
USING PHYSX FOR SIMULATION-BASED ENDOSCOPIC
HARDWARE DESIGN
Felix Dingeldey, Karsten Isakovi
´
c and Ilja Teiche
Fraunhofer Institute for Computer Architecture and Software Technology (FIRST)
Kekul
´
estraße 7, 12489 Berlin, Germany
Keywords:
Surgical simulation, Simulation-based hardware design, PhysX, Physics engine integration.
Abstract:
Computer-assistance becomes increasingly important in minimally invasive surgery. Automation and image-
processing techniques are employed for assisting surgeons with their highly skilled tasks. Our research is
aimed at developing a novel system for laparoscopic surgery consisting of a new type of endoscope and aug-
mented reality components. In order to facilitate the design of the hardware and the algorithms, we developed
a virtual endoscopy simulator. It utilizes the capabilities of NVIDIA’s physics engine “PhysX” for simulating
the physical behavior of soft tissue, instruments, and smoke. In this paper, we present our proposed solu-
tions for modeling the objects using PhysX and discuss possible problems specific to the domain of medical
simulation.
1 INTRODUCTION
Over the last decades, minimally invasive surgery
(MIS) has become more and more important, as it re-
quires smaller incisions and causes less pain to the pa-
tient than open surgeries. However, in MIS surgeons
have to deal with limited orientation and difficult nav-
igation. The 2D camera image of the endoscope pro-
vides the only visual feedback. Additionally, instead
of being able to use their hands, surgeons have to use
long instruments that only give indirect sensation of
the movements and contacts.
The advances in medical imaging and computer
technology let computer-assisted surgery (CAS) find
its way into the operating rooms. Especially for un-
experienced surgeons, CAS has the potential to sup-
port the navigation in novel manners by utilizing tech-
niques from image processing, computer vision, and
virtual or augmented reality (VR/AR).
The “Endoguide” project (Endoguide, 2010) aims
to develop a novel computer-assisted system for la-
paroscopic surgery, consisting of two major parts: a
new type of endoscope with variable viewing direc-
tion and a processing unit for offering VR/AR support
and intuitive user input paradigms. The CAS unit will
be able to automatically capture and stitch panoramic
overview scans that can be augmented with additional
information, such as the current viewing rectangle or
the area already inspected. By tracking the position
and orientation of all instruments and the endoscope,
the system will allow augmenting the camera output
with navigation aids, such as indicators that simplify
the localization of the instruments with the camera.
In addition, the tracking data will allow to overlay in-
formation from patient-specific imaging data, such as
segmented organs, CT/MRI slices, or annotations that
have been added during pre-surgical planning.
We developed a virtual simulator in order to sim-
plify the development of both the new endoscope
hardware and VR/AR algorithms. The simulator sup-
ports representative laparoscopic surgery scenarios,
such as navigating in the abdomen or lifting up tis-
sue. The simulation lets us evaluate hardware con-
cepts before actually realizing, and lets us assess the
robustness, accuracy, and speed of the algorithms be-
ing developed.
The use of simulated camera output and track-
ing data allows to start designing the algorithms very
early, even before data from the real physical system
is available. For detecting possible instabilities of the
image processing due to poor visibility or variations
and movements in the scene, the simulator has to pro-
vide a high degree of visual realism and, additionally,
model the physical behavior of scene objects, such as
instruments, soft tissue, or smoke.
In order to maintain interactive frame rates, we
use the PhysX real time physics engine (Corp., 2009a)
and integrate it into our rendering framework. PhysX
358
Dingeldey F., Isakovi
´
c K. and Teiche I. (2010).
USING PHYSX FOR SIMULATION-BASED ENDOSCOPIC HARDWARE DESIGN.
In Proceedings of the International Conference on Computer Graphics Theory and Applications, pages 358-363
DOI: 10.5220/0002840103580363
Copyright
c
SciTePress

is a production-ready and well-established physics
engine that has been used in various video games,
such as “Batman: Arkham Asylum” or“Unreal Tour-
nament 3” (Corp., 2009b). In fact, the Microsoft
Robotics Development Studio also uses PhysX for
physics simulations (Morgan, 2008).
