
EXPERIMENTAL DATABASE IMPLEMENTATION FOR THE
C
EREBROVASCULAR DISEASES RESEARCH INTEGRATES
TOGETHER DIFFERENT KINDS OF MEDICAL DATA
Petr Vˇcel´ak, Jana Kleˇckov´a
Department of Computer Science and Engineering, University of West Bohemia, Univerzitni 8, Pilsen, Czech Republic
Vladim´ır Rohan
Department of Neurology, University Hospital, Pilsen, Czech Republic
Keywords:
Cerebrovascular diseases, Clinical data, DASTA, Database, DICOM, Medical data, Metadata, Resource De-
scription Framework (RDF), Stroke, Thrombolysis.
Abstract:
The cerebrovascular diseases are one the most common cause of death worldwide. The second most frequent
cause of death in the Czech Republic. The proposed database should notably contribute to the solution of this
complex problem. Its profit is based on medical data interconnection and aggregation of collaborating centers.
There are stored miscellaneous de-indentified medical data in the database such as (1) a set of patient’s clinical
data in a DASTA file format, (2) a set of brain scans like computed tomography in a DICOM files, (3) data
from Safe Implementation of Thrombolysis in Stroke (SITS) register. The experimental database project has
an extensible support for any other fitted data format.
1 INTRODUCTION
Stroke is one of the leading causes of morbidity and
mortality worldwide (Lopez and Blobel, 2009). Large
differences in incidence, prevalence and mortality
have been noted between Eastern and Western Eu-
rope. This has been attributed to differences in risk
factors, with higher levels of hypertension and other
risk factors resulting in more severe stroke in East-
ern Europe (Brainin et al., 2000). Notable regional
variations have also been found within Western Eu-
rope. Stroke is the most important cause of mor-
bidity and long term disability in Europe, and demo-
graphic changes will result in an increase in both inci-
dence and prevalence. It is also the second most com-
mon cause of dementia, the most frequent cause of
epilepsy in the elderly, and a frequent cause of depres-
sion. (Awareness et al., 2008; Rothwell et al., 2005;
O’Brien et al., 2003) The cerebrovasculardiseases are
one the most common cause of death worldwide. It is
the second most frequent cause of death in the Czech
Republic. Cancer incidence and mortality are two to
three times greater in the Czech Republic than in other
developed countries in Europe. (MZ
ˇ
CR – Ministry of
Health of the Czech Republic, 2010;
ˇ
CS
´
U – Czech
Statistical Office, 2010)
The experimental database is as a meta data min-
ing tool used primarily in a research. It allows study-
ing of crucial values and dependencies of particular
parameters in high amount of examinations with clin-
ical data correlations. Captured data and obtained
knowledge should notably contribute to the solution
of complex cerebrovascular brain diseases problem.
Later, the database can be used as a practical educa-
tion and diagnostic tool. The experimental database
concept was announced in (Vˇcel´ak et al., 2009).
2 MEDICAL DATA TYPE
SUPPORT
This paper experiments and hypothesis are based on
an evaluation of heterogeneous medical data. It is a
patient’s clinical data set in a DASTA (Karlova uni-
verzita v Praze – 2. l´ekaˇrsk´a fakulta v Praze (Charles
University in Prague – 2nd Faculty of Medicine),
2010) format and radiological studies in a DICOM
format (National Institute of Neurological Disorders
and Stroke, 2010) of patients affected by a stroke at
111
V
ˇ
celák P., Kle
ˇ
cková J. and Rohan V. (2010).
EXPERIMENTAL DATABASE IMPLEMENTATION FOR THE CEREBROVASCULAR DISEASES RESEARCH INTEGRATES TOGETHER DIFFERENT
KINDS OF MEDICAL DATA.
In Proceedings of the Multi-Conference on Innovative Developments in ICT, pages 111-114
DOI: 10.5220/0003037501110114
Copyright
c
SciTePress

the University hospital in Pilsen. The hospital is a
participant of the Safe Implementation of Thrombol-
ysis in Stroke (SITS) program, an internet-based in-
teractive thrombolysis register (Safe Implementation
of Thrombolysis in Stroke, 2010). Medical doctor
records details about a stroke into the SITS register.
