
CALIBRATION-FREE MARKERLESS AUGMENTED REALITY IN
MONOCULAR LAPAROSCOPIC CHOLECYSTECTOMY
H. Djaghloul
1
, M. Batouche
2
and J. P. Jessel
3
1
Ferhat Abbes University, Setif, Algeria
2
King Saoud University, Kingdom of Saudi Arabia
3
IRIT, Toulouse, France
Keywords:
Markerless augmented reality, Wavelets, Multi-resolution analysis, Evolutionary algorithms, Swarm intelli-
gence.
Abstract:
In this paper we present an augmented reality system for laparoscopic cholecystectomy video sequences en-
hancing. Augmented reality allows surgeons to view, in transparency, occluded anatomical and pathological
structures constructed preoperatively using medical images such as MRI or CT-Scan. The deformable nature
of digestive organs leads to a high dimensionality N-degrees of freedom detection and tracking problem. We
describe a knowledge-based construction method of powerful statistical color models for anatomical structures
and surgical instruments classification. Thanks to a new wavelet based multi-resolution analysis of the virtual
reality models and the anatomical color space; we can detect and track digestive organs to ensure marker-
less laparoscopic monocular camera pose and preoperative 3D model registration. Results are shown on both
synthetic and real data.
1 INTRODUCTION
Augmented reality allows viewing in transparency
anatomical and pathological 3D models reconstructed
preoperatively using medical images (MRI, CT-Scan)
in the surgeon field of view. However, one of the ma-
jor challenges that limits augmented reality massive
use in clinical laparoscopic abdominal surgery is the
difficulty of markerless registration of preoperative
digestive organs 3D models within the laparoscopic
view. Indeed, digestive organs are highly deformable
and variable. Therefore, we believe that a clinical
use of augmented reality in abdominal laparoscopic
surgery system has to ensure markerless registration
with knowledge-based interactive functionalities. In
this paper we propose a novel method to ensure 3D
models alignment and tracking of digestive organs di-
rectly onto laparoscopic cholecystectomy videos.
Cholecystectomy is the first surgical intervention
in the United States with more than a half million op-
erations done each year. Since the first cholecystec-
tomy of Langenbuch(Traverso, 1976), it is established
as the standard procedure for surgical treatment of
gallbladder diseases such as gallstones. Cholecystec-
tomy consists in the complete removal of the gallblad-
der with different techniques such as open or laparo-
scopic(Reynolds, 2001; Litynski, 1999) procedures.
However, the video-assisted laparoscopic cholecys-
tectomy is actually the gold standard technique with
more than 98% of interventions(Bittner, 2004).
The rest of the paper is organized as follows. In
the second section, we present a number of signifi-
cant augmented reality systems and methods applied
to digestive surgery. Next, we describe the proposed
method used for statistical abdominal organs color
model construction and its application to detect and
track anatomical structures and surgical instruments
in order to register the preoperative virtual model us-
ing a new wavelet based multi-resolution analysis of
deformable objects and particles swarm optimization
(PSO). In section 4, experimental results on synthetic
and real data show the effectiveness and robustness of
our method. Finally, we present our conclusion and
perspectives.
2 RELATED WORKS
In the last few years, researchers of augmented real-
ity community have proposed many systems for var-
ious medical domains and applications. According
139
Djaghloul H., Batouche M. and P. Jessel J. (2010).
CALIBRATION-FREE MARKERLESS AUGMENTED REALITY IN MONOCULAR LAPAROSCOPIC CHOLECYSTECTOMY.
In Proceedings of the International Conference on Computer Graphics Theory and Applications, pages 139-142
DOI: 10.5220/0002837801390142
Copyright
c
SciTePress
to tracking devices and methods, augmented reality
systems in digestive surgery can be classified in two
categories, vision-based and hybrid systems. In their
work, (Nicolau et al., 2005b) proposed a low cost
and accurate guiding system for laparoscopic surgery
with validation on abdominal phantom. The system
allows real time tracking of surgical tools and regis-
tration using markers by optimization of a given cri-
terion (EPPC)(Nicolau et al., 2005a). In the other
side, (Feuerstein et al., 2008) propose a hybrid sys-
tem composed of optical and electromagnetic track-
ing systems to determine the position and the ori-
entation of the intra-operative imaging devices, such
as mobile C-arm, laparoscopic camera and flexible
ultrasound, allowing direct superimposition of ac-
quired patient data in minimally invasive liver resec-
tion without need of registration.
