
FROM LEGISLATION TO PRACTICE
A Case Study of Break the Glass in Healthcare
P. Farinha, R. Cruz-Correia
CINTESIS – Centre for Research in Health Technologies and Information Systems, Faculty of Medicine, Porto, Portugal
L. Antunes
Instituto de Telecomunicações, Faculty of Science, Porto, Portugal
Filipe Almeida
Comissão de Ética para a Saúde, Hospital S. João, Porto, Portugal
A. Ferreira
CINTESIS – Centre for Research in Health Technologies and Information Systems, Faculty of Medicine, Porto, Portugal
Keywords: Healthcare legislation, Access control, Break the glass.
Abstract: Recommendations and regulations are available in healthcare to protect sensitive medical information.
These regulations tend to be generic and orient attitudes within the medical practice and are usually not
straightforward to be translated into practice. The main objective of this paper is to present the
implementation of the Break the Glass (BTG) concept in a real healthcare setting in order to enforce the
legislation for genetic information and evaluate the process of translating legislation into the healthcare
practice. The user logs were analysed to assess if the BTG system was working as expected, providing
genetic information confidentiality, as well as if the legislation was being enforced in a controlled and
responsible manner. Results show that the process to translate legislation into practice could be faster and
more efficient. User logs show that in terms of confidentiality the BTG features prevent more non
authorised people from accessing genetic reports. We expect the tendency to be that only users who really
need to access the reports will go through with the process of BTG. Enhancements to the system include the
implementation of the access control management infrastructure within a more robust access control
platform to perform the authentication and authorization processes.
1 INTRODUCTION
Recommendations and regulations are available in
healthcare to protect sensitive medical information
and to guarantee that this type of information is only
accessed and used in specific and justified contexts
(CdMaÉ, 1997) (CoE-Co. 2004). These regulations
tend to be generic and orient attitudes within the
medical practice. However, is not straightforward to
translate these orientations into practice. Many times
this is not even possible. Research shows that
excessive regulation can actually create a barrier that
physicians have to surmount when treating patients
(Ross-Lee et al, 2004). Nevertheless, means need to
be put into place to make that translation possible so
that confidentiality of medical information –
prevention of unauthorized access – is provided.
As an important support tool for consultation,
diagnosis and integration of heterogeneous
information from different places, the Electronic
Medical Record (EMR) (Waegemann, 2003) (Cruz-
Correia et al, 2005) stresses even more the need for
confidentiality and access control. However, security
must not constitute a barrier for a successful use and
integration of EMR into the medical practice but
allow for a controlled yet transparent way of doing
it. With this in view, an EMR was developed and is
in use since 2004 at the 2
nd
biggest hospital in
114
Farinha P., Cruz-Correia R., Antunes L., Almeida F. and Ferreira A. (2010).
FROM LEGISLATION TO PRACTICE - A Case Study of Break the Glass in Healthcare.
In Proceedings of the Third International Conference on Health Informatics, pages 114-120
DOI: 10.5220/0002748201140120
Copyright
c
SciTePress
Portugal – the Hospital S. João (HSJ) - (Cruz-
Correia et al, 2005) (Ferreira et al, 2004). As there
was also the need to provide for an access control
management platform for the EMR, the webcare
platform was developed for this purpose (Farinha et
al, 2006). This platform is based on the role-based
access control model - RBAC (Ferraiolo et al, 2001)
and helps to perform, in an easy and flexible way,
the most basic administrative access control actions.
However, this is not enough in such a hectic
environment. More flexible access control policies
are required not only to improve EMR efficiency but
also to enforce the legislation related to genetic
information (Lei, 2005). This is a Portuguese
legislation and defines how genetic information
must be protected, and what and how healthcare
professionals are authorized to access it during the
course of their work.
In order to do this in a flexible way the
information is restricted to an authorized group of
healthcare professionals previously defined.
However, this access is not entirely denied to all the
other healthcare professionals that may need to
access this information in emergency situations, but
in a controlled way (Rissanen et al, 2004) (Povey,
2000) (Ferreira et al, 2006) (Break-Glass, 2004). We
designated this access by Break the Glass (BTG).