The SITS register contains e.g. therapeutic details,
adverse drug reaction, National Institutes of Health
(NIH) data, computed tomography (CT) description,
death cause description. These data are not structured
in a clinical data from the hospital information sys-
tem. Feedback missing in the SITS register is a huge
disadvantage for a medical doctor. That is why we
are trying to interconnects clinical and imaging data
(Rohan et al., 2007) with SITS the register data in the
experimental database.
Patient’s health records are provided mostly in the
native language text data. A laboratory report is a
structured document used while making a decision,
calculation or graphing. These clinical data can be
stored in a DASTA or a HL7 format. A diagnos-
tic imaging procedures creates multimedia data, that
originates from e.g. electroencephalograph (EEG)
and Computed Tomography (CT).
2.1 DASTA Type
The DASTA is Data Standard abbreviation (Karlova
univerzita v Praze – 2. l´ekaˇrsk´a fakulta v Praze
(Charles University in Prague – 2nd Faculty of
Medicine), 2010). It is a national electronic commu-
nication standard format of a public health service in
the Czech Republic. The DASTA is a Health Level 7
(Health Level Seven, Inc., 2010) standard equivalent
and was developed by the Czech Public Health Infor-
matics and Scientific Information Organization sup-
ported by the Minister of Health of the Czech Repub-
lic. It is based on the XML-based mark-up standard
with XSD schema.
2.2 Health Level Seven Type
Health Level Seven (HL7) is a non-profit organization
involved in the development of international health-
care standards. It is widely used for interchange be-
tween hospitals and physician record systems and be-
tween electronic medical record systems and prac-
tice management systems. (Health Level Seven, Inc.,
2010)
The HL7 Clinical Document Architecture (CDA)
documents are used to communicate documents such
as physician notes and other material (Dolin et al.,
2006). The CDA is an XML-based mark-up stan-
dard intended to specify the encoding, structure and
semantics of clinical documents. The CDA docu-
ment consists of a mandatory textual part and optional
structured parts. The mandatory textual part ensure
human interpretation of the document contents.
The experimental database bargain for the HL7
documents support. The HL7 storage is supported,
but a next layer with HL7 format support is not im-
plemented, at this time.
2.3 DICOM Type
The Digital Imaging and Communications in
Medicine (DICOM) standard has been developed
with an emphasis on diagnostic medical imaging. It
is applicable to a wide range of image and non-image
related information exchanged in clinical and other
medical environments. This standard is widely
used for representing and communicating radiology
images scans and reporting. The DICOM standard is
supported by the National Electrical Manufacturers
Association. (National Institute of Neurological
Disorders and Stroke, 2010)
The searching in the set of DICOM files has some
difficulties. You need a special library to acquire any
information from the DICOM file.
2.4 The SITS Register Data
The SITS register is an academic driven, non-profit,
international collaboration. It is an initiative by the
medical professionals to certify excellence in acute
stroke treatment. The SITS initiated an internet-based
interactive thrombolysis register, to serve as an instru-
ment for clinical centers to follow their own treatment
results and compare with other centers in their coun-
tries and in the collaborating countries. (Safe Imple-
mentation of Thrombolysis in Stroke, 2010)
Treatment results are not available to a medical
doctor for reuse this data in a structured way for a re-
search purpose. We created a set of scripts that down-
load and parse a treatment file report page.
3 DATA PROCESSING
The research is based on an anonymous data only. All
of identification information are necessarily removed
before an upload from the hospital to the experimen-
tal database. The data anonymization process kept
all relationships. That is why we can use so much
more data to research. The patient’s clinical data are
stored together with imaging examinations in a single
database. It can be extended with any other proper
kind of data.
