
STUDY ON EFFECTS OF MICROORGANISM IN
DEPOLYMERIZATION PROCESS OF XENOBIOTIC POLYMERS
BY MODELING AND SIMULATON
Masaji Watanabe
Graduate School of Environmental Science, Okayama University, Okayama, Japan
Fusako Kawai
R & D Center of Bio-based Materials, Kyoto Institute of Technology, Kyoto, Japan
Keywords:
Polymer, Biodegradation, Mathematical modeling, Numerical simulation.
Abstract:
Effects of microorganism in biodegradation process of polyethylene glycol are studied by modeling and simu-
lation. Dynamics of population of microorganism is taken into consideration in modeling of depolymerization
process of exogenous type, and a mathematical model is described. A molecular factor of degradation rate
is obtained by solving an inverse problem, and a time factor of degradation is obtained by analyzing the dy-
namics of population of microorganism. Once the time factor and the molecular factor of degradation rate are
determined, a depolymerization process is simulated by solving an initial value problem.
1 INTRODUCTION
Biodegradation is an essential factor of environmental
protection against accumulation of xenobiotic poly-
mers. It is important not only for water soluble
polymers but also for water-insoluble polymers, be-
cause they are not completely recycled nor inciner-
ated. Microbial depolymerization processes are gen-
erally classified into exogenous type or endogenous
type. In an exogenous type depolymerization pro-
cess, monomer units are separated from terminals
of molecules stepwise. Examples of exogenous de-
polymerization process include the β-oxidation of
polyethylene (PE). In an endogenoustype depolymer-
ization process, molecules are split at arbitrary po-
sitions. Examples of endogenous depolymerization
process include enzymatic degradation of polyvinyl
alcohol PVA. Mathematical models for those depoly-
merization processes were proposed in previous stud-
ies. Microbial depolymerization processes of xenobi-
otic polymers were studied by analysis based on those
models.
In this study, microbial depolymerization process
of polyethylene glycol (PEG) is analyzed. PEG is
one of polyethers expressed by HO(R-O)
n
H, for ex-
ample, PEG: R = CH
2
CH
2
, polypropylene glycol
(PPG): R = CH
3
CHCH
2
, polytetramethylene glycol
(PTMG): R = (CH
2
)
4
(Kawai, 1993). They are uti-
lized for constituents of productsincluding lubricants,
antifreeze agents, inks, and cosmetics. PEG is pro-
