
URINE OUTPUT MONITORING
A Simple and Reliable Device for Monitoring Critical Patients’ Urine Output
Abraham Otero
Department of Information Systems Engineering, University San Pablo CEU, 28668 Madrid, Spain
Teodor Akinfiev, Andrey Apalkov
Center of Automation and Robotics, Technical University of Madrid, Spanish Council for Scientific Research
(CAR UPM-CSIC), La Poveda, Arganda del Rey, 28500 Madrid, Spain
Francisco Palacios
Critical Care Unit, University Hospital of Getafe, Getafe, Carretera Toledo KM 12.500, 28901 Madrid, Spain
Jes´us Presedo
Department of Electronics and Computer Science, University of Santiago de Compostela
15782 Santiago de Compostela, Spain
Keywords:
Biosensors, Urine output, Critical care, Patient monitoring.
Abstract:
Currently, critical care units are equipped with sophisticated commercial monitoring devices capable of sens-
ing most of the patient’ physiological parameters, and of automatically supervising whether the values of the
parameters lie within a preestablished range set by the clinician. The automation of these tasks has discharged
the healthcare staff of a considerable workload. It also avoids human errors, which are common in repetitive
and monotonous tasks.
In all likelihood, urine output is the most relevant physiological parameter that has yet to be sensed or su-
pervised automatically. This paper presents a patent-pending device capable of sensing and supervising urine
output. The device uses reed switches that are activated by a magnet that is attached to a float in order to
measure the amount of urine collected in two containers. When a container fills, it is emptied automatically
using a siphon mechanism and urine begins to collect again. An electronic unit sends the state of the reed
switches via Bluethooth to a PC. From this information, the PC calculates the urine output and supervises the
achievement of therapeutic goals. The end result is a fully automated, simple, inexpensive and accurate urine
meter.
1 INTRODUCTION
Current critical care units are equipped with sophisti-
cated commercial monitoring devices that allow clini-
cians to sense nearly any physiological parameter that
may provide information relevant for interpreting the
patient’s state. In most cases, these devices can also
supervise that the values of the physiological param-
eters they sense remain within a preestablished range
set by the clinician. This range represents the values
considered as normal for each parameter. If a param-
eter does not fall within its acceptable range, the cor-
responding sensing device alerts the healthcare staff
by means of an audible warning (Otero et al., 2009b)
(Hande et al., 2006).
These devices discharge the healthcare staff of a
considerable workload, since they need not continu-
ously supervise if the physiological parameters of ev-
ery patient lie within the acceptable range. They also
avoid human errors, which are common in any repet-
itive and monotonous task such as the one at hand
(Jungk et al., 2002) (Mora et al., 1993).
5
Otero A., Akinfiev T., Apalkov A., Palacios F. and Presedo J..
URINE OUTPUT MONITORING - A Simple and Reliable Device for Monitoring Critical Patients’ Urine Output.
DOI: 10.5220/0003125500050013
In Proceedings of the International Conference on Biomedical Electronics and Devices (BIODEVICES-2011), pages 5-13
ISBN: 978-989-8425-37-9
Copyright
c
2011 SCITEPRESS (Science and Technology Publications, Lda.)

Arguably, the most relevant physiological param-
eter which still is measured and supervised manually
by healthcare staff is the patient’s urine output. Urine
output is the best indicator of the state of the patient’s
kidneys. If the kidneys are producing an adequate
amount of urine it means they are well perfused and
oxygenated. Otherwise, it is a sign that the patient
is suffering from some complication. Urine output is
required for calculating the patient’s water balance,
which is essential in the treatment of burn patients
(Mitra et al., 2006). Finally, it is also used in multi-
ple therapy protocols to check whether the patient re-
acts properly to treatment (Rivers et al., 2001). When
urine output is too low the patient is said to have olig-
uria. If the patient does not produce urine at all, then
he/she is said to have anuria. Sometimes, urine out-
put can be too high; in these cases the patient is said
to have polyuria.
Currently, in critical care units, urine is collected
in a graduated container that is connected to the pa-
tient’s bladder through a Foley catheter. Periodically
the nursing staff manually records the reading of the
container of every patient, and operates a valve which
releases the urine into a larger container. In critical
care units of first world countries, this procedure is
usually performed every hour, 24 times a day, 365
days a year. In the case of emerging countries, often
only the burn patients –for whom urine output moni-
toring is of paramount importance– have this param-
eter registered every hour, while the remaining criti-
cal patients have it recorded every 2 or 3 hours. This
disparity in criteria is due to the more reduced avail-
ability of healthcare staff in the critical care units of
emerging countries.
