
PORTABLE INSTRUMENTATION PLATFORM
FOR ECL-BASED SENSORS AND BIOSENSORS
A. J. Palma, M. A. Carvajal, N. Lopez-Ruiz
ECSens, Department of Electronics and Computer Technology, University of Granada, E-18071 Granada, Spain
J. Ballesta-Claver, M. C. Valencia-Miron, L. F Capitan-Vallvey
ECSens, Department of Analytical Chemistry, University of Granada, E-18071 Granada, Spain
Keywords: Electrochemiluminescence, Portable instrumentation, Photodiode, Luminol, Ruthenium complexes, Screen-
printed electrode.
Abstract: A new portable instrumentation platform for electrochemiluminescence (ECL)-based disposable sensors and
biosensors is described. The reader unit consists of a potentiostat and a photodiode as light-to-current
converter integrated in the same instrument. To check the performance of the instrument as sensors
platform, two transduction chemistries (luminol and tris(2,2’bipyridyl)ruthenium(II)) and two widely used
analytes (hydrogen peroxide and triethylamine) were selected. Additionally, different working modes have
been implemented in the instrument: chronoamperometry and cyclic voltammetry. The calibration functions
obtained show linear dependences with dynamic ranges from 0.01 to 0.07 mg·l
-1
for H
2
O
2
, 0.05 – 10.0 mg·l
-
1
for triethylamine with detection limits of 0.01 mg·l
-1
for H
2
O
2
and 0.03 mg·l
-1
for triethylamine and a
sensor-to-sensor reproducibility (relative standard deviation RSD) around 8.2 % and 3.1 %, respectively at
the medium level of the range.
1 INTRODUCTION
Different techniques have been developed in order to
measure the light resulting from electroluminescence
reactions. Those processes are commonly controlled
by a potentiostat or an amperometric unit (Gautron
et al., 1980; Zheng et al., 2001) which establish
potential differences or electric current flow between
the electrodes of the cell, respectively. When there
are no requirements regarding the size or the power
supply needed for the design, a good solution to
collect the weak photons generated by the ECL
reaction is the use of a photomultiplier tube (PMT)
(Zhou et al., 2004). These photodetectors need a
very high voltage supply, which can reach thousands
of volts. This fact, together with its usually bulky
dimensions, makes the PMT a non convenient
device for the development of portable
instrumentation. Another technique for the detection
of the luminescence that can be found in the
literature is the use of CCD devices, such as CCD
cameras and detectors (Momeni et al., 1999). These
devices have the main disadvantage that for
achieving good resolution they need working
temperature of tens of Celsius degrees below 0º, as
well as, the complexity of its use and processing.
The use of organic and solid-state photodiodes as
photodetectors for the registration of the ECL
radiation is also well stated in the literature (Hemmi
et al., 1995; Hofmann et al., 2005). Photodiodes are
devices of small dimensions with constant
improvements of their features as optical detectors,
thus being easily integrated in a measurement
system. The sensitivity of these photodetectors
depends on the inverse polarization applied to them,
which can vary from few volts to hundreds of volts,
in the case of avalanche photodiodes. Even in the
shot-circuit configuration, this optoelectronic
devices presents very good performace without
requering support polarization circuitry. Therefore,
photodiodes are an appropriate solution for the
development of portable instrumentation to measure
luminescence from ECL reactions, which is the goal
of this work.
The portable instrumentation, here developed,
tries to be a generic electronic platform for the
319
Palma A., Carvajal M., Lopez-Ruiz N., Ballesta-Claver J., Valencia-Miron M. and Capitan-Vallvey L..
PORTABLE INSTRUMENTATION PLATFORM FOR ECL-BASED SENSORS AND BIOSENSORS .
DOI: 10.5220/0003132803190322
In Proceedings of the International Conference on Biomedical Electronics and Devices (BIODEVICES-2011), pages 319-322
ISBN: 978-989-8425-37-9
Copyright
c
2011 SCITEPRESS (Science and Technology Publications, Lda.)

bionanalytes determination using biosensors. This
will be achieved with the inclusion, in a unique
instrument, of the photodiode, the potentiostat and
the reconfigurable electronics which adapts the
analog processing to the ECL signal. Main
advantages of our design lie on portability, low cost
because of the use of a photodiode instead of a
costly or bulky photomultiplier, and the use of a few
microliters of sample analysis.