INNOV 2010 - International Multi-Conference on Innovative Developments in ICT
112
The hospital information system exports a particu-
lar patient with a stroke medical data sets into a single
directory. There are exported DASTA and DICOM
files with unique names. DASTA file links all clinical
event relevant DICOM files. The links are DICOM
file names. The DASTA file can contain the SITS reg-
ister identificator in a comment attribute. Each stroke
corresponds to one SITS register identificator. De-
identified DASTA, DICOM and created SITS-XML
files are raw input files in the experimental system.
All raw input files are processed and extraction of
meta data is done. A new information or knowledge
is referred as a meta data. We decided to use a modern
Resource Description Framework (RDF) model for a
meta data manipulation (W3C, 2010). The raw data
processing tool is extensible. It can be a simple script
that extracts any attribute value or an application with
complex queries, images evaluation, computing, etc.
A processing tool can operate on a meta data extracted
previously, not only on a raw data.
The advantage of our experimental database is the
incremental growing of a RDF model and increasing
knowledgebase. Suppose you are interested in a treat-
ment time delay of a stroke. First, you know nothing,
except the algorithm. So you implement an evalua-
tion of a time interval between a stroke and a treat-
ment’s begin. The RDF model will be extended after
the implemented algorithm execution. After that, you
are able to confirm a treatment time delay impression
on patients treatment result. Other advantage exam-
ple is when you want to search for a set of specific
files based on any conditions then it will be too time
consuming to process the raw data. Apparently it is
a better way to acquire useful data earlier and store
them into the RDF graph. Then the search can be
more quickly and efficiently.
4 HYPOTHESIS AND
EXPERIMENTS
4.1 Data Set Description
The whole stroke data set characteristic in the experi-
mental database is:
• Stroke was in years 2005 - 2009
• Patient count 244; Middle age 68
• Male/Female count 148 / 96
• Male/Female middle age 66.5 / 71
• Male/Female average age 66.1 / 67.2
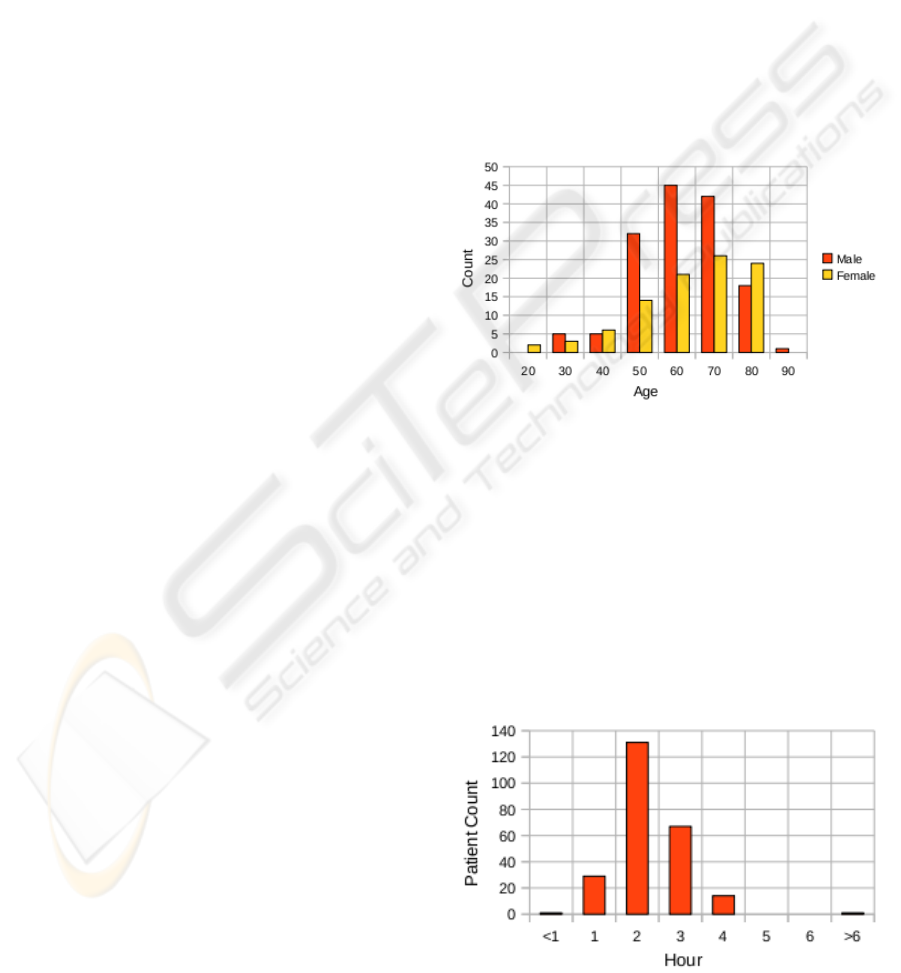
4.2 Patient’s Age and Sex Distribution
Statistic
Advancedage is one of the most significantstroke risk
factors. The incidence of stroke increases exponen-
tially from 30 years of age, and etiology varies by age
(Ellekjær et al., 1997). Our real patient’s data set are
shown on the Figure 1 grouped by age and sex. It
shows us that 95 % of strokes mostly occur in people
age 45 and older. Two-thirds of strokes occur in those
over the age of 65. Similar results are also referred in
(Senelick and Dougherty, 2001; National Electrical
Manufacturers Association (NEMA), 2010). A per-
