
GLYCAEMIA REGULATION PREDICTIVE CONTROL SYSTEMS
PERFORMANCES EVALUATION
A Comparative Study of Neural and Mathematical Models
Nathana¨el Cottin, Olivier Grunder and Abdellah ElMoudni
Laboratoire Syst`emes et Transports, Universit´e de Technologie de Belfort Montb´eliard, rue Thierry Mieg, Belfort, France
Keywords:
Diabetes, Glycaemia regulation, Insulin, NPC, Artificial neural network, Resilient propagation.
Abstract:
Type 1 blood glucose regulation remains a complex problem to simulate. Different blood glucose control
schemes for insulin-dependent diabetes therapies and systems have been proposed in the literature. This arti-
cle presents an adaptative predictive control system for glycaemia regulation based on feedforward Artificial
Neural Networks trained with the resilient propagation (RPROP) method. Experiments performed on a math-
ematical (theoretical) compensation model and our system aim to objectively compare the behaviour of each
approach when both exact and perturbated data are presented. These experiments, which make use of a virtual
patient, not only cover the ANN’s best configuration and training parameters on exact training information,
they also demonstrate the accuracy of the neural approach when up to 20% perturbated data are supplied. As
a result of the experiments on perturbated data, the neural approach gives slightly better evaluations than the
theoretical model. This demonstrates the neural system’s ability to adapt to perturbated environments.
1 INTRODUCTION
Type 1 diabetics suffer from insulin-dependent dia-
betes chronic disorders. Their pancreatic β-cells do
not secrete sufficient insulin, which mostly results in
hyperglycaemia states. Although many researches
have been conducted in the past decades, diabetics
still need practical daily solutions to help them reg-
ulate they blood glucose concentration (BGC).
This work is centered on the presentation of an
adaptive Neural Predictive Control (NPC) system
used to infer a particular patient’s blood glucose regu-
lation metabolism. This system is validated by means
of experimental comparisons against the theoretically
proven closed-loop control mathematical model pro-
posed by (Charpentier et al., 2005) used for blood
glucose regulation. Not only exact raw information
is used to evaluate accuracy of the experimented sys-
tems, up to 20% perturbated raw and test information
allows to verify the adaptability of our NPC approach.
Compared with other works, the proposed system
is designed to adapt to numerous blood glucose reg-
ulation techniques by means of pluggable modules
while conforming to a common regulation process.
This article is organized as follows: next section
presents some related works on closed-loop control
using mathematical models and Artificial Neural Net-
works (ANN) to position the context of this paper.
Section 3 introduces a Global Predictive Control Sys-
tem (GPCS) used for blood glucose regulation and its
derived systems which model the theoretical and neu-
ral approaches to be compared. Next section focuses
on practical experiments results based on a virtual pa-
tient with both exact and perturbated data. Conclu-
sions draw the benefits and drawbacks of each ap-
proach and present some future works.
2 PREDICTIVE CONTROL
SYSTEMS ARCHITECTURES
2.1 Global PCS
Regulating glycaemia aims to fulfill patients with ei-
ther extra glucose or external insulin measurements.
Regulation is handled by a Global Predictive Control
System (GPCS) which takes the following parameters
into account:
• Some patient’s physical information, such as his
age and weight (i.e. miscellaneous parameters);
• A meal identifier, used by the internal regulation
525
Cottin N., Grunder O. and ElMoudni A..
GLYCAEMIA REGULATION PREDICTIVE CONTROL SYSTEMS PERFORMANCES EVALUATION - A Comparative Study of Neural and Mathematical
Models.
DOI: 10.5220/0003138805250528
In Proceedings of the International Conference on Health Informatics (HEALTHINF-2011), pages 525-528
ISBN: 978-989-8425-34-8
Copyright
c
2011 SCITEPRESS (Science and Technology Publications, Lda.)

module to perform initial tasks, before the regula-
tion process starts;
• The glycaemia value before taking the meal (i.e.
pre-meal glycaemia);
• The amount of carbohydrates the patient wishes
to ingest (i.e. initial meal carbohydrates);
• The expected glycaemia value the patient needs
to reach after having ingested the meal carbohy-
drates, assuming that short-term insulin has fully
taken effect (i.e. expected post-meal glycaemia).
As a result, the system estimates the final gly-
caemia (i.e. evaluated post-meal glycaemia) as close
as possible to the patient’s expected glycaemia value.