duced more than any other polyether, and the ma-
jor part of the production is consumed for nonionic
surfactants. PEG is depolymerized by separation of
C
2
compounds, either aerobically or anaerobically
(Kawai, 1995; Kawai, 2002a; Kawai, 2002b). High
performance liquid chromatography (HPLC) patterns
were used in analysis as the weight distribution of
PEG with respect to the molecular weight before and
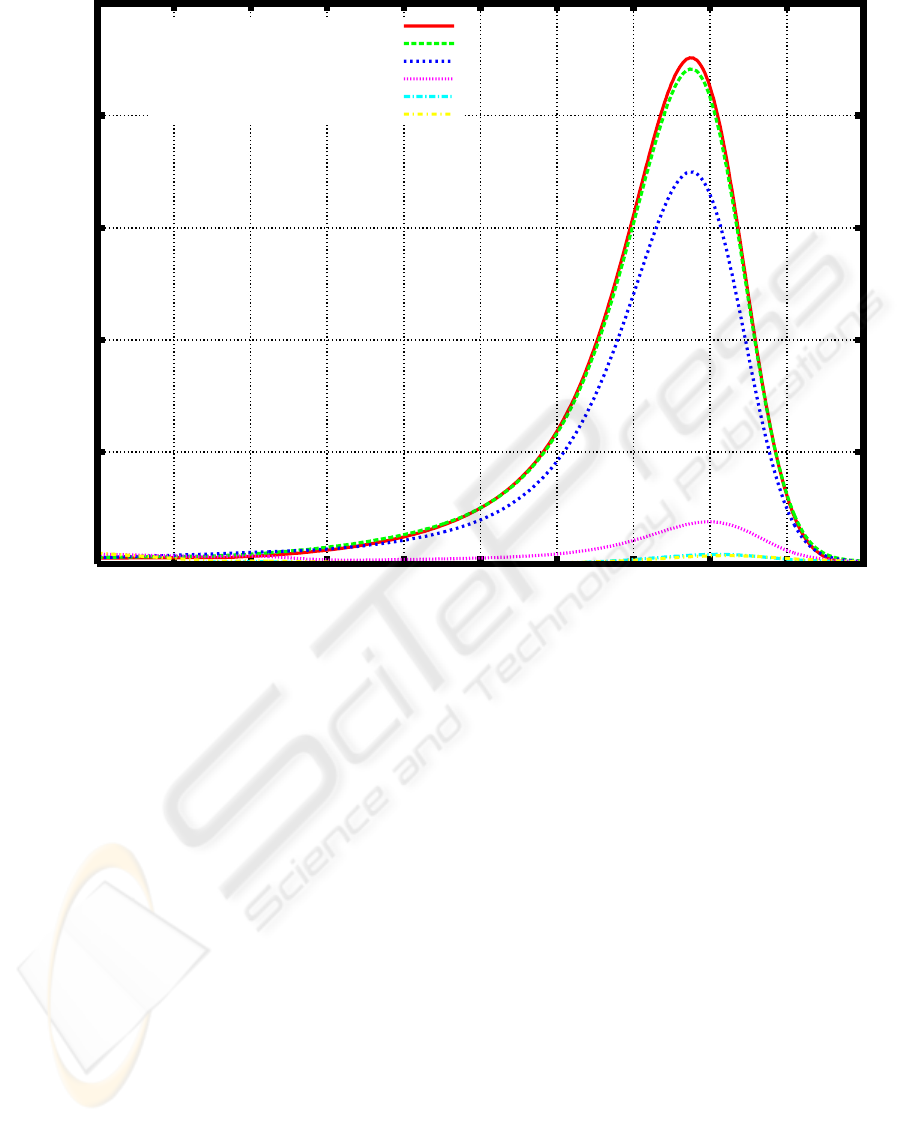
after cultivation of a microbial consortium E1 (Figure
1).
In the previous studies (Watanabe and Kawai,
2004; Watanabe and Kawai, 2005), time indepen-
den degradation rates were assumed. Time depen-
dent degradation rates were considered in a recent
study (Watanabe and Kawai, 2007a). Cubic spline
was used to take the change of microbial population
into consideration (Watanabe and Kawai, 2007b). E
exponential growth in a microbial population was as-
sumed (Watanabe and Kawai, 2009b). The time factor
was also determined by assuming the logistic growth
in the time integral of microbial population (Watan-
abe and Kawai, 2009a). In this study, analysis of
181
Watanabe M. and Kawai F. (2010).
STUDY ON EFFECTS OF MICROORGANISM IN DEPOLYMERIZATION PROCESS OF XENOBIOTIC POLYMERS BY MODELING AND SIMULATON.
In Proceedings of the First International Conference on Bioinformatics, pages 181-186
DOI: 10.5220/0002757601810186
Copyright
c
SciTePress
0
0.005
0.01
0.015
0.02
0.025
3.2 3.3 3.4 3.5 3.6 3.7 3.8 3.9 4 4.1 4.2
COMPOSITION (%)
LOG M
BEFORE CULTIVATION
AFTER 1-DAY CULTIVATION
AFTER 3-DAY CULTIVATION
AFTER 5-DAY CULTIVATION
AFTER 7-DAY CULTIVATION
AFTER 9-DAY CULTIVATION
Figure 1: Weight distribution of PEG before and after cultivation of a microbial consortium E1 (Watanabe and Kawai, 2009a).
biodegradation of PEG is continued. A change of
variable transforms the time dependent model into a
model for which the degradation rate is time indepen-
dent. Techniques developed in previous studies are
applied to solve an inverse problem to determine the
molecular factor of degradation rate for which the so-
lution of an initial value problem satisfies not only the
initial weight distribution but also the weight distribu-
tion after cultivation. The time factor is determined by
assuming change in microbial population due to the
amount of PEG as the sole carbon source. Once the
degradation rate is found, the transition of the weight
distribution was simulated by solving the initial value
problem.
