
SACCADES GENERATION
From the Visual Input to the Superior Colliculus
Wahiba Taouali
Universit
´
e Henri Poincar
´
e - LORIA, Campus Scientifique, Vandoeuvre-l
`
es-Nancy Cedex, France
Nicolas Rougier, Fr
´
ed
´
eric Alexandre
INRIA Nancy - Grand Est Research Center, Villers les Nancy Cedex, France
Keywords:
Superior Colliculus, Dynamic Neural Field, Visual Attention, Ocular Saccades
Abstract:
The superior colliculus is an important structure in the visuomotor pathway of mammals, that is known to be
deeply involved in visual saccadic behavior. We present a model of this structure based on biological data, the
specificity of which is related to the homogeneity of the underlying substratum of computation. This makes
it more suitable to process massive visual flows on a distributed architecture, as it could be requested in a
realistic task in autonomous robotics. The model presented here is embedded in the exogenous part of the
visual pathway, from the retina to the superior colliculus.
1 INTRODUCTION
Displaying an intelligent behavior is often synony-
mous of intelligently exploiting the surrounding en-
vironment. In many animals and animats, this is mas-
sively performed through the visual channel. Partic-
ularly, two adaptive behaviors allow to decrease the
huge amount of information brought by this chan-
nel: visual attention proposes a sequential process-
ing of possible targets; saccadic movements orient the
body and particularly the fovea on regions of inter-
est in the visual scene. The premotor theory of at-
tention (Rizzolatti et al., 1987) stipulates that there
are common processes between these key behaviors
and, more precisely, that they share common neuronal
circuits: Attention would be pre-programming of a
saccade. The importance of these visuomotor behav-
iors and the impact of the premotor theory certainly
explains why there have been so many works to ob-
serve and model the superior colliculus (SC). Indeed,
this small structure in the midbrain of mammals is
known to be implicated in these behaviors. From an
hodological viewpoint, it integrates visual informa-
tion from many sources (cortical or not) in the brain
and sends projections toward the brainstem premo-
tor circuits that trigger saccades (Isa, 2002). From
an anatomical viewpoint, it consists of a set of topo-
logical maps, mapping the surrounding space, from
visual to motor reference frames (Girard and Berthoz,
2005). And from a physiological viewpoint, its inac-
tivation or electrical stimulation confirms its role in
visual attention and saccades (Muller et al., 2005).
Many models have studied the SC and associated
properties (cf. (Girard and Berthoz, 2005) for a re-
view). We just mention here some models underlying
the link to information flows and underlying behav-
ior. The structure of the model described in (Find-
lay and Walker, 1999) underlines that the main task
is to decide when and where the saccade must be
performed. As a consequence, two hierarchical axes
are defined. The When axis (corresponding to the
FEF (Frontal Eye Field) area in the prefrontal cor-
tex) decides when to leave the current fixation point,
whereas the Where axis corresponds to the SC and
implements a spatial competition between candidate
targets. A double-axis model combining FEF and the
SC is also proposed in (Kramer et al., 1999), to ex-
plain the integration of exogenous elements (external
stimuli coming from the retina to the SC) and endoge-
nous elements (internal expectancies or instructions
elaborated in the prefrontal cortex). Later on, (Godijn
and Theeuwes, 2002) proposed a competitive integra-
tion model based on strong experimental evidences at
the behavioral level, indicating that all these elements
(spatial vs temporal processing and integration of ex-
ogenous vs endogenous stimuli) can be integrated in a
176
Taouali W., Rougier N. and Alexandre F..
SACCADES GENERATION - From the Visual Input to the Superior Colliculus.
DOI: 10.5220/0003065501760181
In Proceedings of the International Conference on Fuzzy Computation and 2nd International Conference on Neural Computation (ICNC-2010), pages
176-181
ISBN: 978-989-8425-32-4
Copyright
c
2010 SCITEPRESS (Science and Technology Publications, Lda.)

unique map, seen as a model of the SC. This common
saccade map also includes features generally reported
as physiologically plausible in the SC: a local exci-
tation in the map allowing to combine close stimuli
and a wider inhibition mechanism to trigger a com-
petition between far stimuli. This interaction scheme
explains why it was possible to use such a formal-
ism as Dynamic Neural Field (DNF) (Amari, 1977)
to implement this kind of model, as it is also the case
in (Trappenberg et al., 2001; Schneider and Erlhagen,
2002).