It jointly suggests a (possibly null) short-term insulin
measurement to absorb the meal carbohydrates (in
case of hyperglycaemia) and the total amount of car-
bohydrates the patient should ingest. In case an in-
sulin measurement is predicted, this amount of carbo-
hydrates equals the initial meal carbohydrates value.
This makes sure that extra carbohydrates and insulin
measurements are mutually exclusive results.
2.2 Specializations
To operate glycaemia regulation, the global system
can be specialized in two PCS classes:
• Theoretical PCS (TPCS) which directly imple-
ment mathematical models for glycaemia regula-
tion, without any regulation control loop;
• Commanded PCS (CPCS) which operate by
means of an internal control-command compen-
sation loop to suggest insulin and carbohydrates.
The CPCS overall regulation principle is depicted
by figure 1. A CPCS relies on two key components:
• A predictor, which estimates a glycaemia positive
or negative deviation given carbohydrates and in-
sulin values. It may also take into account some
patient’s miscellanous information to produce a
satisfactory glycaemia prediction;
• An adaptive controller, which modifies the cur-
rent insulin or carbohydrates values based on a
predicted glycaemia. The controller also deter-
mines the control loop stop condition.
The controller uses initial meal carbohydrates
once. This value is then replaced with the regulated
value (although the controller may have keep this ini-
tial value in case it regulates insulin).
3 EXPERIMENTS
This study compares the behaviour and the quality of
the compensation results of an NPC CPCS (i.e. neural
CPCS) against a TPCS. The TPCS is assumed to pro-
vide exact results: a vitual patient, that strictly con-
forms to the mathematical model implemented in the
TPCS, is used to train the neural CPCS.
Then, some perturbations using a normal distribu-
tion are added both on training and test data. This
implies that the TPCS produces errors compared with
the exact data (without perturbations).
Experimentsaredriven on a virtualpatient on both ex-
act and perturbated raw information. This virtual pa-
tient behaves like the theoretical compensation model
proposed by (Charpentier et al., 2005) in case of ex-
act raw information. Use of this virtual patient allows
to determine the neural approach accuracy against the
theoretical model. Indeed, two PCS are compared:
• A theoretical PCS (TPCS), which implements the
mathematical compensation model of the virtual
patient. This PCS provides exact results when ex-
act source raw information is used, which means
that no deviation is produced;
• A neural CPCS, which integrates a neural predic-
tor based on a n− n− 1 (n refers to the number of
inputs of this predictor, conforming to figure 1)
feedforward ANN (Mhaskar, 2005). Input and
hidden layers use the sigmoid activation function
and the output layer uses the hyperbolic tangent
activation function (to represent a signed devia-
tion). This neural CPCS approximates compensa-
tion values based on the function the ANN infers.
Deviations between PCS suggestions and exact
expected compensation values are expressed as fol-
lows:
• Carbohydrates deviations are expressed in grams;
• Short-term insulin deviations are expressed by a
number of unitary doses.
3.1 Neural CPCS Training
The neural predictor of the neural CPCS is trained
from known raw information using supervised train-
ing techniques. This set of raw information is ran-
domly generated, though conforming to the mathe-
matical model of (Charpentier et al., 2005).
A raw information item is basically composed of
the GPCS inputs and outputs values. However, the
final post-meal glycaemia value does not correspond
to the neural PCS estimated glycaemia but refers to a
physical measurement (performed by the patient him-
self or by a medical assistant).
HEALTHINF 2011 - International Conference on Health Informatics
526
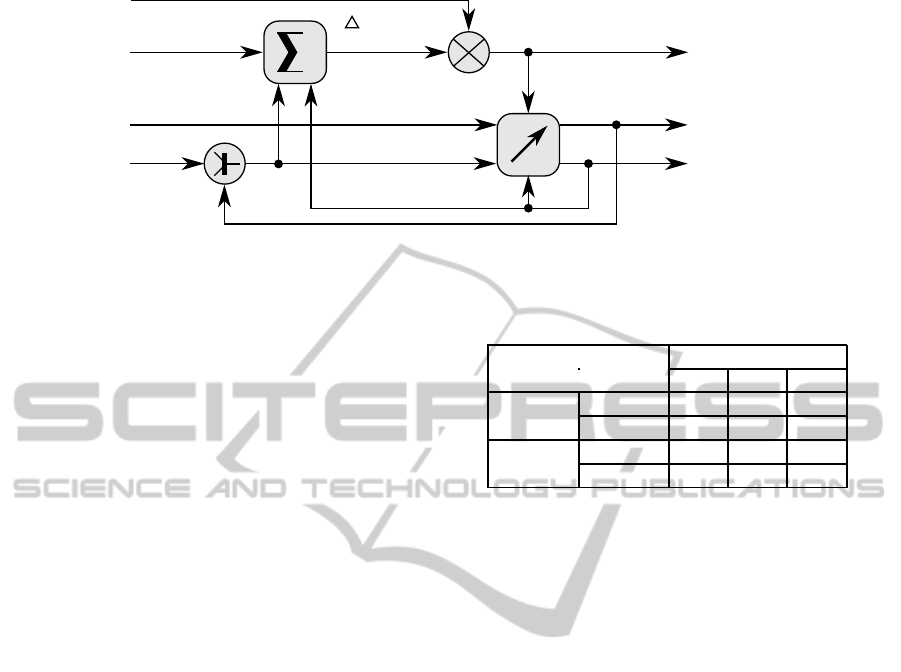
Meal
carbohydrates
Miscellaneous
parameters
(age, weight, ...)
