPMENT OF A BIODIAGNOSTIC DEVICE ASSAY FOR COAGULATION MONITORING.
In Proceedings of the Third International Conference on Biomedical Electronics and Devices, pages 183-186
DOI: 10.5220/0002743001830186
Copyright
c
SciTePress
INR = (observed ratio)
ISI
of thromboplastin
.
Warfarin (Fig. 1) is one of the most commonly
prescribed anticoagulants. It belongs to a group of
coumarins that exert their anticoagulant effect by
interfering with the cyclic interconversion of vitamin
K and its 2,3-epoxide (vitamin K epoxide) (Hart et
al., 2007, Baigent et al., 1998). Warfarin therapeutic
dosage can be affected by several factors and
therefore, it can be difficult to manage (Sick et al.,
2007). The most common complication of warfarin
therapy is bleeding, which occurs in 6 to 39 % of
recipients (Levine et al., 1995). The incidence of
complications varies within this range, depending
upon the clinical indication and the intensity of
anticoagulation. Due to the variability in the
anticoagulant
response to warfarin, regular
monitoring and
dosage adjustment are required to
maintain the INR within the
therapeutic range (Hirsh
et al., 2003).
O
OH
O
O
Figure 1: Chemical structure of warfarin.
The development of microfluidic device platforms is
already an important area for biomedical device
design. Polymer-based microfluidic devices and
their associated materials have gained particular
interest in recent years. Thermoplastics have gained
significant popularity as substrates for the
production of disposable devices for biomedical
applications having low raw material and
manufacturing costs. Their properties which include
thermal stability, ‘mouldability’, precise structural
and morphological control over surface properties,
chemical and biological inertness, good optical and
electrical characteristics and many more are
resulting in the replacement of traditional materials
such as glass, silicon and nitrocellulose as the
foundation of device fabrication.
2 MATERIALS AND METHODS
4Castchips
®
B2.2 (Fig. 2) were injection molded by
Åmic AB (Uppsala, Sweden) in cyclic olefin
polymer (COP) (Zeonor 1020R
®
) to form
micropillars (height 65-70 µm, top diameter ca 50
µm, bottom diameter ca 70 µm, the distance between
the centres of the pillars in a row 85 µm, the distance