3 PROPOSED METHOD
In this section we outline the principal components
of our markerless augmented reality system for la-
paroscopic cholecystectomy. Taking into consider-
ation temporal coherence according to the principal
steps of standard laparoscopic cholecystectomy, the
first component detects all anatomical and patholog-
ical structures in the surgical 2D laparoscopic view
using a statistical color model of digestive organs. As
a result, we have for each organ an initial segmenta-
tion represented by a sparse binary image. Then, false
positives are filtered using an adaptation of particles
swarm optimization (PSO) algorithm. thus, we have
a set of particles with different radius in the 2D image
for each organ.
In laparoscopic cholecystectomy, the most impor-
tant organ is the gallbladder and its vascular sup-
ply. The same principle is applied on preoperative
CT-Scan images to build a particles-based 3D model
of the gallbladder and the liver. The novel pro-
posed wavelet-based multi-resolution analysis allows
to have coarse models either of 3D virtual organs or
2D images. Finally, we make a 2D/3D registration for
each resolution level.
In order to build a statistical color model, a set of
16735 colored laparoscopic images (IRCAD source)
from a video of laparoscopic surgeries is used. The
images have 240 x 320 RGB coded pixels with 256
bins per channel (24 bits per pixel). The video se-
quence is acquired at a frame rate of 30 Hz.
3.1 Anatomical Color Model
According to the cholecystectomy intervention work-
flow step (t), we construct for each anatomical region
(i) a statistical color model using a histogram with 256
bins per channel in the RGB color space. Each color
vector (x) is converted into a discrete probability dis-
tribution in the manner:
P
i,t
(x) =
c
i,t
(x)
∑
N
i,t
j=1
c
i,t
(x
j
)
, t = t
1
. . . t
6
, i = 0 . . . S
t
. (1)
where c
i,t
(x) gives the count in the histogram bin rep-
resenting the rgb color triple (x) and N
i,t
is the total
count of the rgb histogram entries returned by the his-
togram bins number of the structure region (i) dur-
ing the intervention step (t). The number of detected
structures S
t
varies according to the step. According
to the European standard and common laparoscopic
cholecystectomy installation and intervention work-
flow, the number of structures classes is limited to
four. In practice, the step (t) denotes a time interval
represented by a set of consecutive laparoscopic im-
ages t =
h
I
t
v,1
. . . I
t
v,n
i
in the videos (v) that compose
the training dataset.
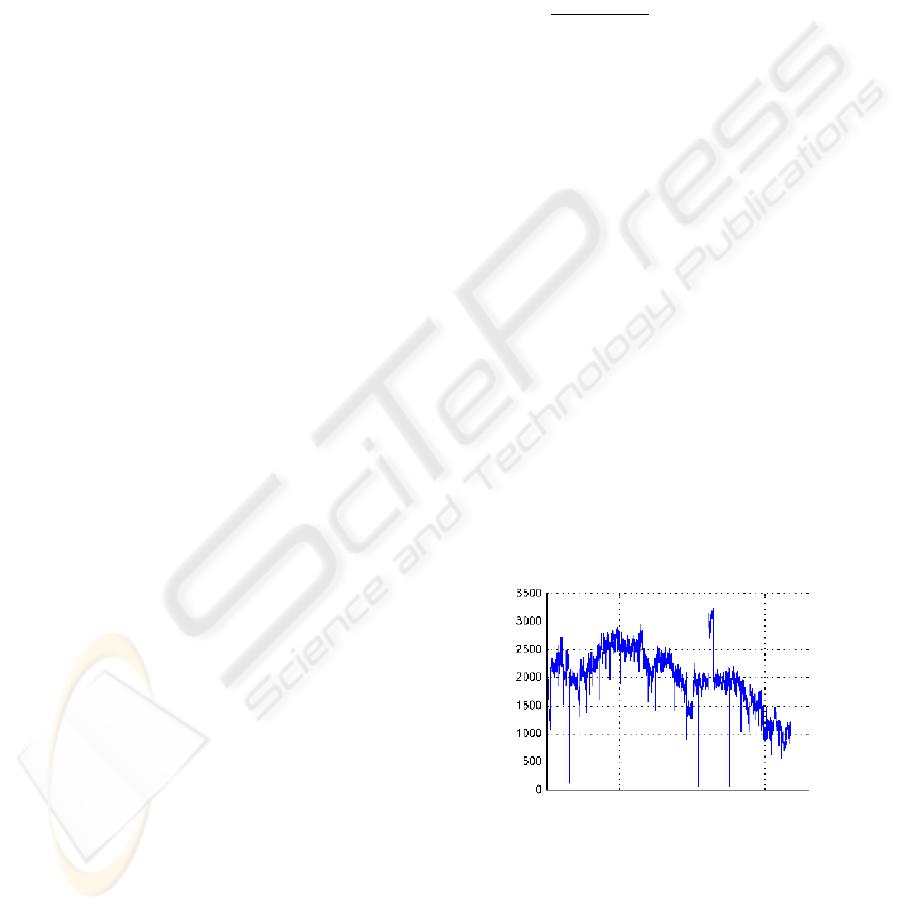
After analysis of the laparoscopic video, we have
observed that it contains at most 10017 RGB color
bins over the whole sequence with a mean of 1997
RGB triples in each frame. Therefore, the RGB his-
togram is mostly empty with 99,94% of the 256
3
RGB
bins that are not used. Figure 1, shows the evolution
of RGB bins count in the training laparoscopic chole-
cystectomy video.