Insulin
Carbohydrates (incl.
extra carbohydrates)
Controller
Predicted
glycaemia
+
+
=
Predictor
Initial
glycaemia
Final glycaemia
Predicted
glycaemia
Expected
glycaemia
Carbohydrates
Insulin
Figure 1: CPCS regulation principle.
Experiments indicate that the best configuration
for the ANN is obtained for 100 training data and
3000 training epochs. Other experiments (not men-
tioned here) prove that using more than 200 training
information does not significantly lower the average
and maximum deviations indices. On the contrary,
a regression phenomenon is observed (i.e. the car-
bohydrates and short-term insulin deviations become
larger when more than 200 training information items
are used).
Given a meal identifier, the corresponding ANN
is trained with the selected raw information items,
whose meal identifier matches the given identi-
fier. The “resilient propagation” training algorithm
(RPROP) with default parameter values suggested
by (Riedmiller and Braun, 1993) is used.
One could have experimented with “well-chosen”
raw information (i.e. deterministic raw information)
which cover a wide range of values and provide all
compensation cases, but this would have two main
drawbacks:
• The experimental results would be slanted as the
ANN would give insignifiant deviation values;
• This case is definitely unrealistic: a patient will
never cover the whole range of possible val-
ues for all parameters (i.e. pre-glycaemia, post-
glycaemia and meal carbohydrates in particular).
3.2 Results Review on Exact Data
The results of this comparison are gathered in table 1.
This table indicates average and maximum regulation
glucose and compensation short-term insulin devia-
tions from exact measurements provided by the math-
ematical model. The neural PCS is trained with ran-
domized exact information.
This table demonstrates that 100 training informa-
tion items lead to the lowest short-term insulin aver-
age and maximum deviations (of 0.05 and 0.17 re-
spectively). The maximum carbohydrates deviation
of 1g is not significant and does not reflect upon the
Table 1: Neural CPCS deviations from expected compensa-
tions using exact training data and 3000 epochs.
Neural PCS Training data
deviations 50 100 200
Carbo- Average 0 0 0
hydrates Max 0 1 0
Insulin
Average 0.18 0.05 0.11
Max 0.56 0.17 0.37
accuracy of the measurements. The widest insulin
deviation obtained for 50 training data reflects the
lack of information to train from. Use of 200 train-
ing data provides still acceptable measurements (i.e.
below 0.5 doses) but a (well-known) regression phe-
nomenon starts to become visible.
3.3 Support for Perturbated Data
Previous experiments were based on exact raw infor-
mation to train the ANN and validate the neural PCS
behaviour. In order to ensure the validity of the neural
PCS in a “real” environment, we use perturbated raw
information for training to simulate devices measures
deviations, patients reading errors and instable sensi-
tivity to insulin deseases. Perturbations apply to pre-
meal and post-meal glycaemia as well as meal carbo-
hydrates on each raw information item. Each pertur-
bation follows to a normal distribution centered on 0
with a 20% deviation maximum and a 10% average
of the initial (i.e. exact) value. This means that each
initial (i.e. exact) value can be modified up to ±20%.
Table 2 gathers theoretical and neural PCS be-
haviours in the presence of perturbated information.
The set of representative initial (exact) values being
perturbated, the TPCS produces deviations from the
exact measurements. The neural CPCS uses pertur-
bated training information randomly generated. Each
deviation is calculated from the exact initial values.