In summary, the SC is undoubtedly an important
integrative structure, to be included in a cognitive
neuroscience modeling approach of visuospatial be-
haviors. Most recent models give a stronger role to
the SC at the price of a more complex internal func-
tioning. Indeed, those models define several kinds of
units, depending on their location on the map, which
is not very consistent with the principle of homogene-
ity in DNF. More precisely, in (Trappenberg et al.,
2001), the reported behavior is obtained with some
units standing for the currently fixated stimulus (con-
sequently in the fovea), other units representing po-
tentially fixated stimuli in the periphery, both kinds
sending inhibition to other units triggering saccades
toward a target. These kinds of units, also exploited
in the model by (Schneider and Erlhagen, 2002), are
presented as representing respectively so-called fixa-
tion, build-up and burst neurons, which are sometimes
reported as parts of the intermediate layer of the SC
(Wurtz and Optican, 1994), though this is still to be
clearly established. In (Godijn and Theeuwes, 2002)
also, the substratum of computation is not homoge-
neous, since the sensitivity of units decreases with
their eccentricity onto the map. This trick is used to
reproduce the observation that the latency of a sac-
cade toward a target, presented together with a dis-
tractor, is longer when the distractor is closer from the
rostral zone of the SC (corresponding to the fovea).
In this paper, we present a model of the SC, based
on DNF formalism, with an identical functioning rule
for all the units in the map. Obtaining such an homo-
geneous substratum can yield fully distributed com-
putation, which is important to design models that can
be used online in robotic visuomotor tasks. Finally,
the proposed model is not an isolated structure but is
a function of exogenous information flows and asso-
ciated geometrical properties.
2 MODEL
We designed a three layers neural network model of
the visual pathway for generating a saccade from the
retina (R) to the superior colliculus (SC) through the
primary visual cortex (V1):
• Retina (R, 256 × 512 units) receives visual input
from a CCD camera.
• Visual cortex (V1, 256 × 256 units) implements
the actual cortical magnification.
• Superior colliculus (SC, 63×63 units) is the place
where salient locations enters competition.
The retina model is restricted to the right visual field
as it is known to be the case in mammals visual path-
way (left visual field projects to right colliculus and
right visual field projects to left colliculus). We used
an image size of 512 × 512 pixels and fed the retina
with a normalized gray-level image of size 512 × 256
pixels.
2.1 Cortical Magnification
The retina, the sensory input space, has a com-
plex structure composed of layers of neurones. The
phenomena of vision begins in the layer of photo-
receptors. Then the flow is transmitted to the gan-
glion cells which are large nerve cells whose cylin-
draxes form the optic nerve. Due to the non homoge-
neous repartition of photo-receptors on the (human)
retina surface, visual acuity decreases from the cen-
ter of the retina (fovea) to the periphery. This prop-
erty is attributed to a variation in the density of photo-
receptors that decreases from the center to the periph-
ery (Marilly et al., 1999). Consequently, the foveal
region benefits from a much higher resolution than pe-
ripheral regions and this property is preserved along
the visual pathway up to early visual areas (Purves,
2004). This is referred as cortical magnification. To
analyze this magnification in a quantitative way, a co-
ordinate system is often defined in the visual field.
The coordinates that are best suited to the visual sys-
tem are polar coordinates (ρ, φ) that characterizes a
position in the visual field by its eccentricity ρ from
the center of gaze and its polar angle φ is measured
for example in relation to the lower vertical merid-
ian. We can therefore define a retinotopic map which
corresponds to the spatial transformation of the image
by the spatial arrangement of the grid of neurons. It
is often approximated by a log-polar transformation
of the spherical image centered on the eye (Robin-
son, 1972). We used a simplified model of the retina
considering only the photo-receptors layer. And for
computational reasons (speed), we did not enforce
the non-uniform repartition of photo-receptors on the
retina surface but we modeled instead a uniform dis-
tribution of neurons onto the retina associated with
a deformed polar coordinate system as proposed by
SACCADES GENERATION - From the Visual Input to the Superior Colliculus
177
(Ottes et al., 1986). Each cortical visual cell is sup-
posed to be connected to a single or several photo-
receptor cells, with respect to a logpolar deformation,
that form its receptive field. So the non uniformity
is caused by the changing size of the receptive fields.