2 MODEL OF EXOGENOUS
TYPE DEPOLYMERIZATION
PROCESS WITH TIME
DEPENDENT DEGRADATION
RATE
In microbial depolymerization of PE, molecules lose
their weight gradually by terminal separations of
monomer units (β-oxidation) until they become small
enough to be absorbed directly into cells. The PE
biodegradation model proposed previously is based
on two essential factors, the gradual weight loss of
large molecules due to β-oxidation and the direct con-
sumption of small molecules by cells. Let t and M
be the time and the molecular weight respectively.
Suppose tha a M-molecule denotes a molecule with
molecular weight M. Let w(t,M) represent the total
weight of M-molecules at time t, and L be the amount
of weight loss due to the β-oxidation. Let the func-
tion ρ(M) represent the direct consumption rate, the
function β(M) represent the β-oxidation rate, which
BIOINFORMATICS 2010 - International Conference on Bioinformatics
182
is the rate of the weight conversion from the class of
M-molecules to the class of (M − L)-molecules due
to the β-oxidation, and α(M) = ρ(M) + β(M). The
equation (1) was proposed to study PE biodegrada-
tion processes (F. Kawai, 2002; M. Watanabe, 2003;
F. Kawai, 2004; M. Watanabe, 2004).
dx
dt
= −α(M) x + β(M + L)
M
M + L
y, (1)
where x = w(t, M), the total weight of M-molecules at
time t, and y = w(t,M+ L), the total weight of M+L-
molecules at time t.
The mathematical model (1) was originally devel-
oped for the PE biodegradation, but it can be viewed
as a general biodegradation model for exogenous de-
polymerization processes. In an exogenous depoly-
merization process of PEG, a molecule is first oxi-
dized at its terminal, and then an ether bond is split.
It follows that L = 44 (CH
2
CH
2
O) for the exoge-
nous depolymerization of PEG. PEG molecules stud-
ied here are large molecules, and they can not be ab-
sorbed directly through membrane into cells. Then
ρ(M) = 0, and α(M) = β(M).
Equation (1) is appropriate for the depolymeriza-
tion processes over the period in which the microbial
population has reached a stationary state. On the con-
trary, the change of microbial population should be
taken into account for the period in which it is still
in a developing stage or in a decreasing stage. Then
degradation rate should be time dependent, and the
exogenous depolymerization model is
dx
dt
= −β(t, M)x+ β(t,M + L)
M
M + L
y. (2)
The solution x = w(t,M) of the equation (2) is asso-
ciated with the initial condition
w(0,M) = f (M) , (3)
where f (M) is the initial weight distribution. Given a
presicribed degradation rate β(t,M), equation (2) and
the initial condition (3) form an initial value problem.
Time factors of the degradation rate such as micro-
bial population, dissolved oxygen, or temperature af-
fect molecules regardless of sizes. Then the degrada-
tion rate should be split into the time dependent part,
which we denote by σ(t), and the molecular depen-
dent part, which we denote by λ(M), and the degra-
dation rate is the product
β(t,M) = σ(t)λ(M) . (4)
The time factor of the degradation rate can be re-
moved by the transformation
τ =
Z
t
0
σ(s) ds. (5)
Let
W (τ,M) = w(t, M) ,
X = W (τ,M) ,
Y = W (τ, M + L) .