The idea is that healthcare professionals are warned
they are not authorized to access that information,
but if it is an emergency, they can still access it
knowing that they will have to justify and face the
consequences later.
The main objective of this paper is to present
the implementation of the BTG concept in a real
healthcare setting in order to enforce the legislation
for genetic information. Further, we evaluate in
generic terms the process of translating legislation
into the healthcare practice and the impact of BTG
use within the same practice.
2 BACKGROUND
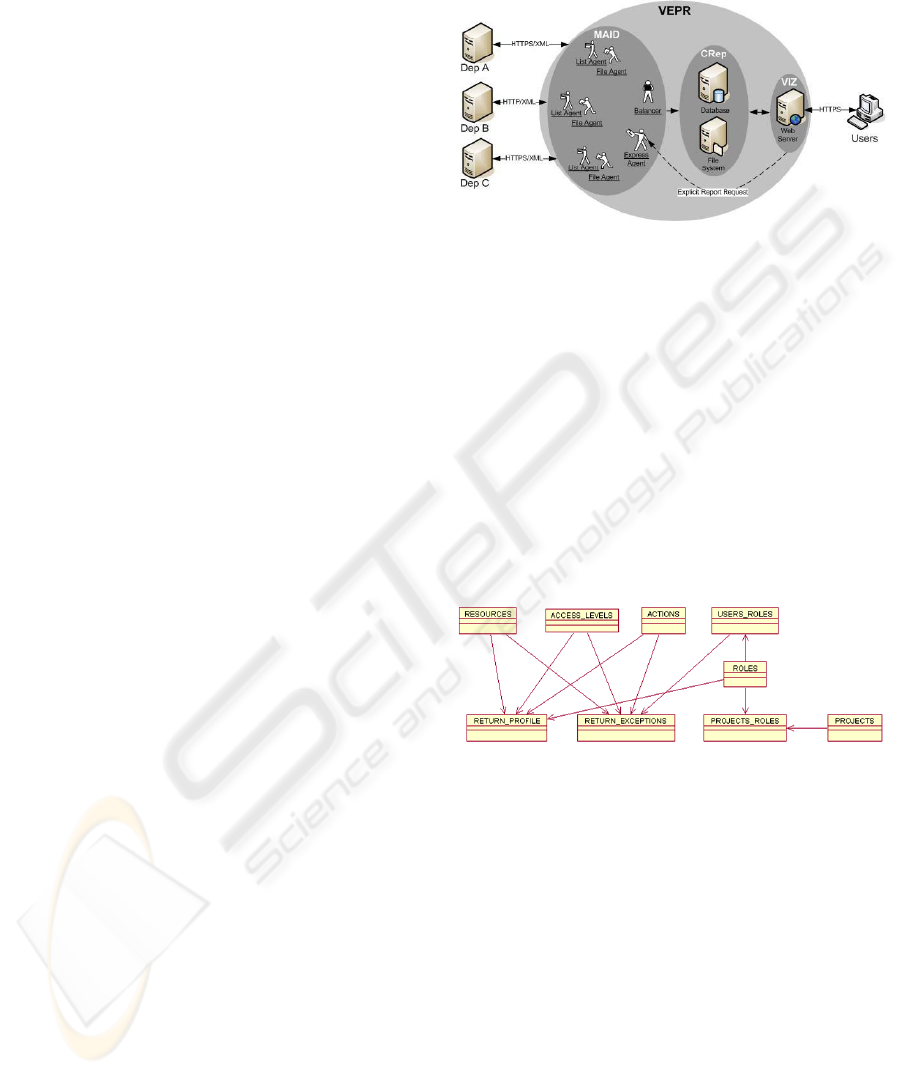
The core of the EMR system is composed by three
modules (VIZ – Viewing modules, MAID - Multi-
Agent system for Integration of Data and CRep –
Central repository) which are presented in Figure 1.
MAID collects clinical reports from various hospital
departments (e.g. DIS A and DIS B), and stores
them on a central repository (CRep) consisting of a
database holding references to these resports. After
searching the database, the users can access the
integrated data of a particular patient through a web-
based interface (VIZ). When selecting a specific
report, its content is downloaded from the central
repository file system to the browser.