After reviewing related work in section 2 and giv-
ing a very brief overview of laparoscopic interven-
tions in section 3, we discuss how to model the dif-
ferent aspects of the laparoscopic simulation using
PhysX in sections 4 and 5. We show results in sec-
tion 6 before drawing a conclusion and giving direc-
tions for future work in section 7.
2 RELATED WORK
Numerous highly specialized and advanced commer-
cial systems are available for practicing various min-
imally invasive interventions, e.g. (Immersion, 2009)
(Simbionix, 2009). (C¸ akmak et al., 2005) showed
how to use their virtual trainer for designing and
testing instruments, such as graspers. Training sys-
tems generally reach a very high degree of visual and
physical realism. However, as proprietary solutions
they are not suitable for our purposes. (Reichenbach,
2009) uses PhysX for simulating and testing the de-
sign of humanoid robots, particularly the rigid body
mechanics. In addition, (Reichenbach, 2009) dis-
cusses how to combine real and virtual sensors by
communicating sensor feedback between the actual
robot and the simulator. (Rieffel et al., 2009) demon-
strate how to utilize PhysX for simulating soft-bodied
robot designs and gaits — where the high deforma-
bility usually requires computationally very complex
models — by empirically determining behavioral pa-
rameters for the soft body system in PhysX.
In the field of medical simulation, (Ermisoglu
et al., 2009) present a first study for using PhysX to
practice the scooping procedure during cervical disc
replacement surgeries. A thorough discussion of how
to utilize PhysX for a virtual laparoscopic training
simulator with haptic feedback is given by (Maciel
et al., 2009). One focus of their work is how to han-
dle the different update rates required for the haptic
feedback (∼1 kHz) and those achieved with PhysX
(∼20 Hz in their implementation).
(Ott et al., 2007) use PhysX’s rigid body engine in
conjunction with an advanced VR haptic workstation
with two data gloves for manipulating physically ani-
mated objects. The haptic feedback is computed from
the deviation of the tracking data of the gloves and
the simulated position of the PhysX rigid body actors
that model the hands. Deviations occur if the actors
Figure 1: Example of a tetrahedral mesh used in PhysX
(left) and its graphical representation (right).
are blocked by other virtual objects in the simulated
scene.
3 BRIEF OVERVIEW OF
LAPAROSCOPIC
INTERVENTIONS
At the beginning of a minimally invasive laparoscopic
intervention, several small incisions are made for in-
serting trocars (basically tubes that seal the cut and
allow inserting instruments or the endoscope). After-
wards, the abdomen is insufflated using CO 2 in or-
der to create a working space between the abdominal
wall and the organs. Next, the instruments and the
endoscope with the camera are inserted through the
trocars.
During the intervention, the surgeon might have
to perform various tasks using different instruments,
such as: grasping, lifting, and cauterizing tissue; clip-
ping arteries; sucking out blood; or suturing lesions
using curved needles.
4 SIMULATION OF ORGANS
AND TISSUE
We simulate all organs and soft tissue using soft bod-
ies, which are simulated on GPU by PhysX. The en-
gine uses a volumetric model for describing the defor-
mation of soft bodies. The edge constraints between
mass vertices are organized in tetraheda. For detect-
ing collisions, a collision sphere is placed around each
vertex. The radius of the spheres (the particle radius)
can be specified per soft body object. Figure 1 shows
an example of a tetrahedral soft body mesh and the
corresponding graphical mesh.
After each simulation step, we update the posi-
tions of the graphical vertices according to the cur-
rent deformation computed by PhysX. For this, each
vertex of the graphics mesh is “linked” to the enclos-
ing (or closest) tetrahedron. The barycentric coordi-
nates of the vertex within the tetrahedron define the
USING PHYSX FOR SIMULATION-BASED ENDOSCOPIC HARDWARE DESIGN
359

influence (i.e. the weight) of each of the four tetra-
hedron’s mass vertices on the graphical vertex. The
update can be formulated as: v
0
=
∑
i = 0
3
b i · t i,
where v
0
denotes the graphical vertex being updated,
b i the barycentric coordinate (weight), and t i the po-
sition of the i’th tetrahedron’s mass vertex of the soft
body. In order to increase the rendering performance,
we update the normals only if the total displacement
has been larger than a user-definable threshold.