2 MATERIALS
The disposable cell consists of a screen printed three
electrodes cell where we can found a round-shaped
graphite working electrode, a graphite counter
electrode and a silver pseudo-reference electrode.
The screen-printed electrode was covered by a thick
overlapping plastic layer with a 50-μl volume hole
in the electrode area to place the sample. The
sensing layer was cover by two types of solutions,
one formed of luminol dissolved in 0.25 M NaCl
and pH 9.0 phosphate buffer 0.2 M with H
2
O
2
as
analyte and another for luminophore Ru(bpy)
3
2+
with
0.25 M NaCl, pH 8.5 Tris buffer 0.2 M and
triethylamine (TEA) as analyte.
3 PORTABLE INSTRUMENT
DESCRIPTION
The system is composed by a photodiode with an
operational amplifier integrated in the same chip, an
electronic amplification stage, a potentiostat and a
microcontroller that controls all elements (Figure 1).
In this instrument, the PIC18F2553 of Michochip
Technologies (USA) has been chosen.
3.1 Instrument Overview
In order to avoid the external illumination
interferences, the electrode is placed into a dark little
drawer attached to the instrument housing. The
output current of the photodiode needs to be
converted into voltage by an I/V amplifier. This
converter can be included into the photodiode case,
in the same chip, or can be external. Before
amplification, the output voltage of the I/V converter
was filtered in order to reduce the electrical grid
interference (Figure 1). The filtered output is
amplified by a Programmable Gain Amplifier
(PGA). The gain of this amplifier can be configured
by the microcontroller, and as consequence, the
dynamic range can be adapted depending on the
emission intensity of the ECL reaction. We used the
PGA103 of Texas Instruments with 1, 10 and 100
gain factors that can be selected easily. Finally the
results are storage in an EEPROM memory.
Microcontroller
ADC
EEPROM
memory
I2C
PGA
Low pass
filter
Electrode
Low pass
filter
Photodiode and
I/V converter
Feedback
resistor net
DAC
Potentiostat
Amplification and
conditioning
circuit
Figure 1: Block diagram of the luminometer.
The potentiostat is the electronics in charge of
starting the ECL reaction by applying voltage
pulses. Basically, it consists of a Digital-to-Analog-
Converter (DAC) and analog circuitry to amplify
and to shift the voltage output. In section 3.2 the
pulse configuration will be described in detail.
Finally, the presented luminometer in this work can