It can even be argued that the monitoring interval
currently employed in first world countries –once ev-
ery hour– also is a compromise between avoiding risk
states for the patient and relieving the nursing staff
of an excessive burden. A system capable of auto-
matically monitoring urine output would decrease the
workload associated with this task and, at the same
time, it would permit supervision to take place on a
more continuous basis.
This paper presents a patent-pending device capa-
ble of sensing and supervising urine output (Akinfiev
et al., 2010). Section 2 reviews related work. Our
device is described in Section 3. It uses reed switches
that are activated by a magnet that is attached to a float
in order to measure the amount of urine collected by
two containers. An electronic unit checks the state
of the reed switches and sends it to a PC, which su-
pervises the achievement of the therapeutic goals that
have been established for urine output. Section 4 dis-
cusses the results of this work. Finally, a series of
conclusions and lines of future extension are given.
2 RELATED WORK
Automating the supervision of urine output is a
problem that has only begun to be addressed by
the biomedical engineering community very recently.
There are several problems that have contributed to
this delay. On the one hand, it requires devices capa-
ble of measuring very small amounts of flowing liq-
uid (up to 3-5 milliliters per hour). This precludes the
use of common industrial solutions such as ultrasound
sensors (Johnson, 1978) or commercial flowmeters.
On the other hand, any component of the device that
is in contact, or may be in contact, with the urine
cannot be reused on other patients and must be re-
placed approximately every 4-7 days for hygienic rea-
sons. Therefore, any component of the device that is
or may be in contact with the urine must be easy to
dispose of, and should have a low price. This pre-
cludes the use of expensive high precision laser based
solutions (Ishida, 1990). Furthermore, contact with
the urine also means that the component is in indi-
rect contact with the patient’s bladder through a Foley
catheter. Therefore, the components that come into
contact with the urine have to be sterilized before use.
Finally, urine contains Uric acid, Sodium, Potassium,
Chlorine and other components which make it corro-
sive, especially for metals.
The first device proposed to automatically mea-
sure urine output was Urinfo 2000, developed by the
Israeli company Medynamix (Hersch et al., 2009).
Urinfo 2000 was designed to automate the hourly
urine output measurement, but not to take more fre-
quent measures. Its operation is based on counting
the number of drops of urine produced by the patient,
and from this count it estimates urine output. The av-
erage error of the device when used to take hourly
measurements was 8% (±25 ml). Urinfo 2000 cannot
supervise therapeutic goals and its readings are not
transmitted to a central station/PC. Thus it requires
the nursing staff to take the urine output measures
from the device’s display, placed next to the patient’s
bedside.
The authors of this paper proposed a device to au-
tomate the supervision of urine output in (Otero et al.,
2009c). Several technical and legal problems pre-
cluded us from moving this device beyond the lab-
oratory validation phase. Using the knowledge we
gained building it, we developed a second device with
the main objective of conducting a series of clinical
studies based on more continuous and accurate mon-
itoring of urine output throughout the stay of patients
BIODEVICES 2011 - International Conference on Biomedical Electronics and Devices
6
in a critical care unit (Otero et al., 2010) (Otero et al.,
2009a). This device uses a high precision scale to
measure the weight of a commercial urine meter. On
the scale’s pan there is a support frame made up of
Bosch profiles that isolates the scale from force trans-
mission from the patient’s bed, and guarantees that
the urine flows properly through the urine meter’s in-
put tube. The maximum measurement error of this
device is under 1.5%.
Currently this device is in use at a research unit
associated with the University Hospital of Getafe, in
Spain. In this research unit a series of experiments
aimed at the study of sepsis in an animal model –pigs–
are being conducted. The aim of these experiments is
twofold. On one hand, we would like to gain a better
understanding of the pathophysiological mechanisms
underlying systemic and renal hemodynamics during
sepsis –hence the interest in a continuous and accu-
rate monitoring of urine output. On the other hand,
we would like to define the optimum monitoring in-
terval for urine output, and to determine the level of
accuracy required for this task.
The high accuracy and acquisition rate of this de-
vice make it ideal for carrying out clinical studies.
However, this device was not intended to be used in
the clinical routine. Its size and operation make it
somewhat tedious to be used in a critical care unit.
Conversely, in using this device we have learned that
its accuracy –1.5%– and measurement interval –up to
10 sec.– are superior to what is required for properly
supervising urine output. Our experience shows that
an accuracy of 5% and a monitoring interval of 5-10
minutes is enough. Therefore, these parameters can
be relaxed to obtain a cheaper and simpler device.
That is the goal of the device presented this paper.