We used equations mapping retinotopic polar coor-
dinates (ρ, φ) onto V1 Cartesian coordinates (x, y).
These equations were first introduced by (Ottes et al.,
1986):
x = B
x
ln(
p
ρ
2
+ 2Aρ|cos(φ)| + A
2
A
) (1)
y = B
y
arctan(
ρsin(φ)
ρ|cos(φ)| + A
) (2)
with A = 3
◦
, B
x
= 1.4mm, B
y
= 1.8mm. These pa-
rameters have been chosen to fit the stimulation map
of the SC given by (Robinson, 1972). A neuron in
the visual cortex fires an action potential when a vi-
sual stimulus appears within its receptive field. But
for any given neuron, it may respond best to a sub-
set of stimuli within its receptive field correspond-
ing to its preferred direction. Neurons with similar
tuning properties (what the neurons respond to) tend
to cluster together but the exact structure is still un-
clear. Then, it is acceptable to assume that V1 has
a retinotopic map similar to the collicular motor map
in (Bear et al., 1996). It means that a cell at a given
position (x, y) in the V1 map is activated by retinal
cells in positions (ρ, φ) according to given equations.
One result of this deformation is that the same stim-
ulus causes a large activation in the V1 map if it is
located near the fovea and smaller activation in pe-
ripheral positions (fig1). Visual receptors of V1 have
been modeled in two dimensions corresponding to an
eye visual hemifield with no connection between the
different receptors.
Figure 1: Cortical magnification from the retina to the
visual cortex distorts geometrical properties of the image
while keeping neighborhood relationship.
2.2 Dynamic Neural Field Theory
Collicular population (the motor layer of one supe-
rior colliculus) has been modeled with respect to the
dynamical neural field theory (Wilson and Cowan,
1973; Amari, 1977; Taylor, 1999) that describes the
evolution of a neural population using equation (see
(Rougier and Vitay, 2006) for details):
τ
∂u(x, t)
∂t
= −u(x, t)+
Z
w(x − y) f (u(y))dy + h + I(x, t)
(3)
where x denotes a location onto the SC; t is time;
u(x, t) denotes the membrane potential of a neural
population at point x and time t; τ is the temporal de-
cay of synapses, f is a sigmoid function computing
the mean firing rate, w is a neighborhood function,
s(x) is the input received at position x and h is the
mean neuron threshold. w has been set as a differ-
ence of Gaussian (DoG) with short-range excitations
and long range inhibitions following anatomical and
physiological data as reported in (Munoz and Istvan,
1998) (see also figure 2):
w(x − y) = Ae
−
|x − y|
2
a
2
− Be
−
|x − y|
2
b
2
(4)
and f has been set as a simple rectification of x. The
input I(x, t) is a direct one-to-one relationship accord-
ing to V1 and SC respective sizes.