Figure 1: Evolution of RGB bins count in the sequence.
3.2 Spherelet : Wavelet-based
Multi-resolution Analysis
In this section we propose a new multi-resolution
analysis of 3D objects modeled as a set of elementary
non intersected particles defined by their centers and
radius. The virtual model of the anatomical structure
GRAPP 2010 - International Conference on Computer Graphics Theory and Applications
140

is subdivided into a set of particles, that we will call
”‘Spherelet”’. The closest greatest sphere to the pre-
operative 3D model gravity center represents the root
of the Spherelet model. The Spherelet root is used
to initiate 2D/3D rigid registration. Hence, the root
definition has to ensure the most stability and less de-
formation during the whole sequence. The root repre-
sents the coarsest resolution level of the virtual model.
We suppose that
S
j
is the Spherelet at the resolution
level (j). We have :
S
j
=
S
j,1
S
j,2
. . . S
j,i
. . . S
j,n
j
t
(2)
where (S
j,i
) is the (i
th
) particle of the virtual model
at the resolution (j).(n
j
) is the length of the Spherelet
at the resolution level (j) denoting its particles count.
The initial resolution level is
S
0
and the coarsest one
is [S
r
] corresponding to the Spherelet root. The rela-
tion between two successive resolution levels is given
by:
S
j+1
= A
j+1
S
j
D
j+1
= B
j+1
S
j
(3)
with
D
j
represents the wavelet detail coeffi-
cients of the resolution level (j):
D
j
=
D
j,1
D
j,2
. . . D
j,i
. . . D
j,n
j
t
(4)
The
A
j
and
B
j
matrices are called the analysis
filters of the resolution level (j).
To reconstruct the superior resolution level we use
two matrices
P
j
and
Q
j
called synthesis filters.
The initial resolution level is given by:
S
j
= P
j+1
S
j+1
+ Q
j+1
D
j+1
(5)
The relation between the analysis and synthesis filters
is formulated by:
[A|B]
t
= [P|Q]
−1
then[A|B]
t
[P|Q] = I (6)
The detail information relative to eliminated parti-
cles contains their inter-distances or volume ratios in
the logarithmic scale. In the simplest case, the trans-
formation to an inferior resolution level (j) of the 3D
Spherelet volumetric model consists in replacing two
particles of the resolution level (j-1) by a representa-
tive one. We have then:
A
j
= [I|0]
B
j
= [−I|I] (7)
The
A
j
filter is used to select elements of the
next inferior resolution level and
B
j
to extract
wavelet coefficients of each level. Hence, the anal-
ysis process is formulated by:
S
r
= A
r
S
r−1
= A
r
A
r−1
. . . A
2
A
1
S
0
D
r
= B
r
S
r−1
= B
r
B
r−1
. . . B
2
B
1
S
0
(8)
Therefore, the synthesis filters are given by:
P
j
= [I|I]
t
Q
j
= [0|I]
t
(9)
Assuming that the initial Spherelet is com-
posed of (2
r
) spheres. We have, S
( j=0)
=
S
0,2
0
S
0,2
1
. . . S
0,i
. . . S
0,2
r
t
with n
( j=0)
= 2
r
and r
gives the number of levels to reach the coarsest repre-
sentation corresponding to the Spherelet root.
3.3 Anatomical Structures Tracking
We propose a tracking by detection method of di-
gestive organs using particles swarm optimization
(PSO). The classical PSO is a global search strat-
egy for optimization problems(Kennedy et al., 1995)
and it is based on the social evolution simulation of
an arbitrary swarm of particles based on the rules of
Newtonian physic. Assuming that we have an N-
dimensional problem, the basic PSO algorithm is for-
mulated by position x
m
(t) and velocity v
m
(t) vectors
representing the time evolution of M particles with
random affected initial positions. Hence, we have:
x
m
(t) = [x
1
(t)x
2
(t). . . x
N
(t)]
T
(10)
v
m
(t) = [v
1
(t)v
2
(t). . . v
N
(t)]
T
(11)
The evolution of the particles in the classical algo-
rithm is done by the following equations:
v
m
(t + 1) = f
m
i
v
m
(t) + f
m
c
[D
c
]
N
(x
m
(t
c
) − v
m
(t))
+ f
m
s
[D
s
]
N
(x
opt
(t
s
) − v
m
(t)) (12)
Thus, the new position of the particle m is given by:
x
m
(t + 1) = x
m
(t) + v
m
(t + 1) (13)
Where (v
m
(t)) and (v
m
(t + 1)) are, respectively, the
past and the new velocity vectors of the particle (m).