Figure 1: Architecture of the EMR system showing the
MAID, the VIZ and the CRep modules.
In order for the access control management
platform, the webcare, to be implemented it is
necessary an authentication procedure where the
user is uniquely identified and associated with his
profile according to the role or groups where he
belongs (i.e. privileges and permissions).
To associate this profile to the user, an
infrastructure to model the relationships between all
the identities that integrate the RBAC model,
including exceptions (accesses with more or less
privileges that are related to specific users and not
only their roles or groups), was created (see Fig. 2).
Figure 2: Entity-relation model for the access control
platform.
This infrastructure includes entities such as users,
roles (which can include subroles), resources, access
levels, actions, projects, the entity that includes the
privileges and connects all of them (return_profile),
and also the entity that does the same for the
exception rules (return_exceptions). This model
implements all the necessary structure as well as the
exceptions needed to generate the profile for a
specific user at the time he/she authenticates to the
system. To retrieve all this information there is a
centralized feature, a procedure, to search the whole
structure and collect all the privileges associated to
the user.
FROM LEGISLATION TO PRACTICE - A Case Study of Break the Glass in Healthcare
115
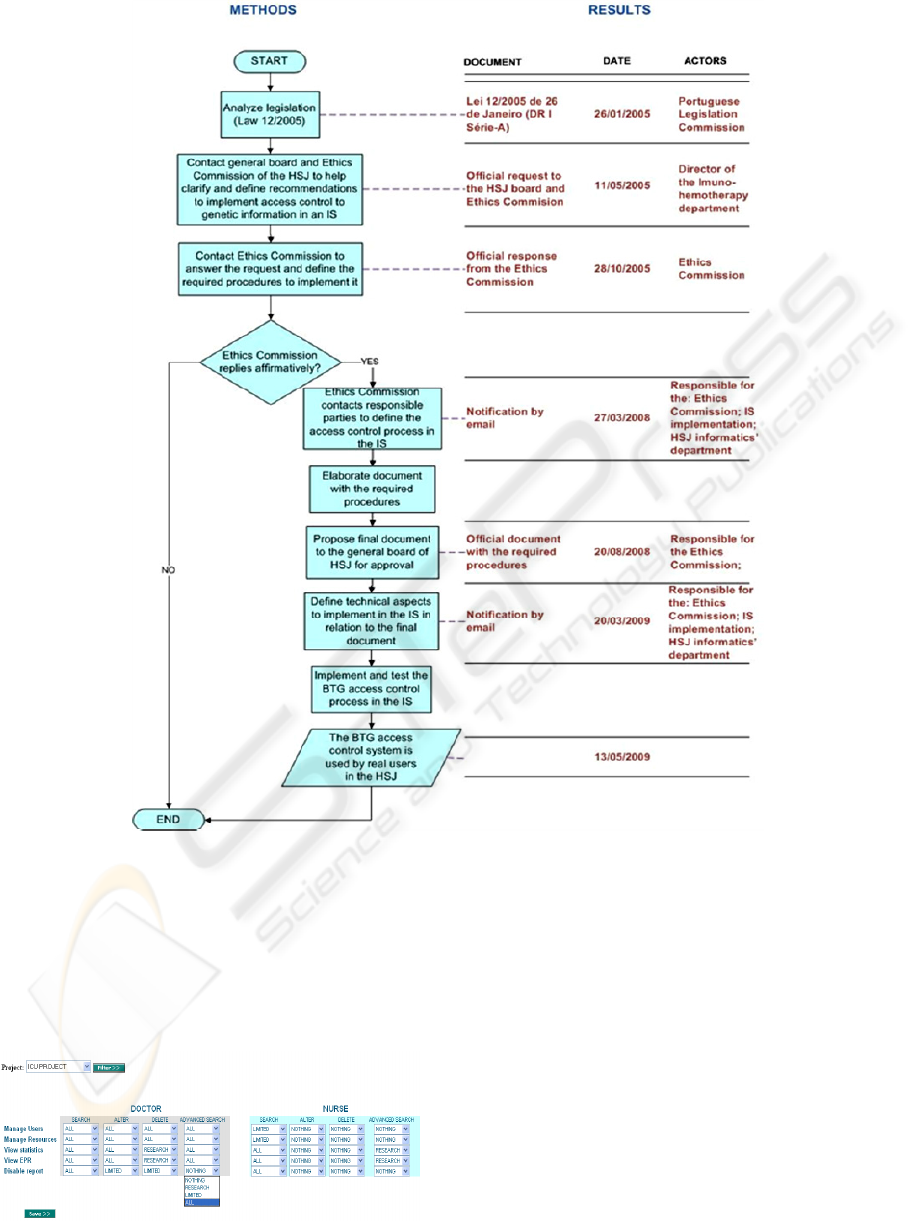
Figure 3: Methods and results from legislation to practice.