Then
dX
dτ
=
dx
dt
dt
dτ
=
1
σ(t)
dx
dt
,
and the equation (2) becomes
dX
dτ
= −λ(M) X + λ(M + L)
M
M + L
Y . (6)
Given the initial weight distribution f (M), solution
the initial value problem is the solution of the equa-
tion (6) subject to the initial condition
W (0, M) = f (M). (7)
Given additional condition
W (T ,M) = g(M) , (8)
solution of the inverse problem is the degradation rate
λ(M) for which the solution of the initial value prob-
lem (6), (7) also satisfies the condition (8). When the
solution W (τ,M) of the initial value problem (6), (7)
satisfies the condition (8), the solution w(t,M) of the
initial value problem (2), (3) satisfies
w(T,M) = g(M) , (9)
where
T =
Z
T
0
σ(s) ds. (10)
The inverse problem (6), (7), (8) was solved nu-
merically with techniques developedin previous stud-
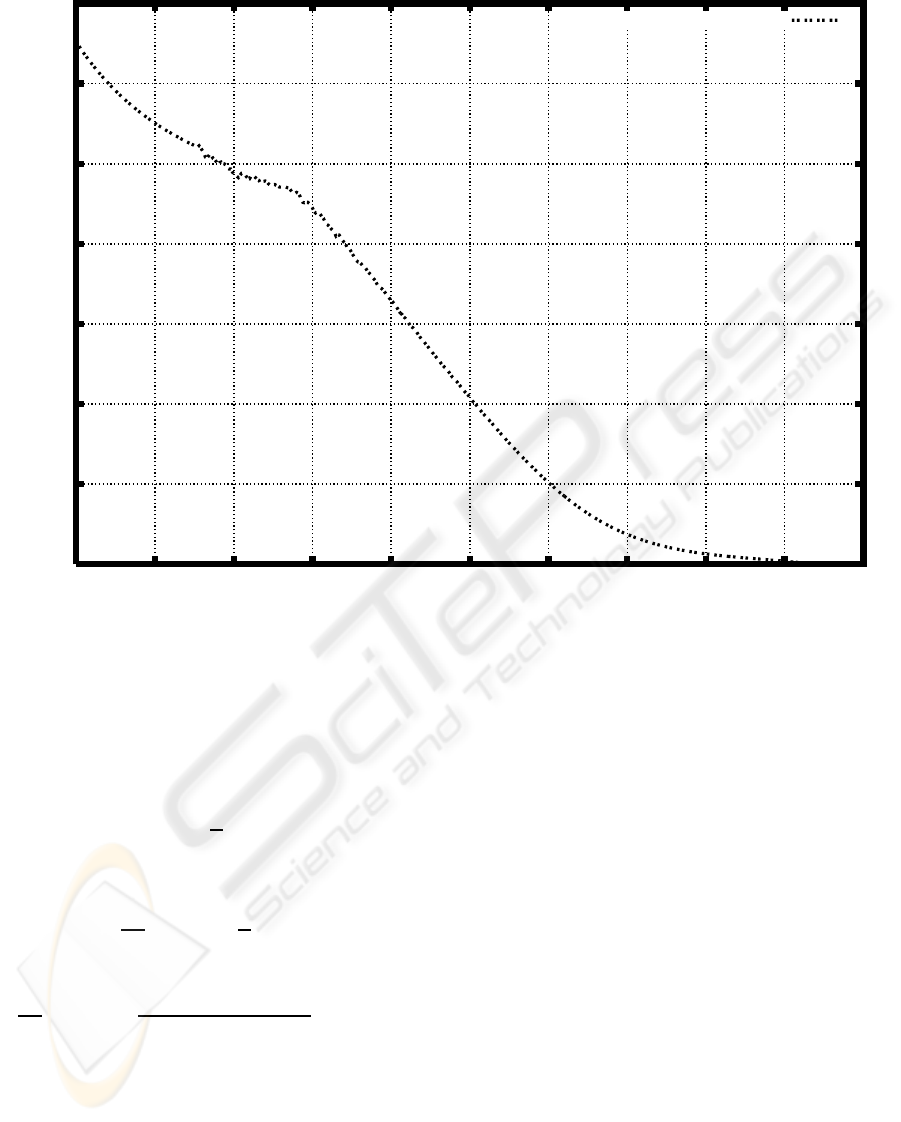
ies. Figures 2 shows the degradation rates based on
the weight distribution before and after cultivation for
three days (Watanabe and Kawai, 2009a).