between the centres of the pillars in a column 185
µm). These facilitated controlled and highly
reproducible capillary filling of liquid samples.
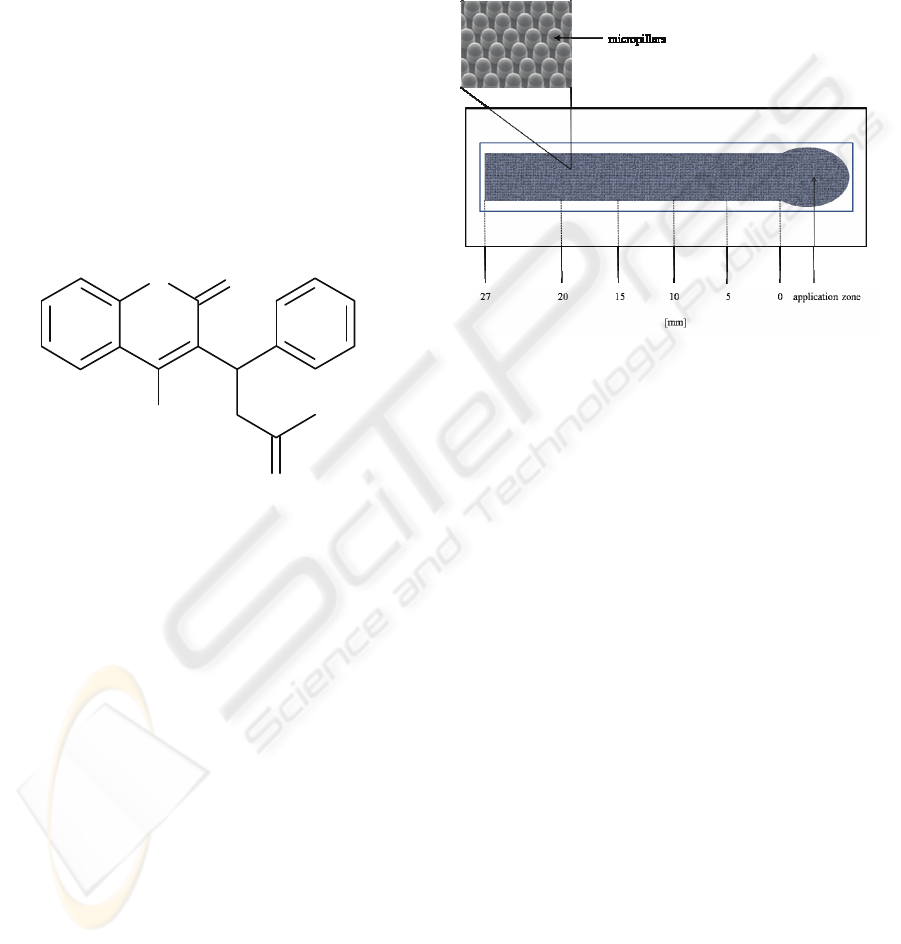
Figure 2: Graphical representation of the B 2.2 micropillar
lateral flow device employed for the detection of
fibrinogen level. The test channel possessed hot-embossed
micropillar structure, as shown in the magnified inset.
The assay platforms were coated with a mixture of
activating reagents by a drop-deposition. 10 μL was
applied and left to dry in under ambient conditions.
Among the active components immobilized on the
assay platform were: activated partial
thromboplastin time (aPTT) reagent, aPTT-SP
(Hemosil), prothrombin time (PT) reagent,
Simplastin HTF (BioMerieux) and Russell’s Viper
Venom (RVV) (Pethapharm). aPTT activator was
ready-to-used solution. PT reagent was reconstituted
in 2- or 4-fold less volume of diluent than suggested
by manufacturer. The activity of RVV solution was
50 U/mL. Positive control was normal clotting,
control plasma (Hemosil), while negative control
consisted of control plasma supplemented with
heparin at a final concentration of 50 U/mL. Such a
high concentration of an anticoagulant was used to
ensure no clotting occurred. The time required for a
test solution to reach each step of a test channel (Fig.
2) was measured. The filling characteristics were
assessed on a basis of obtained filling profiles. In
addition, the device was validated using anonymous
warfarin-treated patient plasma samples with INR
values of 1.1, 2.1 and 9.0.
BIODEVICES 2010 - International Conference on Biomedical Electronics and Devices
184
3 RESULTS AND DISCUSSION
3.1 Assay Chemistry Formulation
The development of the assay was based on the
monitoring of distance traveled by normal clotting
and non-clotting (heparinized) samples. The aim was
to achieve a significant difference in a distance
traveled between these two variants that would allow
identification of minor clotting disorders (slightly
prolonged CT). Substances known to facilitate rapid
clot formation (aPTT, PT, RVV) were employed in
platform development in order to achieve a flow
cessation. The distances traveled by normal and
heparinized samples on chips coated with a variety