be controlled via USB by a computer. An ad hoc
software application has been developed for
allowing the remote configuration and downloading
of results.
3.2 Selection of the Photodiode
We studied two photodiodes without an integrated
I/V converter: S1227-66BR and S1227-1010R
(Hamamatsu, Japan), with an active area of 33 and
100 mm
2
respectively. To achieve a gain factor high
enough, a T net resistor was used as the feedback
converter resulting in an effective resistance of 5.2
GΩ. Others three photodiodes with a built-in I/V
amplifier were tested: the S9269 and S9270
(Hamamatsu, Japan), with an active area of 33 and
100 mm
2
respectively; and the OPT301 (Texas
Instruments, USA), with an area of 5.2 mm
2
. The
S9269 and the S9270 have an internal resistance of
1GΩ and the OPT301 internal resistance is 1MΩ. In
the last one, an additional external T net resistance
was added to reach a value of 5.2 GΩ. The output
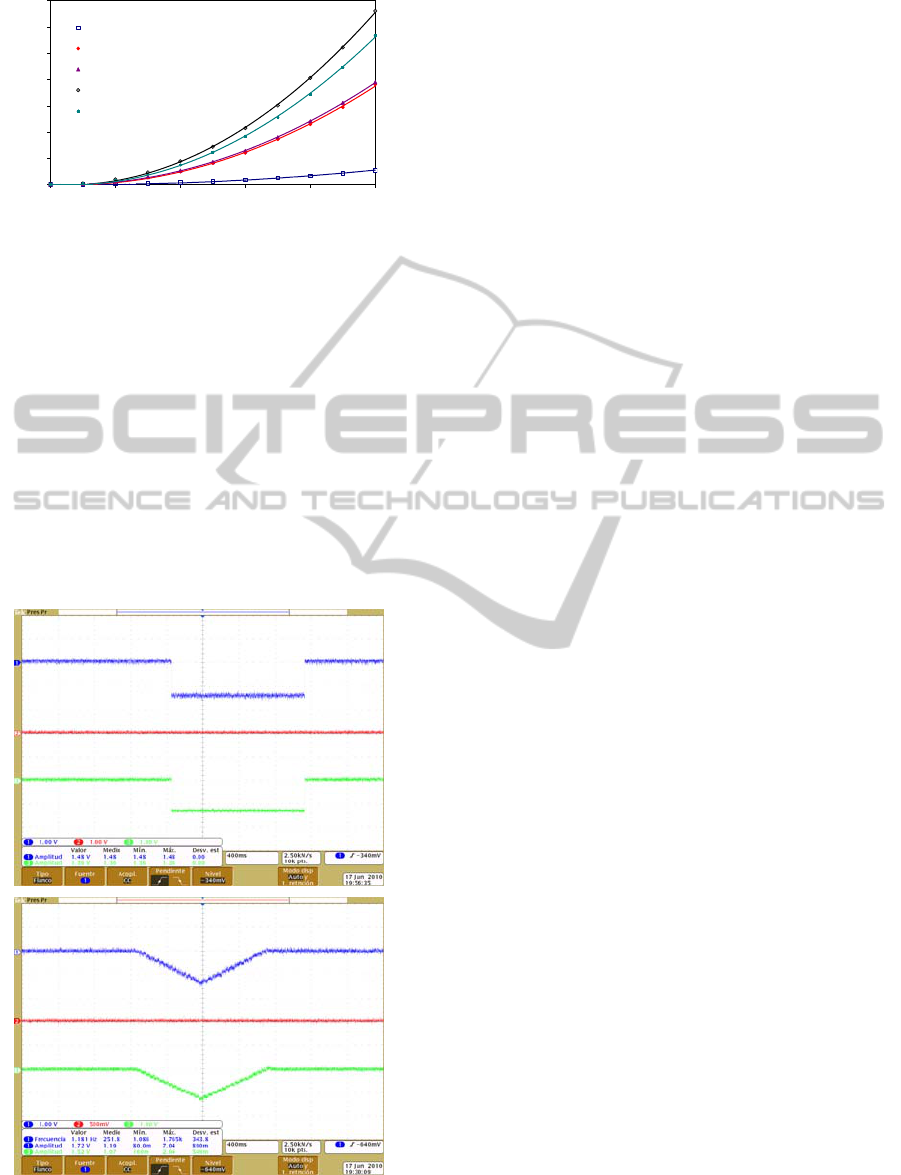
currents of the different photodiodes are plotted in
the Figure 2.
As expected, the most sensitive photodiodes
were the S9270 and the S1228-1010BR due to their
bigger sensitive area. We selected the most sensitive
photodiode, the S9270. In addition, this device
includes a built-in I/V converter, showing better
interference immunity.
BIODEVICES 2011 - International Conference on Biomedical Electronics and Devices
320
0
10
20
30
40
50
60
70
0 20406080100
I
LED
(nA)
I
0
(pA)
OPT301
S1227-66BR
S9269
S9270
S1227-1010BR
Figure 2: Output current of the studied photodiodes.
3.3 Potentiostat
Potentiostat of this instrument is based on a previous
design (Martinez-Olmos et al., 2009) with some
modifications. A reference voltage and bipolar
power supply in order to produce negative and
bipolar pulses were added. Moreover, an additional
resistor has been included between the
complementary electrode (CE) and reference
electrode (RE) to avoid saturation of CE operational
amplifier when the solution is not deposited on the
screen-printed electrode. In this work, we have used
mono-polar pulses in chronoamperometric form for
Ru(bpy)
3
2+
and TEA ECL determination, and a
Figure 3: Output voltages of the potentiostat: RE in blue,
WE in red, and CE in green. (a) Monopolar pulse. (b)
Cyclic voltammetry.
cyclic voltammetry mode for luminol and H
2
O
2
. In
the Figure 3, some voltage waveforms of the
Working-Electrode (WE), Reference-Electrode and
the Complementary-Electrode are plotted.