3 THE DEVICE
In this section we first describe the general operating
principles on which our device is based. Although
we will use the device to measure urine output, the
principles on which it is based are general and could
be used to measure the volume of any liquid flow-
ing through a tube. Then we describe the prototype
we have built, and the calibration procedure we have
carried out to determine its operating parameters. Fi-
nally, we describe how the supervision of therapeutic
goals is carried out.
3.1 General Operating Principles
In our device the urine arising from the patient’s blad-
der is collected through an input tube in a container.
Output
Inputtube
Float
Limiter
Magnet
Topopening
Reedswitch
Controlunit
Floatmovement
tube
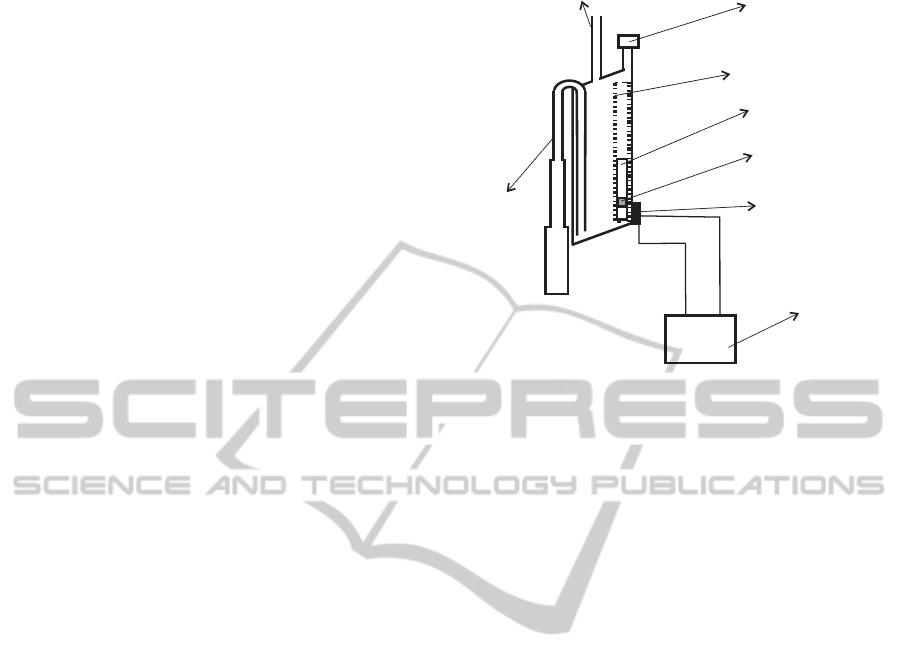
Figure 1: Diagram of the small container of our prototype.
This container is equipped with an output tube that
empties its contents when it gets filled by taking ad-
vantage of the siphon principle (see Figure 1). One
end of the output tube will be placed in the bottom
wall of the container. Ideally the bottom wall of the
container will be inclined or conical and the tube will
be in the lowest part. This favors a more complete
evacuation of the container. Both ends of the output
tube are always open. When the container begins to
receive liquid, the liquid goes up inside of the out-
put tube until it reaches the elbow located in its top
part. When it reaches the elbow, liquid begins to fall
down the portion of the tube located on the exterior
of the container. By the principle of the siphon, the
liquid will flow down the tube and the container will
be emptied.
For the system to function properly, the output
tube should not be too wide. If the tube is too wide,
when the water starts flowing, air could rise through
the tube towards the container, breaking the siphon
principle. On the other hand, to drain the container as
quickly as possible, it is desirable that the tube be as
wide as possible. A compromise between these two
goals can be achieved using a variable diameter tube,
so that its diameter grows (staggered or progressively)
from the elbow of the tube to the end which is outside
the container (see Figure 1). In this way, the principle
of the siphon will work properly because the tube is
narrow enough in its upper part, but when the water
begins to fall it finds a wider tube which provides less
resistance, thereby increasing flow rate.
The container must also be equipped with a fil-
tered top opening that is used to equalize the internal
and external pressures without risk of bacterial con-
tamination. This opening should be located at a height
URINE OUTPUT MONITORING - A Simple and Reliable Device for Monitoring Critical Patients' Urine Output
7

N
S
Open
Close
(a)
(b)
Figure 2: Operation of a reed switch. In the presence of
a magnetic field, the switch is closed. In the absence of
magnetic field, it is open.
H
1
above the elbow of the output tube. The liquid
will not start flowing immediately when it reaches the
elbow height; but it will continue to accumulate in
the container until the height of the column of liq-
uid which is above the elbow height overcomes the
surface tension of the liquid against the walls of the
output tube. The surface tension increases as the di-
ameter of the output tube decreases. The height H
1
and the effective volume of the container V
S
(the vol-
ume of water contained just before the draining starts)
must be determined experimentally.