All accesses are registered in a specific database
structure, separate from the one above. It registers
the user, date and time and also the errors that may
occur during this process. This is easy to do because
the procedure itself can generate exceptions and
insert error information according to the failed
action.
Figure 4: Access control management platform.
As this platform does not handle BTG accesses
some changes needed to be made. These changes are
presented in Section 4.
3 METHODS
Figure 3 presents the methodology used to define
and implement the BTG access control engine from
legislation to practice.
After the implementation the user logs were
analysed so that we could assess if the BTG system
was working as expected and if legislation was
being enforced in a controlled and responsible
manner. We also wanted to evaluate the impact that
HEALTHINF 2010 - International Conference on Health Informatics
116

the BTG systems had on the protection of
confidentiality of patient genetic information.
We did this by analysing logs where users tried
to access patient reports that contained genetic
information. Similar time periods were compared:
the 3 months of BTG access control features usage,
by real users, on a real setting with the same period
of time on the previous year, where no BTG features
were available.
4 RESULTS
4.1 From Legislation to Practice
The necessary steps for a user to perform BTG
within the presented EMR system are the following
(see Figure 5):
1. The healthcare professional tries to access a
patient report within the EMR application
and that report contains genetic information.
2. The webcare platform validates the
healthcare professionals’ credentials.
3. The webcare platform checks within the
database if the pair login/password is correct.
(In the case where the authentication fails, a reject
message is sent from the application to the user and
the request terminates here; if the user is privileged
and can access directly the required report than the
process is done normally)
Figure 3 presents the results from each step of the
applied methodology described in the previous
section.
4.2 BTG Implementation
4. The webcare platform sends back the user
profile that states if the user can BTG or not to
the EMR application.
5. The EMR application asks the healthcare
professional if he/she wants to BTG on that
report, warning about the consequences of
doing that (see Figure 6).
6. If the user chooses to BTG (giving a reason
for it) he/she just needs to press the
appropriate button within the shown interface
(see Figure 6).
7. The EMR application makes the requested
operation to get the report.
8. The report is given to the EMR application.
9. The EMR application shows the report to the
healthcare professional.
Figure 5: BTG steps.
Once the user chooses a report that contains genetic
information, several actions are registered so that the
user is accountable for it afterwards. The system
registers if the user just made a mistake, whether
he/she carries on the BTG procedure or not, and if
so, registering the reason he/she gives to do it.
Several procedures were altered within the
webcare platform in order to do this. These included:
• The creation of a new group of users (11
medical doctors) that comprise the healthcare
professionals that are authorized to access
patient reports that contain genetic information,
according to the Ethics Commission official
document;
• The creation of a new table within the database
(BTG audit table) in order to register
information about who is trying to access
patient reports that contain genetic information
(Table 1);
Table 1: Database table to audit user actions regarding
BTG accesses.
Campo Tipo Descricao
Id Number Unique identifier (primary key)
Timestamp Date
Date & time the BTG popup warning
occurred
Id_sessao Number Session identifier
Id_relatorio Number
Report identifier the user tried to Access
while BTG
Resposta Number
The final option chosen by the user (BTG
or not)
Motivo_opc Number The reason that was chosen for BTG
Motivo String
Reason described by the user for BTG
when the option “Other” is chosen
Besides Table 1, a new attribute was created within
another table that stores all the patient reports within
the database. This new attribute is a Boolean and is
named as “genetics”. It states whether the patient
report contains genetic information or not. This
information is registered automatically from the
moment the patient report is collected and stored in
the database.