Our 3D model of the human abdomen consists of
all important inner organs, bones and muscles. In re-
ality, most abdominal organs are embedded by the
peritoneum (a thin membrane) and connected to the
abdominal wall. In the simulation, however, each or-
gan is represented by a separate soft body and, hence,
the embedding is disregarded. In consequence, the
soft bodies would fall down once the simulation loop
starts. Thus, we attach some of the mass vertices at
the back of the organs at their initial position in world
space. The attachments efficiently keep the soft bod-
ies at their original position, but still allow deforma-
tions of the organs.
4.1 Problems and Drawbacks
A major problem when using PhysX is the lack of
two-way soft body interaction. In the current release
(version 2.8.1), collision detection between two sep-
arate soft body objects is not supported. However, in
our simulator we need to model different organs that
are likely to collide with each other (e.g. the liver and
the gall bladder).
A common approach to tackle this is to introduce
rigid body actors that are placed and attached within
the soft body, as it is also shown in the PhysX sample
applications. During the simulation, each soft body
then collides with the rigid bodies attached to other
soft body objects. However, for the complex (con-
cave) geometry of the organs many small rigid bodies
would need to be attached, which makes the param-
eterization extremely challenging if not impossible,
and results in a highly instable simulation.
Our solution to this problem relies on the self-
collision supported by PhysX (i.e. the collision de-
tection between the object’s own particles) and the
fact that soft body objects may consist of totally dis-
joint groups of tetrahedra. In the simulator, we merge
neighboring organs into a single soft body and enable
self-collision. Although the merged organs then have
the same parameterization, we found this solution to
work very well for our purposes.
Another problem arises from the fact that collision
detection is only performed at the soft body particles
and not at the outer faces of the tetraheda. If the par-
Figure 2: The tips of the instruments available in our sim-
ulator. From top to bottom: The endoscope, the cauterizer,
and the grasper. The red lines depict the virtual wedge we
use for detecting soft body vertices between the two jaws of
the grasper.
ticle radius is too small, thin soft body structures tend
to cut or fall through themselves. If the particle radius
is quite large, overlapping structures seem to hover
on top of each other, since the mass vertices cannot
touch.
For us, these effects have been of particular im-
portance when modeling the small intestine. The lack
of continuous collision detection forced us to partly
connect the tetraedra of thin touching structures.
5 SIMULATION OF
INSTRUMENTS
Our research is motivated by developing a new type
of computer-assisted endoscopy system. At the cur-
rent stage, the simulator supports two kinds of instru-
ments: A grasper for lifting tissue and a cauterizer for
burning tissue. These two actions (lifting and burn-
ing) significantly alter the view and are, therefore,
very suitable for assessing our image processing algo-
rithms (e.g. autofocus control or instrument tracking).
Figure 2 shows the graphical meshes of the two in-
struments and of an endoscope prototype being eval-
uated. The physical simulation relies on kinematic
rigid bodies, whose positions are set explicitly ac-
cording to the user input.
As discussed in section 2, (Ott et al., 2007) use a
pair of kinematic and dynamic actors for calculating
haptic feedback based on the deviation of the actors.
We plan to utilize this method for calculating haptic
feedback for our instruments by connecting additional
dynamic actors to the instruments’ kinematic actors.
5.1 The Endoscope
The geometry of the endoscope consists of a cylindri-
cal shaft with a curved cut-out at the tip, at which a ro-
GRAPP 2010 - International Conference on Computer Graphics Theory and Applications
360

tating prism is located (see figure 2, top). For the en-
doscope, the graphical simulation is much more com-
plex than the physical simulation. In order to be able
to evaluate the concept of the rotating prism camera
optics, we need to model the camera behavior as accu-
rate as possible. For example, we model the complex
movement of the view frustum caused by the internal
reflections in the prism, and apply radial lens distor-
tion, depth-of-field, and chip noise as post-processing
effects.
5.2 The Grasper
Our grasper is composed of a shaft and two jaws that
open and close (figure 2, bottom). In PhysX, the in-
strument is represented by a capsule for the shaft and
two boxes for the jaws. When opening or closing the
grasper, we request PhysX to rotate the boxes of the
jaws around their base at the tip of the shaft.
Pinching can be realized by attaching soft body
vertices that lie between the jaws. Unfortunately,
PhysX does not report contacts between rigid bodies
and soft bodies, which made it necessary to imple-
ment the pinching by hand.