Ideally, the upper wall of the container will also
be inclined or conical in shape. The top opening to
equalize the internal and external pressures should be
placed in the uppermost part of the wall (see Figure
1). This prevents bubbles from forming in the upper
wall of the container, bubbles that would occupy vol-
ume and distort the measures.
One or more reed switches are placed on the out-
side of the container that will receive the urine. A
reed switch contains two or more magnetizable, flexi-
ble, metal reeds hermetically sealed in a tubular glass
envelope whose end portions are separated by a small
gap. Under these conditions, the switch is open (see
Figure 2a). A magnetic field properly applied will
cause the reeds to bend, and the contacts to pull to-
gether, thus closing the switch (see Figure 2b).
A float within a structure designed to limit its
movement so that the float can only move vertically
is located inside of the container, near the container
wall where the reed switches are placed (see Figure
1). The float has a magnet attached which interacts
with the reed switches closing them when the mag-
net is approximately at the same height as each of the
reed switches.
An electronic unit is connected to the reed
switches, continuously checking their state. For the
general case in which there are N reed switches on
the outside wall of the container, the procedure for
measuring the volume of liquid flowing into the con-
tainer is as follows. At least one reed switch should be
located in a position such that when the container is
empty, the magnet located on the float closes the reed
switch. As the liquid begins to flow into the container,
the float, and therefore the magnet, begins to raise. At
some point, the magnet will stop interacting with the
first reed switch and, therefore, it opens. At that point,
a volume V
1
of liquid has flowed into the container.
When enough liquid has been accumulated in the con-
tainer, the magnet will close the second reed switch.
At this point an additional volume V
2
of liquid has
flowed into the container, being the total amount of
liquid accumulated V
1
+V
2
. When the magnet moves
higher, the second reed switch opens again and an ad-
ditional volume V
3
of liquid has flowed –being the to-
tal volume of liquid V
1
+V
2
+V
3
.
In general, when the reed switch N is closed, we
will add the volume V
2N−2
to the the volume of liquid
that has flowed, and when the reed switch N is opened
again, we will add the volume V
2N−1
. When the con-
tainer is emptied, the float with the magnet will go
back to the bottom of the container, and therefore it
will close the first reed switch. At this point, the ef-
fective volume of the container –V
C
– has flowed, and
the measurement procedure is resumed.
The volumes V
2N−2
and V
2N−1
for each reed
switch, and the effective volume V
C
must be deter-
mined experimentally.
3.2 Our Prototype
The general operating principle described in the pre-
vious section suffers from a problem: the volume of
liquid that flows into the container from the time the
container begins to empty through the siphon mecha-
nism, until it is completely empty, will not be mea-
sured. Depending on the specific application and
characteristics of the container, this may or may not
be tolerable. The problem that concern us, the mea-
surement of the amount of urine produced by a pa-
tient, requires an early warning about deviations from
the therapeutic goals. Thus, a small volume container
must be used –approximately 5 ml in the prototype
we have built. A container of such a small size needs
to be emptied a large number of times, which may
make the inability to measure the liquid that flows
during the discharge of the container intolerable if the
patient has polyuria, i.e., the patient is producing a
large amount of urine. If the patient is producing nor-
mal amounts of urine, or if he/she has oliguria, the
amount of urine that will flow during discharge of the
BIODEVICES 2011 - International Conference on Biomedical Electronics and Devices
8
BLUETOOTH
Figure 3: Diagram of our prototype device.
container will be nil or negligible.
It could be argued that in the case of patients that
have polyuria it is not required to have a high degree
of accuracy in the measurement of the urine output.
The more urine is produced, the less important it is
to have an accurate measure; only when the patient
is producing small amounts of urine it is important to
measure accurately (Otero et al., 2010). Therefore,
the operation of the device as is described in the pre-
vious section could be acceptable. In any case, this
problem can easily be solved using two different size
containers, each of them working according to the
principle presented in the previous section. The out-
put of the smaller container is connected to the input
of the larger container. Thus, although the volume of
liquid that flows during the discharge of the first con-
tainer is not measured in the small container, it will
be measured in the larger one.
Chaining two containers as described here causes
another problem. It can happen that when the small
container releases its content, the larger one is nearly
full. In the worst scenario, the first drop that falls
from the small container would trigger the draining
of the large container. Thus, the content of the small
container would not be measured in the large one.
The maximum error this may cause is (V
S
/V
L
)%,
being V
S
and V
L
the effective volumes of the small
and large container, respectively. On average, the
large container will start to drain when the small con-
tainer is emptied halfway. Therefore, the average er-
ror caused by this effect will be ((V
S
/2)/V
L
)%. As
long as V
L
>> V
S
, this error will be small.