FROM LEGISLATION TO PRACTICE - A Case Study of Break the Glass in Healthcare
117

Figure 6: BTG Interface.
Also, on a coding level, there was only the need to
introduce a condition that would check for each
user’s request of a report if that report contains
genetic information and if that user is a member of
the group of healthcare professionals that is
authorized to access these kind of reports. Each time
one of these reports is requested to be seen by the
healthcare professionals, a new record is inserted
within the BTG audit table with identifiers of the
report and the healthcare professional. All this is
registered whether the healthcare professional
chooses to go back, or if the user answers no or yes
to BTG.
All this information is summarized and sent to
the hierarchical superior of those users by email on a
weekly basis. This makes sure that proper
justification or any other disciplinary action can be
taken afterwards. It guarantees that BTG accesses
are properly controlled and taken responsibility for.
4.3 Results Before and After BTG
Implementation
The comparison period comprised 15 weeks of BTG
access control features usage on a real setting
(between the 13
th
of May 2009 and 26
th
of August
2009) with the same period of time on the previous
year, where no BTG features were available
(between the 13
th
May 2008 and 26
th
of August
2008).
The patient reports started to be tagged with a
genetic label (so that they could be identified) on the
27/11/2007, so we can only analyse the obtained
results based on the reports that were stored from
this day onwards. The number of genetic reports that
were marked at the date of 26/08/2008 is 1093,
while on the 26/08/2009 this number had risen to
3274 (2181 more in a year). To this same date the
total number of distinct users of the EMR system is
906.
Table 2: The percentage of accesses to reports containing
genetic information according to the total number of
genetic reports that was available before and after the
BTG system implementation, as well as the number of
distinct users that performed those accesses.
Accesses to reports containing genetic information
Before BTG After BTG
Total of collected genetic reports 1093 3274
% of accesses 21 14
Within authorization group
4 3
Not within authorization group
17 11
No of distinct users accessing 76 135
Within authorization group
4 5
Not within authorization group
72 130
HEALTHINF 2010 - International Conference on Health Informatics
118
After the BTG features started to be used (between
13/05/2009 and 26/08/2009) the total number of tries
to access genetic reports was 471, being 86 from
within the authorisation group while 385 from users
that are not normally authorized to access them.
Table 3 shows within this last number the actions the
users took once they were alerted they were not
authorised to access the report they requested.
Table 3: Number of BTG accesses made to patient reports
containing genetic information (from the users NOT
within the authorisation group).
BTG accesses to reports with genetic information
BTG NO BTG
No of accesses 208 (54%) 177 (46%)
No of distinct users 83 98
Within the 177 users that did not do BTG after
choosing to access a genetic report, 156 selected NO
to BTG while 21 closed the browser without further
action. From the 208 users that selected to perform
BTG, Table 4 describes the most common reasons
the users gave to justify their access.
Table 4: Most common reasons given by the users to
perform BTG (n=208).