3 TIME FACTOR OF
DEGRADATION RATE BASED
ON MICROBIAL POPULATION
Suppose that the time factor σ(t) represents the mi-
crobial population, and that PEG is the sole carbon
source. The total amount of polymer utilized by mi-
croorganisms per unit time at time t is
A =
Z
∞
0
σ(t) λ(M) w(t, M) dM.
The microbial population increases under sufficient
carbon supply when the ratio of the microbial pop-
ulation to the total amount is below a certain lavel
STUDY ON EFFECTS OF MICROORGANISM IN DEPOLYMERIZATION PROCESS OF XENOBIOTIC POLYMERS
BY MODELING AND SIMULATON
183
0
20
40
60
80
100
120
140
3.2 3.3 3.4 3.5 3.6 3.7 3.8 3.9 4 4.1 4.2
DEGRADATION RATE PER DAY
LOG M
DEGRADATION RATE
Figure 2: Degradation rate based on the weight distribution of PEG before and after cultivation of a microbial consortium E1
(Watanabe and Kawai, 2009a).
σ/A < 1/h It decreases under insufficient carbon sup-
ply when σ/A > 1/h. Then the growth rate of micro-
bial population is propotinal to
1− h
σ
A
,
and the microbial population σ(t) is a solution of the
equation
dσ
dt
= k
1− h
σ
A
σ (11)
Note that equation (11) becomes
dσ
dt
= k
1− h
1
R
∞
0
λ(M)w(t, M) dM
σ. (12)
Equation (12) is associated with the initial condition
σ(0) = σ
0
. (13)
Equations (2) and (12) are solved simultaneously
to simulate the transition of weight distrubution of
PEG and evolution of microbial population for σ
0
≈
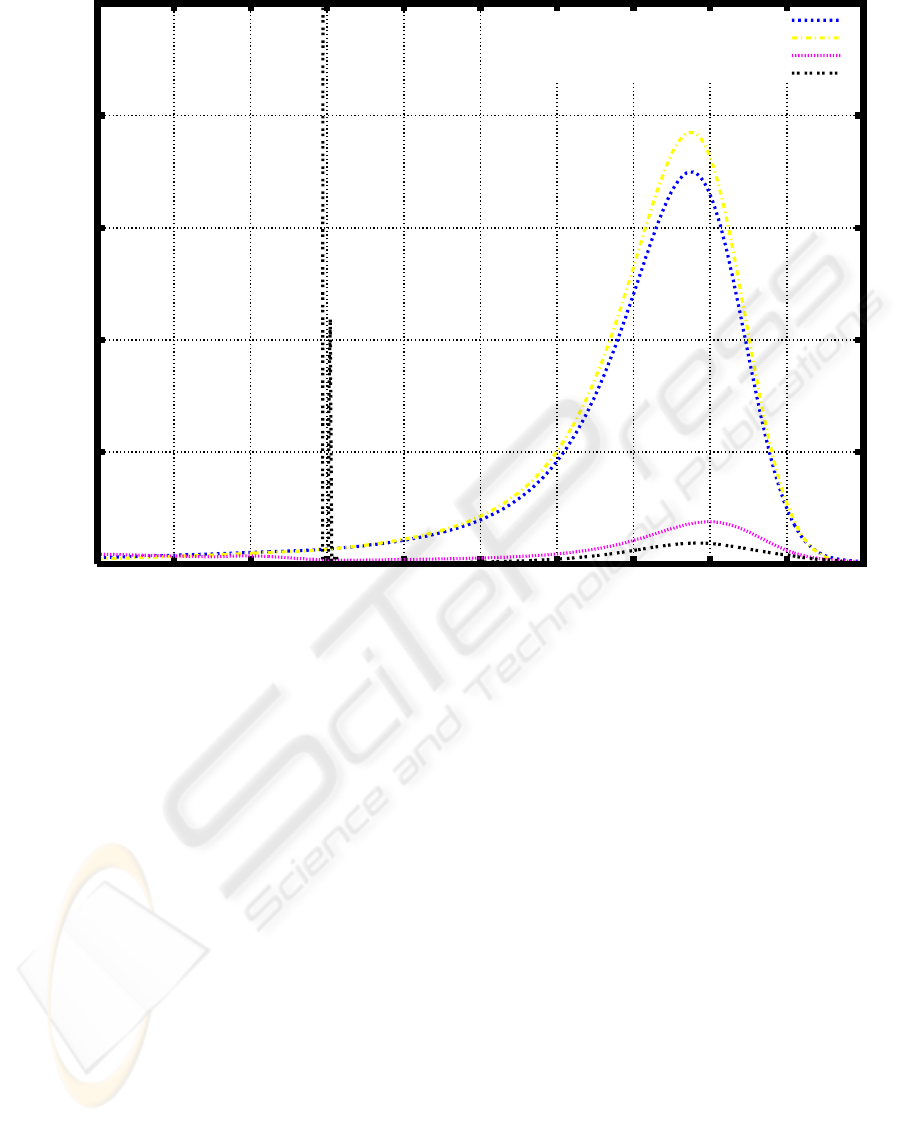
0.029827, k ≈ 51.6, and h = 500.0. Figure 3 shows
the resulsts of simulation for
4 CONCLUSIONS
In a depolymerization process in which microbial
population is an essential factor, the dependence of
the degradation rate on time becomes significant. It
is appropriate to assume that the degradation rate is a