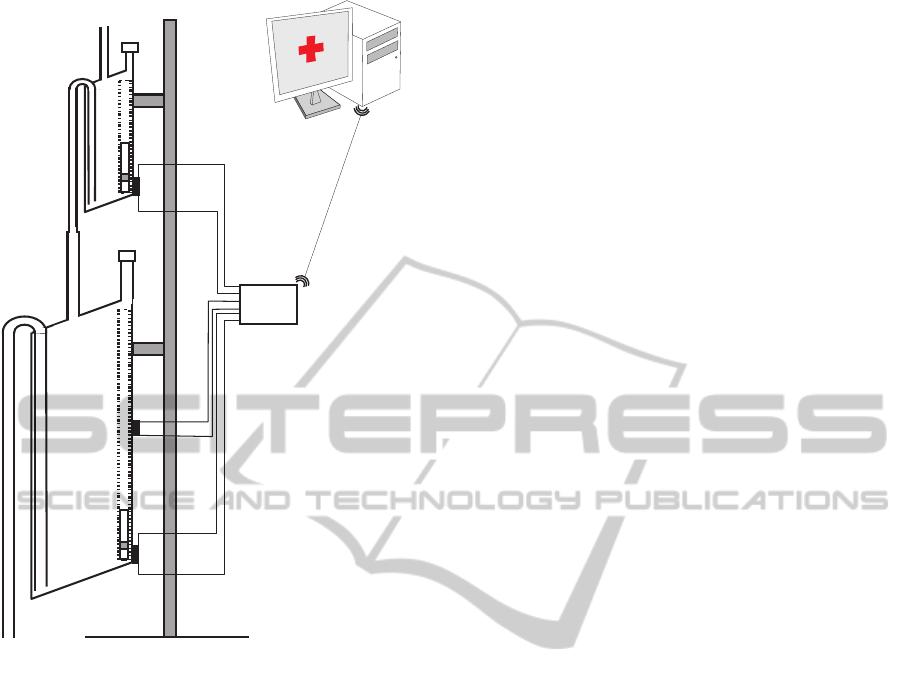
The prototype we have built uses two containers.
The first one was built to have a volume of approx-
imately 5 ml, and the second a volume of approxi-
mately 375 ml (see Figure 3). The first container is
equipped with a single reed switch that is activated
when the float is on its lowest position. The stag-
gered diameter of its output tube increases in three
steps. The output tube is connected to the input tube
of the larger container, which is equipped with two
reed switches. The tube of the large container is con-
nected to a 2.5 liters plastic bag that collects the liquid
once it has been measured.
An electronic unit continuously checks the status
of the reed switches, and reports any change via Blue-
tooth to a Java application that runs on a PC. From
these changes the application calculates the volume
of urine that has flowed and displays a chart with this
information.
3.3 Device Calibration
The calculation of the effective volumes of the con-
tainers –V
S
and V
L
– must be determined experimen-
tally. By effective volume we mean the volume of liq-
uid that triggers the emptying of the container through
the siphon mechanism. This volume will be slightly
higher than the volume corresponding to the height of
the top of the siphon mechanism because the liquid
does not begin to flow until the pressure overcomes
the surface tension force of the liquid against the walls
of the output tube. The smaller the diameter of the
output tube, the greater the height in the column of
liquid required to overcome the surface tension will
be.
The effective volume of each container was deter-
mined separately. A saline solution with properties
similar to those of urine and an dropper were used
to simulate the flow of urine (see Figure 4). Each
of the containers were placed so that they would re-
lease their content on a bowl placed on the plate of a
high-precision industrial scale –a PGW 4502e, built
by Adam Equipment Inc. This scale has an accuracy
guaranteed by the manufacturer of 0.01 g. Given that
the density of the saline solution was known, we can
determine the volume of liquid that the container re-
leases into the bowl from the weight.
URINE OUTPUT MONITORING - A Simple and Reliable Device for Monitoring Critical Patients' Urine Output
9

Saline
solution
Dropper
Plasticbag
Largecontainer
Smallcontainer
Electronicunit
Figure 4: Picture of the prototype device with the saline
solution and eye dropper used in its validation.
The PGW 4502e is equipped with a serial port that
permits querying for readings. We built a program
that periodically takes measurements from the scale.
The program together with the dropper allow us to au-
tomate the process of carrying out multiple measures
of the volume of liquid released by the containers.