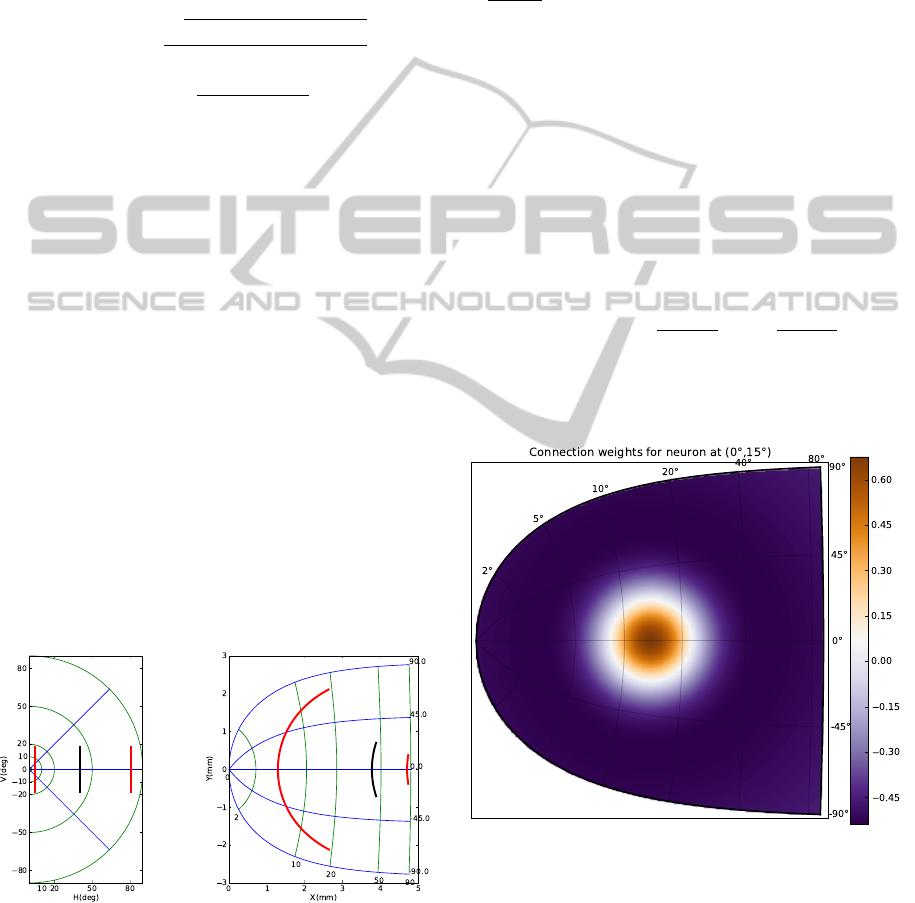
Figure 2: The lateral weights connecting neurons from the
SC is a difference of Gaussian with short-range excitations
and long range inhibitions following anatomical and phys-
iological data. The figure displays incoming weights from
all SC neurons to the neuron at position (0
◦
, 15
◦
).
ICFC 2010 - International Conference on Fuzzy Computation
178
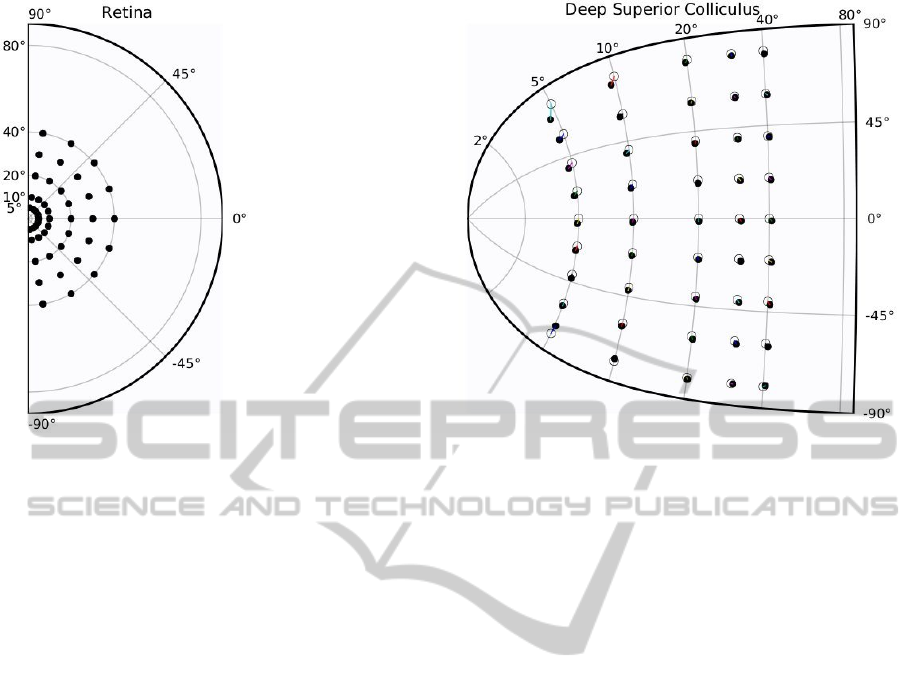
Figure 3: Accuracy of the model of the superior colliculus has been measured using a set of retina targets (black dots on left
figure) that have been sequentially presented to the SC model. For each target and after convergence (difference of activity
between time t and time t + dt is negligible), the center of mass of the collicular activity has been decoded and represented as
a circle on right figure (black dots represent the actual projection of the target in collicular coordinates).