Finding soft body vertices between the jaws could
be implemented by casting rays from the jaws against
the soft bodies. However, it turned out that the ray
casting routine in PhysX is computationally too ex-
pensive and significantly drops the frame rate. As
shown in figure 2 we span a virtual wedge between the
jaws. This wedge defines five planes with which we
basically perform a view-frustum culling operation in
order to find all vertices inside the wedge. Once these
vertices are detected, they are attached to the grasper
and follow it in the continuing simulation.
For the grasping, we attach the vertices as tear-
able, which allows the attachment to break as soon as
the force acting on it becomes large enough. In order
to model forceful grasping when the jaws are almost
closed, the tear factor depends on the opening angle
of the jaws. Once the user opens the grasper, we de-
tach all attached vertices and thereby release the soft
body from the grasper.
5.3 The Cauterizer
The geometry of the cauterizer consists of a tip with
a loop at which a sphere is attached with which the
surgeon can cauterize (figure 2, middle). When push-
ing the tip against an organ, the tissue is burned
and smoke rises. In PhysX, smoke can be modeled
using its GPU-based fluid simulation, which imple-
ments particle-based smoothed-particle hydrodynam-
ics (SPH) (M
¨
uller et al., 2003). During rendering,
each particle can be rendered as textured billboard,
using the positions from the fluid simulation.
In our simulator, we attach a PhysX fluid emit-
ter at the tip of the cauterizer. As soon as the user
activates the cauterizer, we start to check if any soft
body vertex is in immediate proximity of the tip, in
which case we slowly start emitting particles and let
the emitter’s flow rate increase constantly from near-
zero to a maximum. If no soft body vertex is close to
the cauterizer tip, we switch off the emitter again. Ad-
ditionally, we define a fade-out time in order to avoid
sudden disappearance of smoke particles.
6 RESULTS
We implemented our simulator in C++ using DirectX
for rendering and input handling and PhysX version
2.8.1 for the physical simulation. Figure 3 shows a
screenshot of the simulator, which consists of two
windows. On the left, the control window renders
with an overview camera. On the right, the output
of the simulated endoscope camera is presented. The
position and orientation of this camera is controlled
when moving the endoscope geometry. As can be
seen, the simulation takes into account various prop-
erties of the camera, such as field of view, lens dis-
tortion, chip noise, or the image circle that does not
cover the entire chip depending on the zooming level.
For testing our image-processing algorithms, we di-
rect the simulation window onto the secondary moni-
tor port and grab the video on a second computer, as
it would be done with the video stream coming from
the actual endoscope camera.
Our testing platform uses one core of an Intel
Core2 Duo CPU at 3 GHz and 3.2 GB main memory.
For rendering and the PhysX simulation, an NVIDIA
GeForce 9600 GT graphics card with 512 MB RAM
is used. For a scene with roughly 540,000 polygons
and 4,300 tetraheda, the application renders at about
40 frames per second. The physics simulation runs at
about 20 frames per second. Note that we run PhysX
asynchronously for better performance.
As shown in figure 4, our grasper allows to lift or-
gans and inspect the underlying areas. Depending on
the placement of the camera, this can significantly al-
ter the image. In the same way, the implemented cau-
terizer and smoke (see figure 5) also helps testing the
robustness of image-processing algorithms and their
reaction to fast-changing views.