( f
m
i
) is the inertia factor of the particle m, ( f
m
c
) is
its the cognitive factor and ( f
m
s
) is the social factor.
([D
c
]
N
) and ([D
s
]
N
) are the N-dimensional diagonal
matrices composed of statistically independent nor-
malized random variables uniformly distributed be-
tween 0 and 1. (t
c
) is the iteration where the particle
m has reached its best position given by (x
m
). (t
s
) is
the generation that has found its best global particle-
member defined by its components (x
opt
).
The Spherelet root of each organ, mainly that of
the gallbladder, is determined in the 2D UV space of
the image by optimization of the following proposed
cost function:
F
Θ
=
|
1 − k
|
+
|
1 − d
|
(14)
with
k =
α
∑
i, j
I
b
(x)
, (15)
CALIBRATION-FREE MARKERLESS AUGMENTED REALITY IN MONOCULAR LAPAROSCOPIC
CHOLECYSTECTOMY
141

and
d =
∑
i, j
I
b
(x)
x
2
r
, (16)
where, α, models the priori-knowledge and , d, the
density of the particle.
The 2D/3D registrations is ensured by minimiz-
ing the distance between the projection of the 3D
Spherelet (S
3D
) of the virtual model reconstructed us-
ing preoperative slides and the 2D Spherelet (S
2D
)
in the laparoscopic image. Therefore, for each 3D
Spherelet particle we compute the pose using (PSO)
assuming the stability of previously computed coarse
levels registration. For each resolution level, the func-
tion to be minimized is given by:
F
Φ
=
|
Φ
3D
(x) − S
2D
|
(17)
where Φ
3D
is the rendering function of (S
3D
).
4 EXPERIMENTAL RESULTS
First, we have applied the method on synthetic im-
ages (made by hand) to validate detection and track-
ing method (Figure 2).
(a) (b)
Figure 2: Tracking of synthetic gallbladder.
Then, we have tried the method on real laparo-
scopic images as shown below (Figure 3).
(a) Original image. (b) Detection
(c) 2D/3D registration.
Figure 3: Real laparoscopic image augmentation (IRCAD).
5 CONCLUSIONS
In this paper we presented a novel method for aug-
menting images of video based laparoscopic chole-
cystectomy. A new statistical color model is proposed
to detect anatomical and pathological structures. A
new criterion is used to detect and track organs using
particles swarm optimization. A new wavelet based
multi-resolution analysis of 2D laparoscopic images
and 3D particles-modeled objects is used to register
the preoperative model within the real scene. Exper-
iments have shown the effectiveness of the proposed
method.
REFERENCES
Bittner, R. (2004). The standard of laparoscopic chole-
cystectomy. Langenbeck’s Archives of Surgery,
389(3):157–163.
Feuerstein, M., Mussack, T., Heining, S., and Navab, N.
(2008). Intraoperative laparoscope augmentation for
port placement and resection planning in minimally
invasive liver resection. IEEE Transactions on Medi-
cal Imaging, 27(3):355.
Kennedy, J., Eberhart, R., et al. (1995). Particle swarm op-
timization. In Proceedings of IEEE international con-
ference on neural networks, volume 4, pages 1942–
1948. Piscataway, NJ: IEEE.
Litynski, G. (1999). Endoscopic surgery: the history, the
pioneers. World journal of surgery, 23(8):745–753.
Nicolau, S., Garcia, A., Pennec, X., Soler, L., and Ayache,
N. (2005a). An augmented reality system to guide
radio-frequency tumour ablation. Computer Anima-
tion and Virtual Worlds, 16(1):1–10.
Nicolau, S., Goffin, L., and Soler, L. (2005b). A low
cost and accurate guidance system for laparoscopic
surgery: Validation on an abdominal phantom. In
Proceedings of the ACM symposium on Virtual real-
ity software and technology, page 133. ACM.
Reynolds, W. (2001). The first laparoscopic cholecystec-
tomy. JSLS, Journal of the Society of Laparoendo-
scopic Surgeons, 5(1):89–94.
Traverso, L. (1976). Carl Langenbuch and the first chole-
cystectomy. Am J Surg, 132(1):81–2.
GRAPP 2010 - International Conference on Computer Graphics Theory and Applications
142