product of a time factor and a molecular factor. In an
environmental setting, the time factor should also de-
pend on other factors such as temperature or dissolved
oxygen. Once these essentials are taken into consider-
ation, the exogenous depolymerization model will be
applicable to assess the biodegradabilityof xenobiotic
polymers in the environment.
Subjects in study of microbial depolymerization
of xenobiotic polymers by modeling and simulation
in next steps includes development of numerical tech-
niques to identify the values of parameters which
appear in the equation (11). Results of simulation
should be compared with experimental results con-
cerning the transition of weight distribution of the
polymer and the microbial population to verify nu-
merical results.
BIOINFORMATICS 2010 - International Conference on Bioinformatics
184
0
0.005
0.01
0.015
0.02
0.025
3.2 3.3 3.4 3.5 3.6 3.7 3.8 3.9 4 4.1 4.2
COMPOSITION (%)
LOG M
AFTER 3-DAY CULTIVATION
SIMULATION: 3 DAY
AFTER 5-DAY CULTIVATION
SIMULATION: 5 DAY
Figure 3: Numerival simulation of weight distribution and experimental results .
ACKNOWLEDGEMENTS
The authors thank Ms Y. Shimizu for her technical
support. This work was supported by JSPS KAK-
ENHI 20540118.
REFERENCES
F. Kawai, M. Watanabe, M. S. S. Y. Y. S. (2002). Ex-
perimental analysis and numerical simulation for
biodegradability of polyethylene. Polymer Degrada-
tion and Stability, 76:129–135. doi: 10.1016/S0141-
3910(02)00006-X.
F. Kawai, M. Watanabe, M. S. S. Y. Y. S. S. H.
(2004). Comparative study on biodegradability of
polyethylene wax by bacteria and fungi. Poly-
mer Degradation and Stability, 86:105–114. doi:
10.1016/j.polymdegradstab.2004.03.015.