son’s risk of dying if he or she does have a stroke in-
creases with age. However, it is evidence that stroke
can occur at any age.
Figure 1: Patient’s Age and Sex distribution over the stroke
data set.
4.3 rt-PA Treatment Time Delay
The last basic example of data usage is a treatment
time delay evaluation. It is a time interval between
a stroke and an rt-PA treatment begin. The distribu-
tion of the treatment time delay by hours is shown
on a Figure 2. Any more complex query evaluation
research can continue with this values, e.g. we can
identify dependency between the treatment time de-
lays and a treatment success.
Figure 2: Treatment time delay. It is a time interval between
a patient’s stroke and begin of an rt-PA treatment.
EXPERIMENTAL DATABASE IMPLEMENTATION FOR THE CEREBROVASCULAR DISEASES RESEARCH
INTEGRATES TOGETHER DIFFERENT KINDS OF MEDICAL DATA
113

5 CONCLUSIONS
The collaboration vision of medical doctor and com-
puter programmer team were established. The medi-
cal doctor informs what to do with the data and how
to interpret data for the research purposes. The com-
puter programmer creates and implements a right al-
gorithm. The experimental database is ready to new
participants, now.
The project benefits from the medical data rela-
tionships. Related data and a RDF data model is the
project base. Meta data stored in a RDF format are ac-
quired by raw and previously processed medical data
processing. By this way we create information and
build a knowledge base. The knowledge base speed
up and ease searching for resources. A user can take
advantage of this meta data referred to the raw medi-
cal data. It is better to querying in a huge amount of
data. Generally, the meta data can contain any data
type. With RDF model we can refer to any data type.
Basic part of the project is implemented and all
ordinary medical data types are supported. Currently,
we are working on a new data mining algorithms that
extends the project knowledge base.
ACKNOWLEDGEMENTS
The work presented in this paper was supported
by the project Czech Science Foundation number
106/09/0740.
REFERENCES
Awareness, P., Referral, E., Transfer, P., Care, S., Imaging,
D., Principles, G., Tests, B., Prevention, P., Pressure,
H., Smoking, C., et al. (2008). Guidelines for Man-
agement of Ischaemic Stroke and Transient Ischaemic
Attack 2008. Cerebrovasc Dis, 25:457–507.
Brainin, M., Bornstein, N., Boysen, G., Demarin, V., et al.
(2000). Acute neurological stroke care in Europe: re-
sults of the European Stroke Care Inventory. Euro-
pean Journal of Neurology, 7(1):5–10.
Dolin, R., Alschuler, L., Boyer, S., Beebe, C., Behlen, F.,
Biron, P., and Shabo Shvo, A. (2006). HL7 clinical
document architecture, release 2. Journal of the Amer-
ican Medical Informatics Association, 13(1):30.
Ellekjær, H., Holmen, J., Indredavik, B., and Terent, A.