3 RESULTS
3.1 Output Decoding
One of the questions related to the superior colliculus
concerns the proper way to decode the output. Since
the amplitude and direction of a saccade depend on
the activity of the neural population in the deep SC
(Sparks et al., 1990), different ways of SC output eval-
uation have been proposed in the past:
• winner-take-all where the most active site indi-
cates the direction
• summation(McIlwain, 1976; Sparks et al., 1976)
where all activities of active neurons are summed
with weights determined by their individual labels
• weighted average (Lee et al., 1988) using a nor-
malization according to the number of active neu-
rons
These three evaluation schemes are equivalent in the
case of a normally activated population but differ
when there is a deactivation or an over-activation.
We’ve retained the last decoding scheme because we
modeled the superior colliculus using a dynamic neu-
ral field and it is thus ensured that a stereotyped ac-
tivity profile emerges anytime corresponding to the
most salient location of the V1 area. Furthermore, this
stereotyped activity possesses a Gaussian shaped two-
dimensional profile and it is possible to find its center
of mass. We have been testing the accuracy of this
coding scheme by feeding the model with standard
Gaussian shaped stimuli (aperture 0.016
◦
) at different
locations (see figure 3). Despite the magnification ef-
fect, one can see that the model has a high precision
in the standard saccadic range (−30
◦
to +30
◦
, 0 to
50). We also tested the inactivation of a subpart of the
colliculus to check that we obtain both hypo-metric
and hyper-metric saccades as reported in (Robinson,
1972) but those results are not presented in this paper.
3.2 Natural Images Processing
We have also tested the model using natural images
taken from a color CCD camera. No image process-
ing has been performed on the image but a conver-
sion to a gray-level representation. Figure 4 exhibits
an example where a subpart of a computer keyboard
has been shot. This allows to illustrate the main fea-
ture of the proposed model. If one look closely at
the half retina representing the keyboard (upper left
part of the figure), one can see that several letters (O,
P, L, M are eligible for attention focus and an ocu-
lar saccade. However, the retinotopic projection onto
the model of the V1 area reduces quite naturally this
set to letters O and L. The model of the SC is thus
confronted with a choice between these two locations
and the dynamic field theory, as it has been introduced
in the previous section, ensures that only one location
remains after competition. However it is hard to spec-
SACCADES GENERATION - From the Visual Input to the Superior Colliculus
179
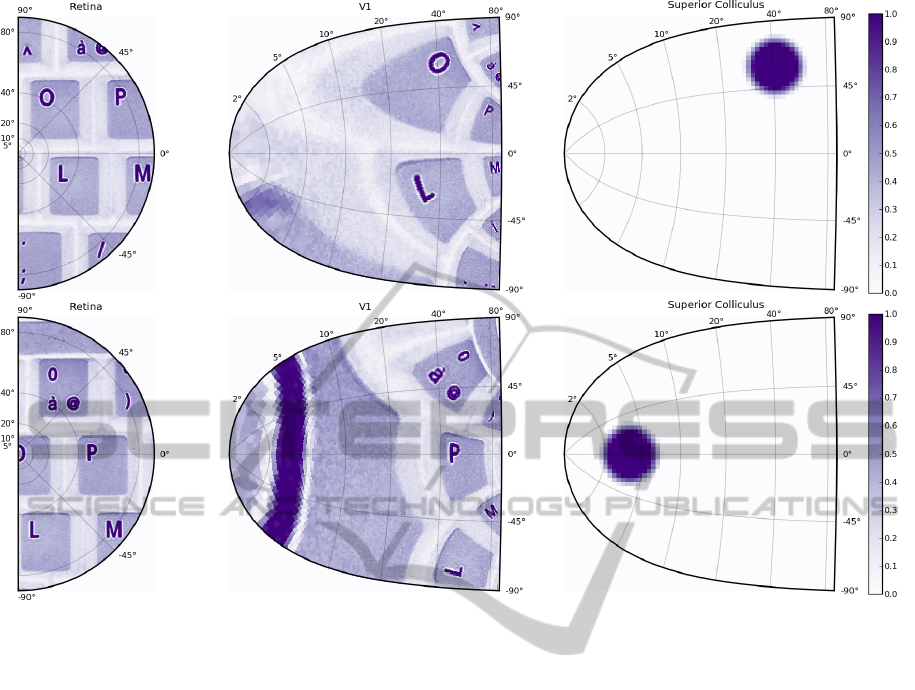
Figure 4: An image of a computer keyboard has been captured using a color camera (resolution 1024×1280) and transformed
into a normalized gray level image. Upper figure. The right half of the image is presented to the half retina area which in turn
feeds the V1 area where retinotopy is applied following equation 2. The colliculus area enters a competition stage where most
salient locations are eligible for final activation and after some iterations, the competition ends up onto the O letter that is thus