Kawai, F. (1993). Biodegradability and chemical structure
of polyethers. Kobunshi Ronbunshu, 50(10):775–780.
In Japanese.
Kawai, F. (1995). Breakdown of plastics and polymers by
microorganisms. Advances in Biochemical Engineer-
ing/Biotechnology, 52:151–194.
Kawai, F. (2002a). Microbial degradation of polyethers. Ap-
plied Microbiology and Biotechnology, 58:30–38. doi:
10.1007/s00253-001-0850-2.
Kawai, F. (2002b). Xenobiotic polymers. In Imanaka, T.,
editor, Great Development of Microorganisms, pages
865–870, Tokyo. NTS. Inc. In Japanese.
M. Watanabe, F. Kawai, M. S. S. Y. Y. S. (2003). Computa-
tional method for analysis of polyethylene biodegra-
dation. Journal of Computational and Applied
Mathematics, 161(1):133–144. doi: 10.1016/S0377-
0427(03)0051-X.
M. Watanabe, F. Kawai, M. S. S. Y. Y. S. S. H.
(2004). Analytical and computational techniques
for exogenous depolymerization of xenobiotic poly-
mers. Mathematical Biosciences, 192:19–37. doi:
10.1016/j.mbs.2004.06.006.
Watanabe, M. and Kawai, F. (2004). Analysis of biodegrad-
ability for polyethylene glycol via numerical simula-
tion. Environmental Research and Control, 26:17–22.
In Japanese.
Watanabe, M. and Kawai, F. (2005). Numerical simula-
tion of microbial depolymerization process of exoge-
nous type. ANZIAM J., 46(E):C1188–C1204. Proc.
STUDY ON EFFECTS OF MICROORGANISM IN DEPOLYMERIZATION PROCESS OF XENOBIOTIC POLYMERS
BY MODELING AND SIMULATON
185

of 12th Computational Techniques and Applications
Conference, CTAC-2004, Melbourne, Australia in
September 2004, Editors: Rob May and A. J. Robert
(http://anziamj.austms.org.au/V46/CTAC2004/Wata).
Watanabe, M. and Kawai, F. (2007a). Mathematical study
of the biodegradation of xenobiotic polymers with ex-
perimental data introduced into analysis. ANZIAM J.,
47:C665–C681. Proceedings of the 7th Biennial En-
gineering Mathematics and Applications Conference,
EMAC-2005, Melbourne, Editors: Andrew Stacey
and Bill Blyth and John Shepherd and A. J. Roberts,
(http://anziamj.austms.org.au/V47EMAC2005/
Watanabe).
Watanabe, M. and Kawai, F. (2007b). Numerical study of
biodegradation of xenobiotic polymers based on ex-
ogenous depolymerization model with time dependent
degradation rate. Journal of the Faculty of Environ-
mental Science and Technology, Okayama University,
12:1–6.
Watanabe, M. and Kawai, F. (2009a). Mathematical analy-
sis of microbial depolymerization processes of xeno-
biotic polymers. In Mercer, G. N. and Roberts,
A. J., editors, Proceedings of the 14th Biennial Com-
putational Techniques and Application Conference,
CTAC2008, volume 50 of ANZIAM J., pages C930–
C946. (http://anziamj.austma.org.au/ojs/index.php
/ANZIAMJ/article/view/1465).
Watanabe, M. and Kawai, F. (2009b). Modeling and simu-
lation of biodegradation of xenobiotic polymers based
on experimental results. In BIOSIGNALS 2009, Sec-
ond International Conference on Bio-inspired Systems
and Signal Processing, Proceedings, Porto - Portugal,
14-17 January, 2009, pages 25–34. INSTICC Press.
BIOINFORMATICS 2010 - International Conference on Bioinformatics
186