Using this set up we took 200 measurements of
the volume of liquid released by the small container,
and 50 measures of the volume of liquid released by
the large container. From these measures we calcu-
lated the effective volumes of the containers: 5.87 ±
0.32 ml and 376.72 ± 1.11 ml (mean ± standard de-
viation). At the time of writing, the authors are work-
ing to determine the volumes V
L
1
, V
L
2
and V
L
3
, i.e., the
volumes corresponding with the opening of the first
reed switch of the large container, with the closing
of the second reed switch, and whit the opening of
second reed switch, respectively. This is challenging
because it requires measuring with high accuracy the
volume of liquid in the container before the liquid is
released, without interfering with the normal opera-
tion of the device. These volumes are not necessary
for the system to work correctly, but they would al-
low us to correct the measurements obtained from the
small container –which are less precise–, not just one
time but four times for each release cycle of the large
container.
3.4 Therapeutic Goals Supervision
The state of the reed switches placed in the containers
is sent from the electronic unit to a Java application
installed on a PC. From these states and from the val-
ues of V
S
and V
L
, the amount of liquid flow can be
calculated. The Java application allows the health-
care staff to inspect a graph showing urine output,
and to set the therapeutic goals for the urine output.
These therapeutic goals are represented with the aid
of the Fuzzy Set Theory, a tool which has proved its
value for handling and representing medical knowl-
edge (Barro et al., 2001).
We shall introduce some basic concepts of the
Fuzzy Set Theory. Given a discourse universe R we
define a fuzzy value C by means of a possibility distri-
bution π
C
defined over R (Zadeh, 1975). Given a pre-
cise value x ∈ R, π
C
(x) ∈ [0,1] represents the possibil-
ity ofC being precisely x. A fuzzy number (Kaufmann
and Gupta, 1984) is a normal (∃x ∈ R, π
C
(x) = 1)
and convex (∀ x, x
′
, x
′′
∈ R, x
′
∈ [x,x
′′
], π
C
(x
′
) ≥
min{π
C
(x),π
C
(x
′′
)}) fuzzy value. Normality and con-
vexity properties are satisfied by representing π
C
, for
example, by means of a trapezoidal representation. In
this way, C = (α,β,γ,δ), α ≤ β ≤ γ ≤ δ, where [β,γ]
represents the core, core(C) = {x ∈ R| π
C
(x) = 1},
and ]α,δ[ represents the support, supp(C) = {x ∈
R|π
C
(x) > 0}.
A fuzzy number C can be obtained from a flexi-
ble constraint given by a possibility distribution π
C
,
which defines a mapping from R to the real interval
[0,1]. A fuzzy constraint can be induced by an item
of information such as “x has a high value”, where
“high value” will be represented by π
C=high
. Given
a precise number x ∈ R, π
C=high
(x) ∈ [0,1] represents
the possibility of C being precisely x; i.e., the degree
with which x fulfills the constraint induced by “high
value”.
Physicians are accustomed to expressing the ther-
apeutic goals for urine output in milliliters of urine
produced per kilogram of patient body mass per hour
–ml/kg · h. Our tool allows them to indicate the
weight of the patient -P- and the therapeutic goals rep-
resented by the trapezoidal possibility distribution π
U
.
π
U
can be interpreted as a computational projection of
the piece of clinical knowledge “adequate UO”. The
minimum and maximum values acceptable for urine
BIODEVICES 2011 - International Conference on Biomedical Electronics and Devices
10

output are the beginning and the end of the support of
the distribution, respectively. If the patient produces
less urine than the amount corresponding with the be-
ginning of the support, the patient is clearly in olig-
uria. If he/she produces more urine than the amount
corresponding with the end of the support, the patient
is clearly in polyuria. The beginning and end of the
core are the limits of the interval within which ideal
values of urine output lie.
If u
i
is the urine output in ml/kg· h, the degree to
which the therapeutic goals established by the clin-
ician are being met is π
U
(u
i
). If π
U
(u
i
) = 1, the
urine output is within the range of ideal values. If
π
U
(u
i
) = 0, either the urine output is less than the
minimum acceptable value -the patient has oliguria or
anuria-, or greater than the maximum acceptable –the
patient has polyuria. In both cases, the program pro-
duces an audible warning. The closer π
U
(u
i
) is to 1,
the closer the amount of urine produced by the patient
is to the ideal value, and the closer π
U
(u
i
) is to 0, the
closer the patient is to oliguria or polyuria.
In the tool π
U
(u
i
) is represented by a color code
used when drawing the graph of urine output. Red
represents the null compatibility –the patient is clearly
in oliguria or in polyuria–, followed by orange, yel-
low, green and black, which represents the total com-
patibility -the urine output lies within the range of
ideal values. Therefore, the urine output graph pro-
vides instant visual feedback on the patient’s state.