As table 2 states, both theoretical and neural PCS
show they limits as some predicted measurements are
unacceptable. This is due to the fact that perturbations
GLYCAEMIA REGULATION PREDICTIVE CONTROL SYSTEMS PERFORMANCES EVALUATION - A
Comparative Study of Neural and Mathematical Models
527

Table 2: Deviations for theoretical and neural PCS from ex-
pected compensations (with original non perturbated data)
using perturbated training data and 3000 epochs.
TPCS and neural Th. Training data
CPCS deviations PCS 50 100 200
Carbo- Average 0 0 0 0
hydrates Max 2 4 4 4
Insulin
Average 1.12 1.10 0.95 1.10
Max 3.29 3.22 1.84 3.20
on initial values introduce a dynamic modification of
the initial sensitivity to insulin which cannot be mod-
elled by the TPCS: the mathematical formulaes lead
to generate errors proportional to the deviation intro-
duced by the perturbation. On the contrary, the neural
CPCS, thanks to its neural predictor, demonstrates its
ability to suggest more acceptable evaluations.
Once again, the neural CPCS trained with 100
data seems to provide the best measurements: in this
case, the maximum insulin deviation is divided by
a factor of 1.8 between the TPCS and the neural
CPCS. The results of table 2 demonstrate that the neu-
ral CPCS suggests either comparable or more accu-
rate values than the TPCS. The neural CPCS is able
to genealize an average compensation function from
perturbated training data and give good approxima-
tions, whereas the TPCS is more rigid. The neural
CPCS ability to approximate the compensation func-
tion even when perturbated data are used is mostly
due to the training process tuning (in terms of num-
ber of training data and maximum number of epochs)
which reduces the noise generated by perturbations
on training data.
4 CONCLUSIONS
An adaptive GPCS has been proposed. Theoretical
and neural variants of this global system (TPCS and
neural CPCS, respectively) have been presented and
compared using both exact and up to 20% perturbated
data. Each datum is randomly generated, though con-
forming to the theoretical model proposed by (Char-
pentier et al., 2005). As a result, the neural approach
appeared to be accurate and adaptable to each pa-
tient’s reaction to insulin and glucose. This study
highlights that NPC techniques provide similar mea-
surement to empirical models and are able to handle
perturbations. The main drawback of NPC lies in the
amount of training data required to perform an ac-
ceptable training. This can be problematic when the
system does not have access to enough information.
Use of predetermined ANN based on a type 1 diabetes
database is investigated.
Compared with other type 1 diabetes closed-loop
control systems in the literature (Takahashi et al.,
2008), the Global PCS architecture is highly modu-
lar thanks to programming interfaces which allow to
define its effective behaviour.
Although this study demonstrated that the neu-
ral predictor provides acceptable glycaemia devia-
tions, complementary work is still necessary to eval-
uate other ANN configurations and activation func-
tions. The neural CPCS can be used “as is”. How-
ever, further testing is needed in case of meals recov-
ery (which occurs when a patient eats while the previ-
ous meal short-term insulin has not fully taken effect).
Long-term insulin regulation is currently left for fu-
ture works as the corresponding controlled and math-
ematical models are much more complex due to the
combined short-term and long-term insulin effects.
Future works will also concern the definition of train-
ing data selection heuristics. These heuristics will be
used to retain the most relevant training information
and leave unnecessary information out.
Finally, similar experiments could also be pro-
posed on systems which make use of Support Vector
Machine (SVN) techniques in place of ANN.
ACKNOWLEDGEMENTS
Jointly to the University of Technology of Belfort-
Montbeliard (UTBM), France, this work is supported
by the European Center for Diabetes Studies, Stras-
bourg, France and The ACTIMAGE Group, France.
REFERENCES
Charpentier, G., Virally, M., Varroud-Vial, M., Hochberg,
G., Riveline, J.-P., Stevenin, C., Lejeune, M., Re-
queda, E., and Benoit, A. (2005). Libert´e Alimentaire
et Diab`ete. Centre Hospitalier Sud Francilien.
Mhaskar, H. (2005). Approximation properties of a mul-
tilayered feedforward artificial neural network. Ad-
vances in Computational Mathematics, 1(1):61–80.
Riedmiller, M. and Braun, H. (1993). A direct adapta-
tive method for faster backpropagation learning: The
RPROP algorithm. In ICNN’93, IEEE International
Conference on Neural Networks, pages 563–568.
Takahashi, D., Xiao, Y., and Hu, F. (2008). A survey of
insulin-dependent diabetes-part ii: control methods.
International Journal of Telemedicine and Applica-
tions, 2008:1–14.
HEALTHINF 2011 - International Conference on Health Informatics
528