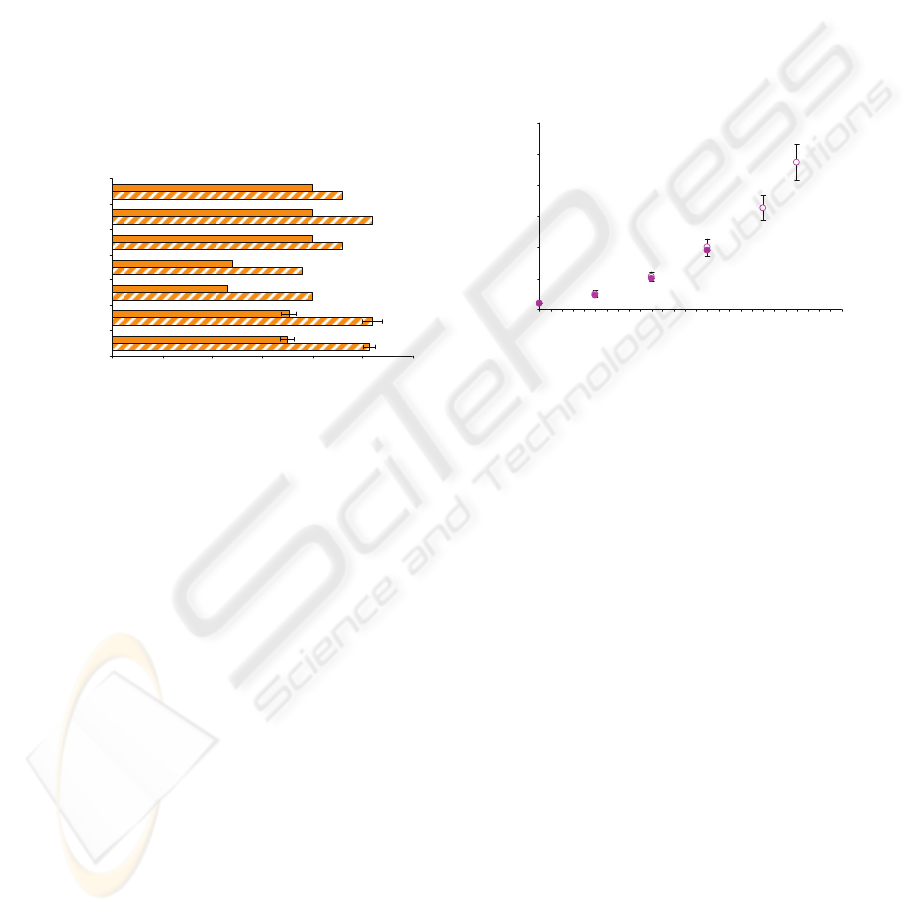
of activator combinations are illustrated in Fig. 3.
0 5 10 15 20 25 30
4xPT:RVV:water (1:1:2)
4xPT:RVV (1:1)
4xPT:RVV (2:1)
4xPT:RVV (3:1)
4xPT:water (1:1)
4xPT:RVV:water (1:1:1)
aP TT:RVV (1:1)
Ratio of immobilized reagents
Distance travelled [ mm]
Figure 3: Distance traveled by normal clotting (plain) and
non-clotting, strongly heparinized (striped) plasma
samples. Test channels were coated with clotting
activators at different ratios.
The difference in the distances traveled by clotting
and non-clotting samples was between 3 and 8.5 mm
depending on a formulation used. The use of the
aPTT reagent did not allow rapid clot formation. The
difference of 3 mm would not allow a precise
differentiation between samples of varying
anticoagulant activity. Similarly, 1:1 mixtures of 4-
fold concentrated PT with water with or without
RVV did not result in good discrimination between
samples of different clotting abilities. Dried mixture
of 4-fold concentrated PT and RVV at ratios of 3:1
and 2:1 yielded a short distances traveled of 12 and
11.5 mm for clotting and 19 and 20 mm for non-
clotting samples. The significant decrease in a
distance traveled was probably not an effect of an
enhancement in clotting, but was more likely due to
high concentration of immobilized PT reagent. The
deposition of high protein concentration (tissue
thromboplastin) and phospholipids could result in a
change of surface properties such as roughness and
wettability. It has been noticed that the distance was
short not only for clotting sample but also for the
negative control sample, for which no clotting
occurred. Therefore, the reduction in the distance
traveled was of no benefit because of the changes
introduced to the surface properties. Formulations
composed of 4-fold concentrated PT and RVV with
or without water dilution at a ratio of 1:1 or 1:1:2
proved to be best at yielding a significant difference
in distance. Fill times were measured at each stage
of the channel coated with these formulations. In
both situations they looked similar as illustrated in
an example in Fig. 4. It has been shown that the
filling profile was very similar for clotting and non-
clotting samples. The flow of a clotting sample was
rapidly arrested at between 15 – 20 mm.