The instrument can configure the applied pulses
by varying the maximum voltage value (positive and
negative), the rise and fall times.
4 EXPERIMENTAL RESULTS
4.1 Measurement Conditions
The instrumentation is equipped with the
electrochemical tools for the different types of ECL
analytical signals. The intensity of the pulse of the
collected light shows a direct relationship with
peroxide or triethylamine concentration. However, it
is necessary to study several pulses in time of the
same concentration to see the profile of the obtained
peaks to discriminate and eliminate the intensity of
the blank signals or by the necessity to obtain
reproducible emission peaks. In the case of
ruthenium complex system, the signal is obtained
working at chronoamperometric mode generating 10
pulses. The measurement conditions were: a) applied
potential (1.3 V); b) time between pulses, being 30 s
for better sensitivity; c) pulse time with 1.5 s. The
analytical signal was the average of the last four
pulses due to blank signal generates overlapping
intensity peaks in the first pulses.
For the luminol system, the ECL signal is more
reproducible if we use a fast cyclic voltammetry. For
that purpose, we generate 4 pulses configured as
follows: a) voltage range: from 0 to 1 V; b) time
between pulses: 30 s; c) rise time: 3s; d) fall time:
3s. The analytical signal was the different pulse
maxima for each peroxide concentration. The best
analytical parameter corresponds to the maximum of
the third pulse. The sample volume in the screen-
printed electrode was studied, selecting 50 µl as
optimum volume.
4.2 Analytical Parameters
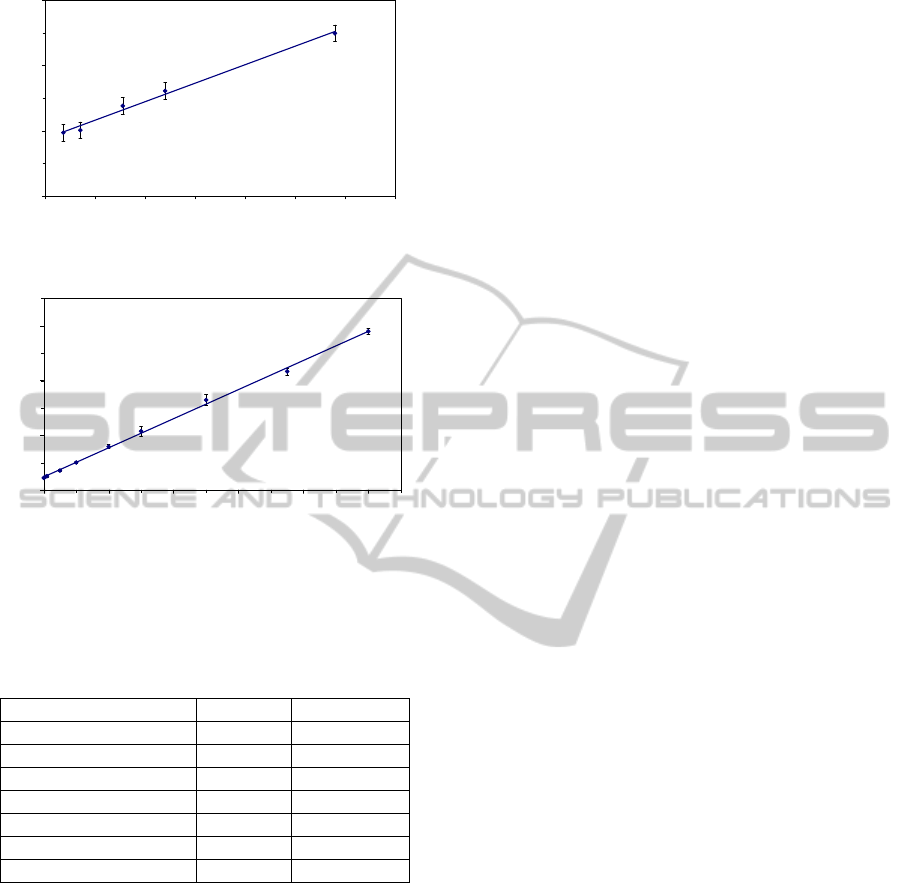
For the evaluation of the presented luminometer,
two calibrations were performed with the two
chemistries involved in this work. For H
2
O
2
determination we obtain a linear response from 0.01
to 0.07 mg·l
-1
using 178 mg·l
-1
of luminol solution in
all cases (Figure 4). In the case of TEA
determination, we obtain a linear calibration from
0.05 to 10 mg·l
-1
using a 374 mg·l
-1
concentration of
the luminophore Ru(bpy)
3
2+
(Figure 5).