The tool also generates an audible warning when
the small container is not filled within the maximum
time allowed by π
U
. This time is given by α · P/V
S
,
α being the beginning of the support of π
U
, P the pa-
tient’s weight, and V
S
the volume of the small con-
tainer.
4 DISCUSSION
Given that the effective volume of the small container
is 5.87 ± 0.32 ml (mean ± standard deviation), in
95% of cases the amount of urine that is really re-
leased when the container is emptied will be in the
range 5.87 ± 0.64 ml (mean ± 2·standard devia-
tion). Therefore, 95% of the measures derived from
the small container will have an error of 10.9% or
less. The purpose of this small container is to pro-
vide an early warning if the patient’s urine output is
not within the therapeutic goals: depending on the pa-
tient’s weight and state this container should be filled
within 5 and 12 minutes, if the patient is within the
limits of the therapeutic goals. If this does not occur,
the healthcare staff is alerted by an audible warning.
To obtain more accurate measures of the total
urine output, we rely on the second container. In 95%
of cases, the amount of urine released by this con-
tainer will be within the range 376.72 ± 2.22 ml. On
the other hand, on average half of the effective volume
of the small container will not be registered. Thus,
the error in the measures in 95% of the cases will be
5.87/2+2.22=5.16; 1.37% of its volume. This error is
low, especially when compared to errors committed
by the nursing staff when they take measures visually,
which has been reported to be as high as 26% (Hersch
et al., 2009).
Our device is capable of providing feedback on
the status of the patient’s kidneys more frequently
than is currently available in critical care units –
hourly. For a patient of 80 kilos, which should pro-
duce at the very least least 40 milliliters of urine per
hour, our device could warn of a deviation from the
therapeutic goals in less than 10 minutes – the time
in which the small container should be filled if the
patient produces urine within the therapeutic goals.
Therefore, it has the potential of improving patient
outcome. Given that it provides feedback on the pa-
tient’s state at shorter time intervals, it can allow the
clinician to react more promptly to complications in
the state of the patient.
The containers needed to build our device are not
more complex than the containers used in commer-
cial urine meters, which often require a small con-
tainer embedded within a large container, valves that
communicate both container with each other and with
a plastic bag, mechanisms to prevent the containers
from overflowing, etc. However, the price of our man-
ufacturing our device is slightly higher than the price
of the commercial urine meters because of the addi-
tion of four pieces: two floats, and two magnets. The
rest of the pieces that are part of our solution do not
have to be discarded because they are not in direct
contact with the patient’s urine, nor do they suffer sig-
nificant degradation caused by its operation. Thus,
their cost can be amortized over a long period of time
and their impact on the overall cost of the solution is
negligible.
Despite its higher cost, the device has the potential
of producing significant economic savings for the in-
stitutions that provide healthcare services. Currently,
a nurse must visit each of the patients’s beds of the
critical care unit to manually record urine output ev-
ery hour. The nurse must put on gloves since he/she is
going to manipulate body fluids, walk to the patient’s
bed, take the measure visually, write it down, open
the valve that releases urine from the graduated con-
tainer to the plastic bag, wait for the urine to drain,
close the valve and check if the plastic bag needs to
be emptied. This procedure takes at least 2 minutes.
URINE OUTPUT MONITORING - A Simple and Reliable Device for Monitoring Critical Patients' Urine Output
11

In a small critical care unit with only 10 patients, this
means 20 minutes per hour; 8 hours a day. Typically,
nurses work in shifts of six hours. Therefore, each day
one and a third nurses’ shift are required only for tasks
related to monitoring urine output. Even a partial au-
tomation of these tasks has the potential of yielding
significant economic savings.
5 CONCLUSIONS AND FUTURE
WORK
We have built a device capable of automatically sens-
ing and supervising the urine output of critical care
patients. The device comprises two containers of dif-
ferent volumes, a small one that receives the urine
coming from the patient’s bladder, and a greater vol-
ume container in which the first container releases
its content when it gets full. Both containers release
their content automatically when they are filled using
a siphon mechanism.
The containers are equipped with reed switches
that are activated by a magnet that is attached to a float
located inside the containers. These reed switches al-
low us to identify the instants at which they get filled
with urine. An electronic unit sends via Bluethooth
the information provided by the reed switches to a PC
which calculates the urine output from the switches’
state, and supervises the achievement of the thera-
peutic goals established by the clinician. The error
in measuring the patient’s urine output is under 2%.
The large container is the one which allows us to ob-
tain this high accuracy, while the small one permits
an early warning of deviations from the therapeutic
goals.