Reasons to perform BTG %
I have urgency in seeing the requested information
although I’m not normally allowed to do it
50%
Write own reason 32%
I should belong to the group that can access genetic
information
18%
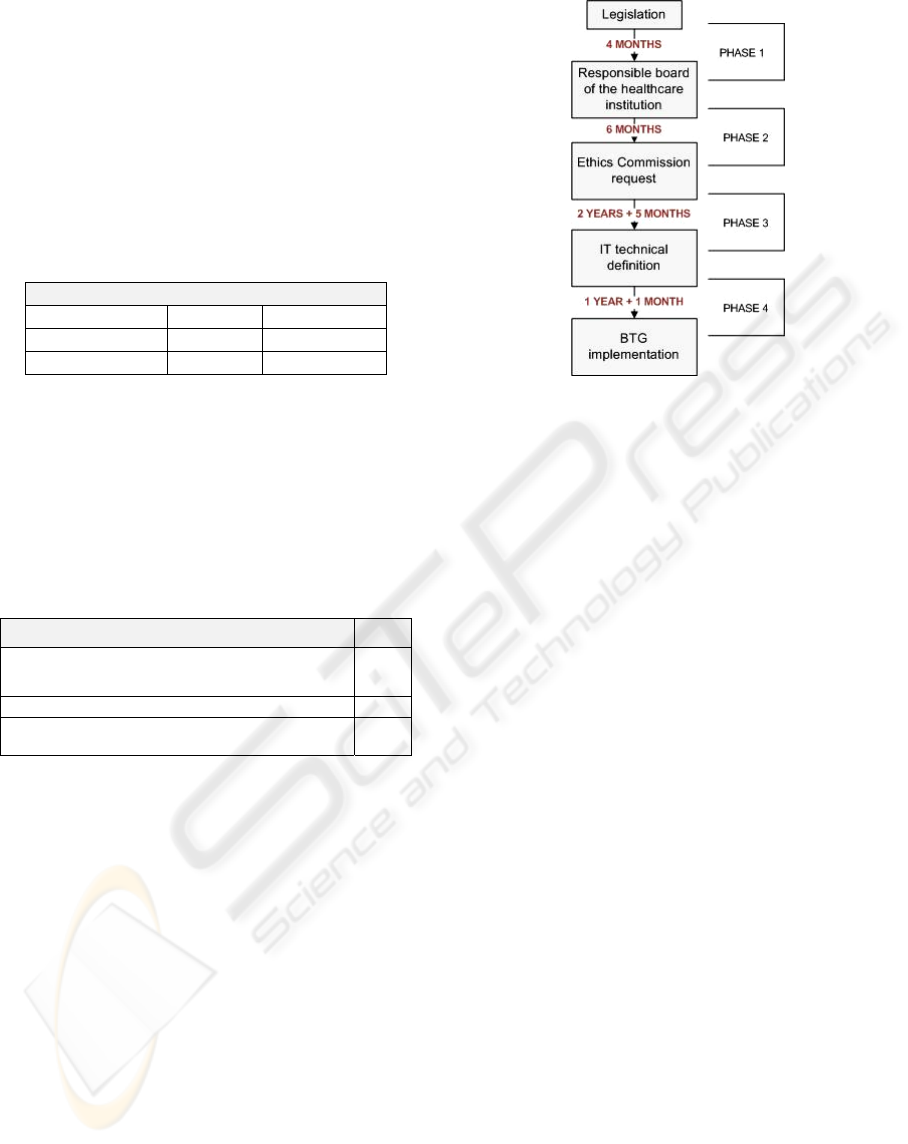
5 DISCUSSION
The healthcare legislation for genetic information
was published in January 2005 and its
implementation in practice took, on the whole, 4
years and 4 months (see Figure 7). The process took
more time in phases 3 & 4, which include the
definition of the regulatory (2 years and 5 months)
and technical specifications (1 year and 1 month).
Figure 7 presents the main phases of this process. In
all of the phases we believe is possible to fasten the
process.
Phases 1 & 2 are more logistic intensive and
therefore should be accomplished in a swifter
fashion.
Although being the hardest to do, we think that
phase 3 is the one that needs more attention. It
should be possible to fasten the process of
translating legislation into regulations that can be
Figure 7: Timeframe of the methodology from legislation
to practice.
implemented in an EMR. In addition, the definition
of what to implement can be faster if the meetings
with the technical people are made earlier in the
process. 4 years to enforce legislation is, in our
view, a long time. The institutions and systems must
be ready to do this in an easier and more efficient
way. We believe that the whole process could have
taken place in possibly half the time. This study
helped in identifying where the major problems can
be located and where improvements can be made.
Regarding the technical implementation of the
BTG concept, this was an easy and fast process
because it was integrated within an EMR platform
that was already in use in the healthcare practice and
was implemented in a modular and flexible way.
Only a few changes were needed to adopt the BTG
concept and this allowed for the long period spent in
defining the procedural regulations to be enforced,
to be shortened at this stage.
