
USING GRANGER CAUSALITY TO CHARACTERISE
BIDIRECTIONAL INTERACTIONS IN THE HUMAN BRAIN
DURING INDUCTION OF ANAESTHESIA
Nicoletta Nicolaou, Julius Georgiou
Holistic Electronics Research Lab, KIOS, Dept. of Electrical and Computer Engineering
University of Cyprus, Kallipoleos 75, 1678 Nicosia, Cyprus
Saverios Houris, Pandelitsa Alexandrou
Anaesthesiology Department, Nicosia General Hospital, 215 Old Nicosia-Limassol Rd, 2029 Nicosia, Cyprus
Keywords: Anaesthesia monitoring, Electroencephalogram, Granger causality, Synchronisation, Bidirectional
interaction.
Abstract: General anaesthesia is a reversible state whereby conscious experience is disrupted and reflexes to afferent
stimuli are depressed. The precise method of action of anaesthetic agents is still largely unknown. However,
the administration of anaesthetics causes observable changes in the electrical brain activity (EEG), the study
of which can provide an insight into the mechanism of action of general anaesthesia. This paper investigates
the patterns of bidirectional interactions that are manifest in brain activity during anaesthetic induction with
propofol. Granger Causality is applied to the EEG of patients scheduled for surgery under general
anaesthesia as a means of characterising the interactions between different brain areas prior and after the
administration of the anaesthetic agents. Strong unidirectional information flow between frontal and
posterior areas was found to occur shortly after anaesthetic induction.
1 INTRODUCTION
General anaesthesia (GA) is a reversible state of
unconsciousness and depression of reflexes to
afferent stimuli, induced by the administration of
chemical agents (Hammeroff, 2006). Desirable
supplements of it include immobility (analgesia),
loss of conscious awareness and amnesia. Since the
mechanism by which consciousness emerges is still
not fully understood, the mechanism by which
general anaesthetics prevent consciousness is also
largely unexplained. One approach to understanding
this critical mechanism is to look for invariant
changes that manifest themselves in observables of
the human brain, such as the electroencephalogram
(EEG), as patients lose and regain consciousness
under the effect of various anaesthetic agents. The
appearance of spindle-like waves and background
slow δ (1.5-3.5Hz) activity is probably the most
prominent EEG sign of GA (Bennett et al., 2009). In
general, the EEG shows signs of decreased fast
activity (α and β rhythms) and increase of the slow
and large-amplitude δ and θ components as the
depth of anaesthesia increases. In very deep
anaesthesia the EEG may develop a peculiar pattern
of activity known as burst suppression, during which
alternating periods of normal to high activity and
low voltage (or even isoelectricity) are observed
(Rampil, 1998).
The changes in the EEG observed under GA are
also important for monitoring the depth of
anaesthesia. Lately devices that monitor the depth of
anaesthesia are utilised during surgery to provide
additional information concerning the general state
of hypnosis of the patient, including anaesthetic
overdose or even potential regaining of
consciousness during surgery. The latter is a serious
concern as the incidence of awareness ranges from
as low as 0.11% for general surgery (Ranta, 2002),
up to an astonishing 20% for trauma surgery (Myles
et al., 2003). The rates of awareness are affected by
a number of factors, such as the patient gender, the
type of surgery, the anaesthetic agent administered,
faults in the anaesthetic apparatus, and individual
188
Nicolaou N., Georgiou J., Houris S. and Alexandrou P..
USING GRANGER CAUSALITY TO CHARACTERISE BIDIRECTIONAL INTERACTIONS IN THE HUMAN BRAIN DURING INDUCTION OF
ANAESTHESIA.
DOI: 10.5220/0003148601880194
In Proceedings of the International Conference on Bio-inspired Systems and Signal Processing (BIOSIGNALS-2011), pages 188-194
ISBN: 978-989-8425-35-5
Copyright
c
2011 SCITEPRESS (Science and Technology Publications, Lda.)

differences in pharmacokinetics (Ranta, 2002, Myles
et al., 2003). This is one of the most distressing
aspects of surgery as in the majority of times the
patients are unable to alert the anaesthetist that they
have regained consciousness during surgery, and are
in pain, due to the routine co-administration of
neuromuscular blocking agents with the anaesthetic
agents.
The most widespread commercial monitors of
hypnosis currently in use are the BIS monitor
(Aspect Medical Systems, Natick, MA) (Sigl and
Chamoun, 1994) and Datex-Ohmeda S/5
TM
Entropy
Module (originally by Datex-Ohmeda Division,
Instrumentation Corp., Helsinki; now with GE
Healthcare) (Viertiö-Oja et al., 2004). Such devices
operate by extracting a number of features from the
EEG in order to deduce the relative depth of
hypnosis, which is then easily visualised as a
number from 0-100 (100: fully awake, 0: no
activity). Even though these devices offer additional
support to the work of the anaesthetist, they still
suffer from some important reliability issues
(Russell, 2006, Barr et al., 1999, Dahaba, 2005,
Messner et al., 2003).