considered the most salient location of the visual scene according to its location and activation. Lower figure. A saccade has
been simulated to center the O letter into the center of the fovea and the colliculus now focuses onto a subpart of the O letter
that appears to be the newly most salient location of the new visual scene.
ify the exact conditions that make the model to focus
on the O instead of the L letter in the given example
and the spatially compact shape of the O is certainly
to be taken into account. This example also under-
lies the inherent difficulty in temporally organizing
ocular saccades without any top-down control. If we
were to let the model only reacts to its environment,
it would certainly focus on the most salient location
without ever exploring other point of interest (from
a behavioural point of view). If the actual saccade
brings into view another salient location, the model
would jump again to the new location (provided we
inhibited the foveal region to prevent the model to
be stuck forever on this single location) but in such
a case, nothing would prevent the model from going
to location A then location B and the again location A,
being trapped in a cycle. Exploring the whole visual
scene thus requires some kind of top down control to
be able to dynamically inhibit visited location once
they have been focused in order to favor other loca-
tions. This is out of scope of the present article but
this has been already made in a less realistic model
(Fix et al., 2006).
4 DISCUSSION
In many visuomotor behaviors, the superior collicu-
lus is reported as an important multimodal map, in-
tegrating various kinds of exogenous and endogenous
information. Most of the models of this structure in-
sist on its internal structure and function, but rarely on
its implication in the main information flows, which
is important from a behavioral point of view.
The model of the superior colliculus presented in
this paper has two specificities: (i) It is based on a
bio-inspired homogeneous local functioning rule. (ii)
It is linked with the external world through an infor-
mation flow coming from the retina. In ongoing and
future works, these specificities will be developed fur-
ICFC 2010 - International Conference on Fuzzy Computation
180

ther. Concerning the first aspect, the model has been
designed together with colleagues from neuroscience
and its justification with regard to biological data is
being also prepared for publication. Concerning the
second aspect, we have only considered for the mo-
ment exogenous inputs coming from the retina. Fur-
ther works will consider endogenous inputs convey-
ing such information as instructions, goals or motiva-
tions from other neural structures.
REFERENCES
Amari, S.-I. (1977). Dynamics of pattern formation in
lateral-inhibition type neural fields. Biological Cyber-
netics, 27(2):77–87.
Bear, M., Connors, B., and Paradiso, M. (1996). Neuro-
science: Exploring the Brain. Lippincott Williams &
Wilkins.
Findlay, J. and Walker, R. (1999). A model of saccade gen-
eration based on parallel processing and competitive
inhibition. Behavioral and Brain Sciences, 22(4):661–
674.
Fix, J., Vitay, J., and Rougier, N. (2006). A computational
model of spatial memory anticipation during visual
search. In Anticipatory Behavior in Adaptive Learn-
ing Systems.