We have already been able to evaluate different
aspects of the endoscope prototype with our simula-
tor. For example, we found that the rotation of the
endopscope camera has to use at least eight steps, and
USING PHYSX FOR SIMULATION-BASED ENDOSCOPIC HARDWARE DESIGN
361
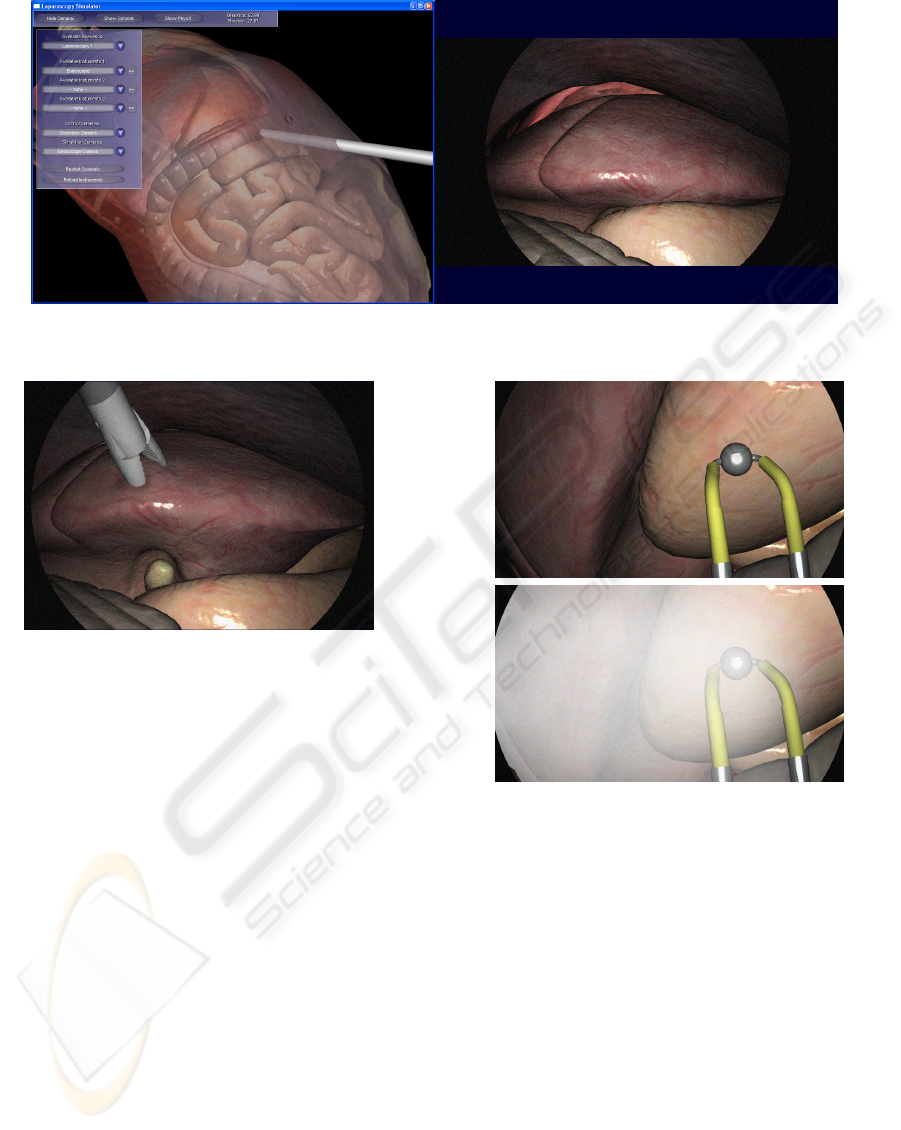
Figure 3: Screenshot of our simulator showing the control window (left) and the simulated output of the endoscope camera
(right).
Figure 4: Screenshot of our simulator demonstrating the use
of the grasper for lifting organs. The camera view corre-
sponds to figure 3 (right).
should ideally be continuous. Otherwise, the surgeon
might lose orientation. In contrast, for zooming it is
sufficient to provide only a few levels, which will sim-
plify the design of the electric motors.
7 CONCLUSIONS AND FUTURE
WORK
In this paper, we showed how to use NVIDIA’s
physics engine PhysX for realizing a laparoscopic
simulator that we use for evaluating hardware con-
cepts and algorithms for a novel computer-assisted
endoscopy system. We utilize the real time capabil-
ities of PhysX for simulating soft bodies (organs),
rigid bodies (instruments and the endoscope), and flu-
ids (rising smoke).
The major drawback of the current release of
PhysX (version 2.8.1) is the lack of both collision de-
tection between different soft body objects (two-way
interaction) and collision detection on outer faces of
the soft bodies. In consequence, we were forced to
Figure 5: Screenshot of our simulator demonstrating the use
of the cauterizer and the rising smoke.
implement several workarounds. For our purposes of
evaluating the endoscope hardware, the entailed re-
duction of physical plausibility is not very critical.
For a medical training simulator, however, we assume
the current limitations to be much more important.