0
10
20
30
40
50
60
0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27
Distance travelled [mm ]
Time [s]
Figure 4: Fill time profiles obtained for normal clotting
(filled symbols) and non-clotting (empty symbols) plasma
samples tested in a channel coated with 4-fold
concentrated PT reagent, RVV and water mixed at a ratio
of 1:1:2 (n=3).
3.2 Validation with Patient Samples
The platforms coated with 4-fold concentrated PT +
RVV (1:1) and 4-fold concentrated PT + RVV +
water (1:1:2) were selected for further validation
using patient samples. Three patient samples with
different INR values were tested: 1.1, 2.1 and 9.0.
Results obtained for normal clotting and heparinized
(50 U/mL) plasma samples and for patient plasma
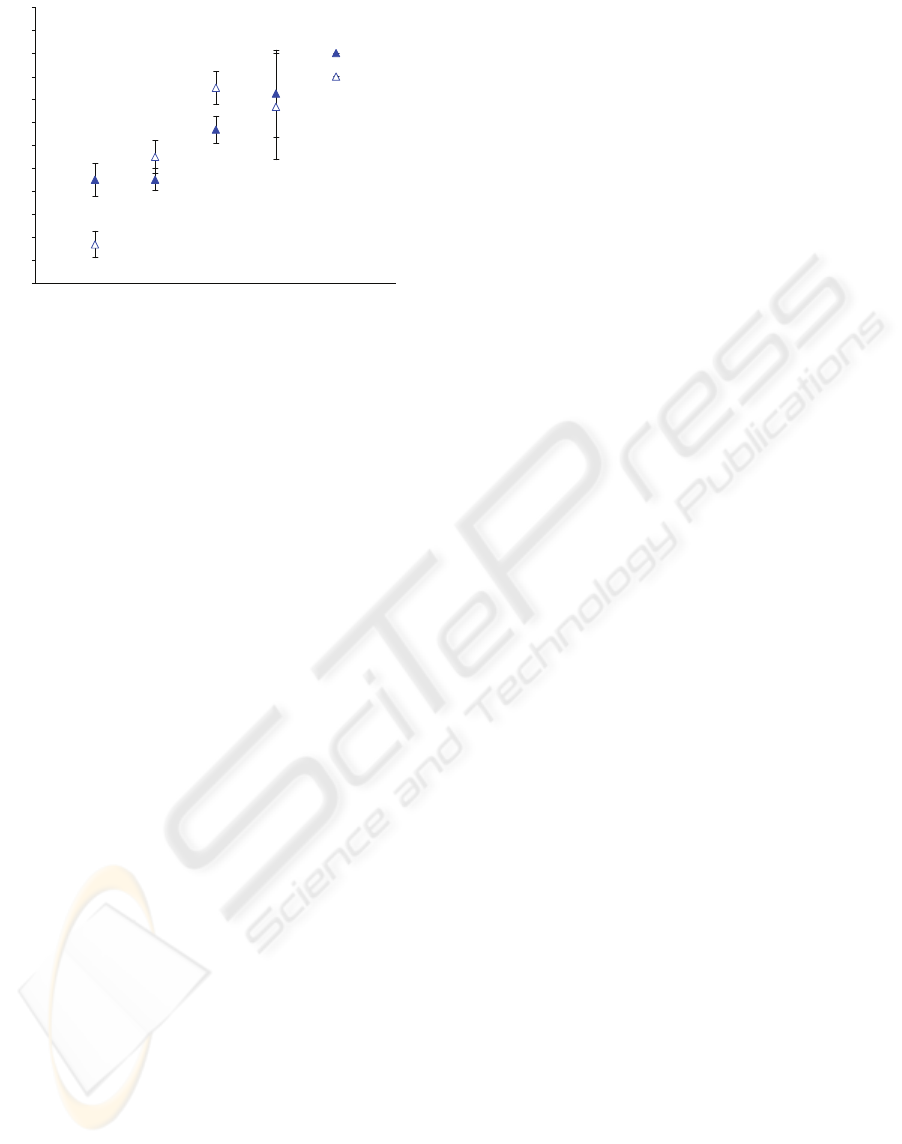
samples are shown in Fig. 5. The 1:1 PT:RVV
showed good discrimination of INR at the lower
range (1.0 to 2.1) but due to assay variability it was
unable to discriminate higher values (INR 9.0 and
non-clotting controls). However, PT:RVV at 1:1.2
showed poor differentiation between INR 1.0 and
1.1, but was better at distinguishing INR 1.1, 2.1, 9.0
and non-clotting controls. However, variability again
made it difficult to distinguish INR 9.0 from other
values due to the long and variable times which
result.