Girard, B. and Berthoz, A. (2005). From brainstem to cor-
tex: computational models of saccade generation cir-
cuitry. Progress in Neurobiology, 77(4):215–251.
Godijn, R. and Theeuwes, J. (2002). Programming of en-
dogenous and exogenous saccades: evidence for a
competitive integration model. Journal of experimen-
tal psychology: human perception and performance,
28(5):1039–1054.
Isa, T. (2002). Intrinsic processing in the mammalian su-
perior colliculus. Current opinion in Neurobiogy,
12(6):668–677.
Kramer, A., Irwin, D., Theeuwes, J., and Hahn, S. (1999).
Oculomotor capture by abrupt onsets reveals concur-
rent programming of voluntary and involuntary sac-
cades. Behavioral and Brain Sciences, 22(4):689–
690.
Lee, C., Rohrer, W., and Sparks, D. (1988). Population
coding of saccadic eye movements by neurons in the
superior colliculus. Nature, 332(6162):357–360.
Marilly, E., Mercier, A., Coroyer, C., Faure, A., and
Cachard, O. (1999). Proprits d’un pr-processeur de
vision fovale. Dix-septime colloque GRETSI.
McIlwain, J. (1976). Large receptive fields and spatial
transfor- mations in the visual system. Int. Rev. Phys-
iol., 10:223–248.
Muller, J., Philiastides, M., and Newsome, W. (2005). Mi-
crostimulation of the superior colliculus focuses atten-
tion without moving the eyes. Proceedings of the Na-
tional Academy of Sciences, 102(3):524–529.
Munoz, D. and Istvan, P. (1998). Lateral inhibitory interac-
tions in the intermediate layers of the monkey superior
colliculus. J. Neurophysiol., 79:1193–1209.
Ottes, F., Gisbergen, J. V., and Eggermont, J. (1986). Visuo-
motor fields of the superior colliculus: a quantitative
model. Vision Res, 26(6):857–873.
Purves, D. (2004). Neurosciences. De Boeck, second edi-
tion.
Rizzolatti, G., Riggio, L., Dascola, I., and Umil, C. (1987).
Reorienting attention across the horizontal and verti-
cal meridians: evidence in favor of a premotor theory
of attention. Neuropsychologia, 25(1):31–40.
Robinson, D. (1972). Eye movements evoked by collicu-
lar stimulation in the alert monkey. Vision Research,
12(11):1795–1808.
Rougier, N. and Vitay, J. (2006). Emergence of atten-
tion within a neural population. Neural Networks,
19(5):573–581.
Schneider, S. and Erlhagen, W. (2002). A neural field model
for saccade planning in the superior colliculus: speed-
accuracy tradeoff in the double-target paradigm. Neu-
rocomputing, 44-46:623–628.
Sparks, D., Holland, R., and Guthrie, B. (1976). Size and
distribution of movement fields in the monkey supe-
rior colliculus. Brain Res., 113(1):21–34.
Sparks, D., Lee, C., and Rohrer, W. (1990). Population cod-
ing of the direction, amplitude, and velocity of sac-
cadic eye movements by neurons in the superior col-
liculus. Cold Spring Harbor symposia on quantitative
biology, 55:805–811.
Taylor, J. (1999). Neural bubble dynamics in two dimen-
sions: foundations. Biological Cybernetics, 80:393–
409.
Trappenberg, T., Dorrisn, M., Munoz, D., and Klein, R.
(2001). A model of saccade initiation based on the
competitive integration of exogenous and endogenous
signals in the superior colliculus. Journal of Cognitive
Neuroscience, 13(2):256–271.
Wilson, H. and Cowan, J. (1973). A mathematical theory of
the functional dynamics of cortical and thalamic ner-
vous tissue. Biological Cybernetics, 13(2):55–80.
Wurtz, R. and Optican, L. (1994). Superior colliculus cell
types and models of saccade generation. Current
Opinion in Neurobiology, 4(6):857–861.
SACCADES GENERATION - From the Visual Input to the Superior Colliculus
181