In the future, we will evaluate alternatives to
PhysX, such as “Bullet Physics” (Coumans, 2009) or
the “SOFA” framework (Allard et al., 2007). Further-
more, we want to improve our simulator and increase
its realism by including other aspects of laparoscopic
interventions, such as cutting tissue. We also plan
to model bleedings caused by the cutting using SPH
fluids, as proposed by (van der Laan et al., 2009).
Besides this, we will integrate haptic feedback into
our application that applies forces on the input device
when pushing against objects in the scene.
GRAPP 2010 - International Conference on Computer Graphics Theory and Applications
362

ACKNOWLEDGEMENTS
The “Endoguide” project is funded by the Federal
Ministry of Education and Research, Germany (pro-
motion reference 01IM08005D).
REFERENCES
Allard, J., Cotin, S., Faure, F., Bensoussan, P.-J., Poyer,
F., Duriez, C., Delingette, H., and Grisoni, L. (2007).
SOFA – an open source framework for medical simu-
lation. In Medicine Meets Virtual Reality (MMVR’15),
Long Beach, USA.
C¸ akmak, H. K., Maaß, H., and K
¨
uhnapfel, U. (2005).
VSOne, a virtual reality simulator for laparoscopic
surgery. Minimally Invasive Therapy and Allied Tech-
nologies, 14(3):134–144.
Corp., N. (2009a). Physx. Retrieved 10/29/2009, from
http://www.nvidia.com/object/physx new.html.
Corp., N. (2009b). Physx games list. Retrieved 10/29/2009,
from http://www.nzone.com/object/
nzone physxgames home.html.
Coumans, E. (2009). Physics Simulation Forum - View
topic - Bullet 2.75 beta1: GPU, SPH fluids pre-
view, new constraints. Retrieved 11/15/2009, from
http://bulletphysics.org/Bullet/phpBB3/
viewtopic.php?f=18&t=3625&start=0.
Endoguide (2010). Endoguide. Retrieved 02/03/2010, from
http://www.ia-vt.de/index.php?id=20.
Ermisoglu, E., Sen, F., Kockara, S., Halic, T., Bayrak, C.,
and Rowe, R. (2009). A scooping simulation frame-
work for artificial cervical disk replacement surgery.
In Proceedings of SMC ’09. IEEE.
Immersion, C. (2009). Surgical simulator: The
laparoscopyvr virtual-reality system. Retrieved
10/29/2009, from http://www.immersion.com/
markets/medical/products/laparoscopy.
Maciel, A., Halic, T., Lu, Z., Nedel, L. P., and De, S.
(2009). Using the physx engine for physics-based vir-
tual surgery with force feedback. The International
Journal of Medical Robotics and Computer Assisted
Surgery, 5:341–353.
Morgan, S. (2008). Simulating the world with Microsoft
robotics studio. MSDN Magazine, 6.
M
¨
uller, M., Charypar, D., and Gross, M. (2003). Particle-
based fluid simulation for interactive applications. In
Proceedings of SCA ’03, pages 154–159, Aire-la-
Ville, Switzerland. Eurographics Association.
Ott, R., De Perrot, V., Thalmann, D., and Vexo, F. (2007).
MHaptic: a haptic manipulation library for generic
virtual environments. In Proceedings of CW ’07,
pages 338–345, Washington, DC, USA. IEEE.
Reichenbach, T. (2009). A dynamic simulator for humanoid
robots. Artificial Life and Robotics, 13:561–565.
Rieffel, J., Saunders, F., Nadimpalli, S., Zhou, H., Has-
soun, S., Rife, J., and Trimmer, B. (2009). Evolving
soft robotic locomotion in PhysX. In Proceedings of
GECCO ’09, pages 2499–2504, New York, NY, USA.
ACM.
Simbionix, L. (2009). Lap mentor laparoscopic
surgery simulator for general surgery, gynecol-
ogy and urology. Retrieved 10/29/2009, from
http://www.simbionix.com/LAP
Mentor.html.
van der Laan, W. J., Green, S., and Sainz, M. (2009). Screen
space fluid rendering with curvature flow. In Proceed-
ings of I3D ’09, pages 91–98, New York, NY, USA.
ACM.
USING PHYSX FOR SIMULATION-BASED ENDOSCOPIC HARDWARE DESIGN
363