The main reason behind some of the problems
faced by existing monitors can be pinpointed to the
fact that they utilise a number of empirical measures
from the EEG, which are then combined in a
proprietory way into a single number denoting the
depth of anaesthesia. The information utilized is,
thus, representative of the characteristics of the
observed activity and not of the physiological
process that occurs during anaesthesia. Strong
evidence for this provide reported incidences of
awareness despite monitors displaying adequate
depth of anaesthesia (Rampersad and Mulroy, 2005,
Mychaskiw et al., 2001), and the inability of some
monitors to distinguish between the EEG of an
anaesthetized patient and the EEG of somebody who
is asleep (Russell, 2006, Sleigh et al., 1999). The
latter is not surprising considering that sleep and
anaesthesia share some common mechanisms (Voss
and Sleigh, 2007). However, despite the large
similarities, there are fundamental differences in the
particular physiological mechanisms of the two
processes which a true monitor of anaesthetic depth
should be able to identify. Thus, a successful
monitor should extract information from the
observed activity that is representative of the deeper
interactions and which reflects the physiological
changes that occur from administration of the
anaesthetic agents as these are manifest in the
observed activity. In other words, the measures
utilised must be based on ‘neurobiologic phenomena
that represent the necessary and sufficient conditions
for consciousness in a specific individual’ (Hudetz,
2008).
In recent years it has been shown that measures
which characterise the interactions between different
brain areas can provide an insight into how
integration of information is achieved in the brain
during various cognitive tasks. One such measure is
Granger causality, a linear measure quantifying the
bidirectional interaction between two time series.
Even though Granger causality has provided useful
information from EEG activity in a number of
applications (see (Pereda et al., 2005) and references
within), it has yet to be applied in the study of
general anaesthesia. In this work, the interactions
between different brain areas during induction of
anaesthesia are investigated using Granger causality.
Anaesthetic induction is important as one can readily
study the point of loss of consciousness that occurs
from the administration of a bolus of anaesthetic
agent. Such information is important in subsequent
monitoring of anaesthetic depth and identification of
potential regaining of consciousness during surgery.
2 METHODOLOGY
2.1 Dataset
The data has been collected from 10 male patients
(mean age 34.6±18) undergoing general and
urological surgery at Nicosia General Hospital,
Cyprus. The study has been approved by the Cyprus
National Bioethics Committee and patients gave
written informed consent for their participation.
Participants were not taking any medication acting
on the central nervous system and were of normal
weight. EEG data was collected using the 24-
channel configuration of the TruScan32 system
(Deymed Diagnostic) at a sampling rate of 256Hz.
Electrodes were placed at positions Fp1, Fp2, F7,
F3, Fz, F4, F8, T3, C3, Cz, C4, T4, T5, P3, Pz, P4,
T6, O1 and O2, according to the International 10/20
system. Data was recorded with an FCz, and ground
was located on the head. No filtering was performed
during data collection. Data recording commenced
while patients were still awake prior to
administration of the anaesthetic agents, continued
through loss of consciousness, during the entire
surgery, and until patients regained consciousness at
surgery end. During the recording event markers
were manually inserted to indicate important events,
such as administration of anaesthetic agents.
USING GRANGER CAUSALITY TO CHARACTERISE BIDIRECTIONAL INTERACTIONS IN THE HUMAN
BRAIN DURING INDUCTION OF ANAESTHESIA
189

GA was administered by the anaesthetist in
charge following standard procedures. All patients
were preoxygenated prior to anaesthesia induction
with a propofol bolus. During induction boluses of
fentanyl (for analgesia) and varying quantities of
neuromuscular blocking agent (cisatracurium) were
also administered. Maintenance of GA was achieved
with a constant intravenous administration of
propofol at concentrations ranging between 20-
40ml/h, except in 1 patient were sevoflurane was
used for maintenance. Given that data collection was
performed during actual surgery, propofol
concentrations for induction varied based on specific
patient characteristics and the type of subsequent
surgery.
In these investigations we are only interested in
studying induction of anaesthesia, and subsequently
the point at which patients lose consciousness. Loss
of consciousness was defined as the point at which
the patient stopped responding verbally to
commands by the anaesthetist and occurred some
seconds after administration of the anaesthetic bolus.
2.2 Granger Causality
The investigation of causal relationships is of great
interest, particularly when dealing with
neurophysiological data. Granger Causality (GC)
has been developed explicitly tailored to allow
inferences about causality between two time series
to be made (Granger, 1969). Wiener defined
causality as: “for two simultaneously measured
signals, if one can predict the first signal better by
incorporating the past information from the second
signal than using only information from the first one,
then the second signal can be called causal to the
first one” (Wiener, 1956). This was later given a
mathematical formulation by Granger through the
use of univariate and bivariate autoregressive
models (AR). According to Granger: for two signals,
A, and B, if A is influenced by B, then the addition of
past values of B in the regression of A will improve
its prediction. This can be assessed from the
variances of the prediction errors of the fitted
univariate and bivariate AR models.