The cost of our device is slightly higher than the
cost of the commercial devices currently used in mon-
itoring urine output. However, the device has the po-
tential to save costs for the institutions that provide
health services by freeing a considerable amount of
time for the healthcare staff. Furthermore, it pro-
vides a more continuous supervision of the urine out-
put than is currently carried out in critical care units,
which may help improve patient outcomes.
As future work, we intend to take advantage of
all the state changes of the reed switches of the large
container to correct the urine output measures while
the large container is been filled. Currently, this cor-
rection is only performed when the large container re-
leases the urine. We also will start to use our device
in animal tests conducted in a research unit associated
with Getafe University Hospital. After this phase, we
intend to use it in a pilot test in the Intensive Care Unit
of this hospital.
ACKNOWLEDGEMENTS
We would like to acknowledge the support by the
Ministry of Science and Innovation of Spain, the Eu-
ropean Regional Development Fund of the European
Commission under the grant TIN2009-14372-C03-
03. T. Akinfiev acknowledges the financial support
received from CSIC under the project “New actua-
tors with high efficiency and control algorithms for
automation and robotics”. A. Apalkov acknowledges
the financial support from Ministry of Science and In-
novation of Spain under Juan de la Cierva Program.
REFERENCES
Akinfiev, T.; Apalkov, A.; Otero, A.; Palacios, F. Device for
measuring the amount of liquid that flows and proce-
dure for its measurement. Patent Pending, 2010. ES
P201031227.
Barro, S., Mar´ın, R., Palacios, F., and Ru´ız, R. (2001).
Fuzzy logic in a patient supervision systems. Artifi-
cial Intelligence in Medicine, 21:193–199.
Hande, A., Polk, T., Walker, W., and D.Bhatia (2006). Self-
powered wireless sensor networks for remote patient
monitoring in hospitals. Sensors, 6(9):1102–1117.
Hersch, M., Einav, S., and Izbicki, G. (2009). Accuracy and
ease of use of a novel electronic urine output monitor-
ing device compared with standard manual urinome-
ter in the intensive care unit. Journal of Critical Care,
24:629–633.
Ishida, S. (1990). Liquid level indicator using laser beam.
us patent 4938590.
Johnson, S. J. (1978). Liquid level measurement device.
United States Patent 3693445.
Jungk, A., Thull, B., and Rau, G. (2002). Fuzzy Logic
in Medicine, chapter Intelligent Alarms for Anaes-
thesia Monitoring Based on Fuzzy Logic Approach.
Physica-Verlag.
Kaufmann, A. and Gupta, M. (1984). Introduction to Fuzzy
Arithmetic. Van Nostrand Reinhold Company Inc.
Mitra, B., Fitzgerald, M., Cameron, P., and Cleland, H.
(2006). Fluid resuscitation in major burns. ANZ Jour-
nal of Surgery, 76:35–38.
Mora, F., Passariello, G., Carraylt, G., and Pichon, J.
(1993). Intelligent patient monitoring and manage-
ment systems: A review. IEEE Engineering in
Medicine and Biology.
Otero, A., Akinfiev, T., Fern´andez, R., and Palacios, F.
(2009a). A device for automatic measurement of crit-
ical care patient’s urine output. In 6th IEEE Inter-
national Symposium on Intelligent Signal Processing,
pages 169–174.
Otero, A., Akinfiev, T., Fern´andez, R., and Palacios, F.
(2010). A device for automatically measuring and su-
pervising the critical care patients urine output. Sen-
sors, 10(1):934–951.
BIODEVICES 2011 - International Conference on Biomedical Electronics and Devices
12

Otero, A., F´elix, P., Barro, S., and Palacios, F. (2009b). Ad-
dressing the flaws of current critical alarms: a fuzzy
constraint satisfaction approach. Artificial Intelligence
in Medicine, 47(3):219–238.
Otero, A., Panigrahi, B., Palacios, F., Akinfiev, T., and
Fern´andez, R. (2009c). Recent Advances in Biomed-
ical Engineering, chapter A prototype device to mea-
sure and supervise urine output of critical patients,
pages 321–324. Intech.
Rivers, E., Nguyen, B., e Havstad, S., Ressler, J., Muzzin,
A., Knoblich, B., Peterson, E., Tomlanovich, M., and
Group, E. G.-D. T. C. (2001). Early goal-directed ther-
apy in the treatment of severe sepsis and septic shock.
New England Journal of Medicine, 345:1368–1377.
Zadeh, L. (1975). The concept of a linguistic variable and
its application to approximate reasoning. Information
Science, 8:199–249. Part 1.
URINE OUTPUT MONITORING - A Simple and Reliable Device for Monitoring Critical Patients' Urine Output
13