The results of implementing and using the BTG
features showed that there is a significant decrease
in the percentage of accesses to genetic reports when
the BTG features are available, even when the
number of genetic reports available are much higher
(almost triple). There is a similar decrease in non
authorized people accessing those reports. Further,
from the unauthorized users that tried to access
genetic reports, almost half of them decide not to go
through with it. This means that the BTG features
can filter these non authorized accesses that would
normally not be prevented. We expect that the
tendency will be that only users who really need to
access the reports will go through with the process
of BTG.
FROM LEGISLATION TO PRACTICE - A Case Study of Break the Glass in Healthcare
119

The most common reason given by the users that
perform BTG is that they have urgency to do it. This
reason needs to be more detailed. Also, the
justification process that happens afterwards needs
to complement the reasons given in an efficient and
coherent way.
Limitations for this study include the few data
that was available as the system had only been in use
in a real setting since May 2009 and the fact that
genetic reports were only identified from November
2007, when the EMR system has been in use since
October 2004. Moreover, the users of the system
still need to get familiar with this feature because in
the beginning they may think it is an application
error that does not let them access what they
normally did, and try it several times in a row. Also
to take into account is the fact that, at the moment,
only medical doctors are using the EMR system. Its
use will need to be more scrutinised when other
healthcare professionals will start accessing it as
well.
Future research to continue the improvement of
this BTG system includes a thorough analysis of the
justification process, to make sure accountability
really works. Another enhancement to this system
will be the implementation of the access control
model within a more robust access control platform
and not only the usage of a database to perform the
authentication and authorization process. Further, we
want to implement the BTG system into similar
domains that require BTG features to conform to
legislation, or any other regulations and needs, in
order to enhance the process from legislation to
practice.
REFERENCES
Break-glass: An approach to granting emergency access to
healthcare systems, 2004. White paper, Joint –
NEMA/COCIR/JIRA Security and Privacy Committee
(SPC).
Cruz-Correia R., Vieira-Marques P., Costa P., Ferreira A.,
Oliveira-Palhares E., Araújo F., et al., 2005.
Integration of Hospital data using Agent Technologies
– a case study. AICommunications special issue of
ECAI. 18(3):191-200.
Farinha P., Ferreira A., Cruz-Correia R., 2006. Gestão de
acessos e recursos para estudos clínicos multicêntricos
on-line. Actas da 1ª Conferência Ibérica de sistemas e
Tecnologia de Informação. 1: 631-640.
Ferraiolo, D. & Sandhu, R. & Gavrila, S. & Kuhn, R. &
Chandramouli, R. (2001). Proposed NIST Standard for
Role-based Access Control. ACM Transactions on
Information and systems security. 4(3):224-274.
Ferreira A., Cruz-Correia R., Costa-Pereira A., 2004.
Securing a Web-based EPR: An approach to secure a
centralized EPR within a hospital. Proceedings of the
6th International Conference on Enterprise
Information Systems. 3: 54-9.
Ferreira A., Cruz-Correia R., Antunes L., Farinha P.,
Oliveira-Palhares E., Chadwick D W., Costa-Pereira
A., 2006. How to break access control in a controlled
manner? Proceedings of the 19th IEEE Symposium on
Computer-Based Medical Systems. 847-851.
Lei nº 12/2005. Informação genética pessoal de saúde.
Membres CdMaÉ, 1997. Protection des Données
Médicales. Recommendation n° R (97) 5.
Ministers CoE-Co. 2004. On the impact of information
technologies on health care – the patient and Internet.
Recommendation Rec (2004) 17.
Povey D., 2000. Optimistic security: a new access control
paradigm. Proceedings of the 1999 workshop on New
security paradigms. ACM Press. 40-45.
Rissanen E., Firozabadi S., Sergot M., 2004. Towards a
Mechanism for Discretionary Overriding of Access
Control. Proceedings of the 12th International
Workshop on Security Protocols, Cambridge.
Ross-Lee B., Weiser M., 1994. Healthcare Regulation:
Past, present and future. JAOA – Healthcare policy.
94(1):74-84.
Waegemann C., 2003. EHR vs. CPR vs. EMR. Healthcare
Informatics online.
HEALTHINF 2010 - International Conference on Health Informatics
120