(
a
)
(
b
)
PORTABLE INSTRUMENTATION PLATFORM FOR ECL-BASED SENSORS AND BIOSENSORS
321
0.0
4.0
8.0
12.0
0.01 0.02 0.03 0.04 0.05 0.06 0.07 0.08
H
2
O
2
(mg·l
-1
)
Li
g
ht
i
n
t
ens
it
y
(
a.u.
)
Figure 4: H
2
O
2
calibration curve.
0
200
400
600
0246810
TEA (mg l
-1
)
gy()
Figure 5: TEA calibration curve.
As we can see in Table 1, the analytical
parameters show a good linearity in both cases
Table 1: Analytical characteristics.
Parameter TEA
H
2
O
2
Linear range (mg·l
-1
) 0.05 – 10.0 0.01 – 0.07
Intercept (a.u.) 47.2 2.4
Slope (l·a.u.·mg
-1
) 57.3 113.1
r
2
0.999 0.992
Detection limit (mg·l
-1
) 0.03
0.01
RSD blank (%) 1.4 % 4.2 %
RSD sample
*
(%) 3.1 % 8.2 %
*
In the middle of the linear range
5 CONCLUSIONS
A novel portable instrumentation based on a
photodiode and a simple potentiostat is presented,
suitable for ECL measurements. In the same
instrument, is possible to work in
chronoamperometric and cyclic voltammetry modes.
This paper represents a first step in this research and
further work will be done to test it for biological
fluids such as uric acid and cholesterol.
ACKNOWLEDGEMENTS
This work has been funded by Junta de Andalucía,
Spain (Projects P09-FQM-5341, P08-FQM-3535).
REFERENCES
Gautron J., Dalbera J. P., Lemasson P., 1980.
Electroluminescence of the electrolyte/n-zinc selenide
junction under anodic polarization. Surf.Sci. 99, 300-
308.
Zheng, X., Zhang, Z., Li, B., 2001. Flow injection
chemiluminescence determination of captopril with in
situ electrogenerated Mn3+ as the oxidant.
Electroanalysis 13, 1046-1050
Zhou, H., Zhang, Z., He, D., Hu, Y., Huang, Y., Chen, D.,
2004. Flow chemiluminescence sensor for
determination of clenbuterol based on molecularly
imprinted polymer. Anal.Chim.Acta. 523, 237-242.
Momeni, N., Ramanathan, K., Larsson, P. O., Danielsson,
B., Bengmark, S., Khayyami, M, 1999. CCD-camera
based capillary chemiluminescent detection of retinol
binding protein. Anal.Chim.Acta. 387, 21-27.
Hemmi, A., Yagiuda, K., Funazaki, N., Ito, S., Asano, Y.,
Imato, T., Hayashi, K., Karube, I., 1995. Development
of a chemiluminescence detector with photodiode
detection for flow-injection analysis and its application
to L-lactate analysis. Anal.Chim.Acta. 316, 323-327.
Hofmann, O., Miller, P., Sullivan, P., Jones, T. S., de
Mello, J. C., Bradley, D. D. C., de Mello, A. J., 2005.
Thin-film organic photodiodes as integrated detectors
for microscale chemiluminescence assays.
Sens.Actuators, B. B106, 878-884.
Martínez-Olmos A., Ballesta-Claver J., Palma A.J.,
Valencia-Mirón M.C. and Capitán-Vallvey L.F., 2009
A Portable Luminometer with a Disposable
Electrochemiluminescent Biosensor for Lactate
Determination Sensors 9 7694-7710.
BIODEVICES 2011 - International Conference on Biomedical Electronics and Devices
322