DEVELOPMENT OF A BIODIAGNOSTIC DEVICE ASSAY FOR COAGULATION MONITORING
185
Non-clotting
sample
INR 9.0
INR 2.1
INR 1.1
INR 1.0
15
16
17
18
19
20
21
22
23
24
25
26
27
INR value
Distance travelled [mm]
Figure 5: Distances travelled by samples with INR of 1.0
(normal clotting control), 1.1, 2.1, 9.0 and non-clotting.
Chips containing 4-fold concentrated PT:RVV at a 1:1
ratio (empty symbols) and 4-fold concentrated PT : RVV :
water at 1:1:2 ratio (filled symbols) were used for testing
(n=3).
4 CONCLUSIONS
The principle of a point-of-care lateral flow device
for the anticoagulant therapy monitoring has been
shown. The device platform made of cyclic poly
olefin polymer and coated with an optimized
mixture of activating agents has been shown a
reliable tool for an assessment of blood clotting
properties. A mixture of Russell’s Viper Venom and
Prothrombin Time reagents allowed rapid clot
formation which resulted in the cessation of sample
flow. Significant differences in the distance traveled
between a normal clotting and a strongly heparinized
plasma sample were shown. The presence of an
anticoagulant (warfarin) in a patient plasma sample
delayed clot formation and therefore resulted in a
prolonged distance traveled in comparison to a
normal clotting control. The device requires further
optimization in order to obtained better recognition
between samples of different clotting statuses.
However, the idea of an anticoagulant dose
monitoring using the lateral flow device for the
distance traveled measurement was shown to be
viable.
ACKNOWLEDGEMENTS
This material is based upon works supported by the
Science Foundation Ireland under Grant No.
05/CE3/B754.
REFERENCES
Ansell, J., Hirsh, J., Poller, L., Bussey, H., Jacobson, A.,
Hylek, E., 2004. The pharmacology and management
of the vitamin K antagonists. In Chest, 126, 3, 204S-
233S.
Baigent, C., Collins, R., Appleby, P., 1998. ISIS-2: 10
year survival among patients with suspected acute
myocardial infarction in randomised comparison of
intravenous streptokinase, oral aspirin, both, or
neither. In British Medical Journal, 316, 1337-1343.
Hart, R. G., Pearce, L. A., Aguilar, M. I., 2007. Meta-
analysis: Antithrombotic therapy to prevent stroke in
patients who have nonvalvular atrial fibrillation. In
Annals of Internal Medicine, 146, 857-867.
Hirsh, J., Fuster, V., Ansell, J., 2003. American Heart
Association American College of Cardiology -
Foundation guide to warfarin therapy. In Journal of
the American College of Cardiology, 41, 1633-1652.
Levine, M. N., Raskob, G., Landefeld, S., Hirsh, J., 1995.
Hemorrhagic complications of anticoagulant
treatment. In Chest, 108, 276S-90S.
Sick, P. B., Schuler, G., Hauptmann, K. E., 2007. Initial
worldwide experience with the Watchman left atrial
appendage system for stroke prevention in atrial
fibrillation. In Journal of the American College of
Cardiology, 49, 1490-1495.
www.argatroban.com
www.roche.com
www.who.int/en
BIODEVICES 2010 - International Conference on Biomedical Electronics and Devices
186