(1997). Epidemiology of stroke in Innherred, Norway,
1994 to 1996: incidence and 30-day case-fatality rate.
Stroke, 28(11):2180.
Health Level Seven, Inc. (2010). What is hl7? Online,
2010-03-02. http://www.hl7.org/about/index.cfm.
Karlova univerzita v Praze – 2. l´ekaˇrsk´a fakulta v Praze
(Charles University in Prague – 2nd Faculty of
Medicine) (2010). Data Standard (DASTA). Online,
2010-03-02. http://dasta.lf2.cuni.cz/.
Lopez, D. and Blobel, B. (2009). A development framework
for semantically interoperable health information sys-
tems. International Journal of Medical Informatics,
78(2):83–103.
MZ
ˇ
CR – Ministry of Health of the Czech Republic (2010).
Ministerstvo zdravotnictv´ı
ˇ
Cesk´e Republiky: Vˇestn´ık
ˇc. 2/2010: P´eˇce o pacienty s cerebrovaskul´arn´ım
onemocnˇen´ım
ˇ
Cesk´e republice. Online, 2010-03-01.
http://legislativa.mzcr.cz/File.ashx?id=233&name=
V%C4%9Bstn%C3%ADk %20%%C4%8D 02
2010.pdf.
National Electrical Manufacturers Association (NEMA)
(2010). Stroke: Hope Through Research. On-
line, 2010-03-02. http://www.ninds.nih.gov/disor-
ders/stroke/detail stroke.htm.
National Institute of Neurological Disorders and Stroke
(2010). Digital Imaging and Communications
in Medicine (DICOM). Online, 2010-03-02.
http://medical.nema.org.
O’Brien, J., Erkinjuntti, T., Reisberg, B., Roman, G.,
Sawada, T., Pantoni, L., Bowler, J., Ballard, C., De-
Carli, C., Gorelick, P., et al. (2003). Vascular cogni-
tive impairment. The Lancet Neurology, 2(2):89–98.
Rohan, V., Sevcik, P., Polivka, J., Ambler, Z., Kreuzberg,
B., and Ferda, J. (2007). Klinick´y pohled na
v´ypo
ˇ
Cetn´ı tomografii u akutn´ı ischemie mozku (A
clinical Approach to Computed Tomography in Acute
Cerebral Ischemia).
ˇ
Cesk´a a slovensk´a neurologie a
neurochirurgie.
Rothwell, P., Coull, A., Silver, L., Fairhead, J., Giles, M.,
Lovelock, C., Redgrave, J., Bull, L., Welch, S., Cuth-
bertson, F., et al. (2005). Population-based study of
event-rate, incidence, case fatality, and mortality for
all acute vascular events in all arterial territories (ox-
ford vascular study). The Lancet, 366(9499):1773–
1783.
Safe Implementation of Thrombolysis in Stroke (2010).
About safe implementation of thrombolysis in stroke.
Online, 2010-03-02. http://www.acutestroke.org.
Senelick, R. and Dougherty, K. (2001). Living with stroke:
a guide for families. Health South Press.
Vˇcel´ak, P., Pol´ıvka, J., Maule, P., Kratochv´ıl, P., and
Kleˇckov´a, J. (2009). Experimental database system
for the vascular brain diseases research. In Neuroin-
formatics 2009: 2nd INCF Congress of Neuroinfor-
matics, Pilsen, Czech Republic. Frontiers in Neuroin-
formatics. Frontiers Research Foundation.
W3C (2010). Resource Description Framework (RDF):
Concepts and Abstract Syntax. Online, 2010-03-02.
http://www.w3.org/TR/rdf-concepts/.
ˇ
Cesk´y – Czech Statistical Office (2010).
ˇ
Cesk´y stati-
stick´y ´u´rad:
´
Umrtnost´ı tabulky (Death-rate Statis-
tics). Online, 2010-03-02. http://www.czso.cz/csu/
redakce.nsf/i/umrtnostni tabulky.
INNOV 2010 - International Multi-Conference on Innovative Developments in ICT
114