For the univariate case,
()
)()(
1
teitacta
a
P
i
ai
+−=
∑
=
(1)
()
)()(
1
teitbctb
b
P
i
bi
+−=
∑
=
(2)
where
(
)
biai
cc
are the estimated univariate AR
coefficients for the AR model of order P, and
)(
ba
ee
are the residuals (prediction errors) of the AR
process. For the bivariate AR case,
() ()
)()(
11
teitbditacta
ab
P
i
abi
P
i
abi
+−+−=
∑∑
==
(3)
() ()
)()(
11
teitaditbctb
ba
P
i
bai
P
i
bai
+−+−=
∑∑
==
(4)
where
(
)
abiabi
dc
and
)(
baab
ee
are as for the
univariate AR case. Granger Causality can then be
defined as:
2
/
2
_/
ln
ABA
AA
AB
GC
σ
σ
=
→
(5)
If by using past values of B the prediction of A is
improved, then the variance of the prediction errors
of the bivariate AR model,
()
abABA
evar
2
/
=
σ
, will
be smaller than the variance of the univariate AR
model,
(
)
aAA
evar
2
_/
=
σ
. Thus,
AB
GC
→
will
increase. If, however, the past of B does not improve
the prediction of A, then
2
/
2
_/ ABAAA
σσ
≈
, and
AB
GC
→
will be close to zero. Similarly,
BA
GC
→
is
defined accordingly. If both
AB
GC
→
and
BA
GC
→
are high, then this indicates a bidirectional coupling
or feedback relationship between A and B.
In order to characterise the direction and strength
of coupling between A and B one can look at the
difference between the GC values:
BAAB
GCGCD
→→
−
=
(6)
⎩
⎨
⎧
→<
→>
stronger is coupling ofstrength ,0
stronger is coupling ofstrength ,0
BA
AB
D
In this way, changes in the direction of coupling can
be readily identified by following the changes in the
sign of D. However, if D is close to zero, then one
can deduce that there either exists bidirectional
coupling of similar strength or no coupling at all. In
this case, one must look at the individual GC values
to identify which of the two scenarios holds.
In the following investigations AR models of
order 6 were utilised. This choice was guided both
by the literature (Tseng et al., 1995, Vaz et al., 1987)
and by preliminary investigations which showed that
the use of higher order models did not have an effect
on the results.
BIOSIGNALS 2011 - International Conference on Bio-inspired Systems and Signal Processing
190
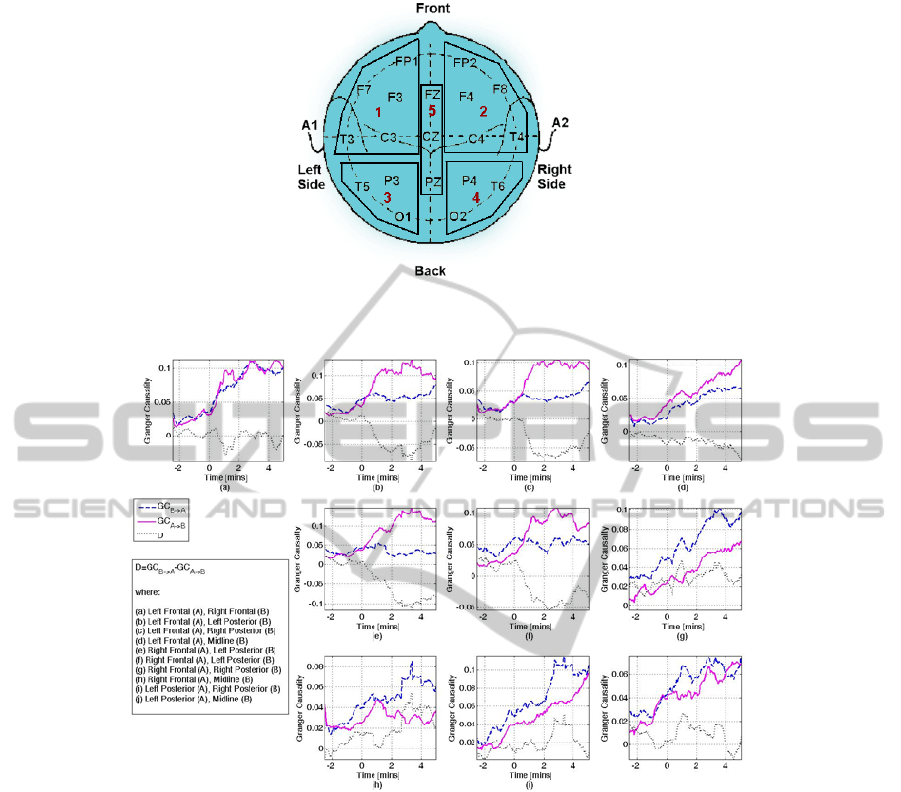
Figure 1: The five brain areas defined and the electrodes contained in each grid. The brain areas were left (grid 1) and right
(grid 2) frontal area, left (grid 3) and right (grid 4) posterior area, and midline area (grid 5).
Figure 2: This figure shows the changes in the direction of coupling, estimated as the difference between
AB
GC
→
and
BA
GC
→
. The legend indicates which area is considered as B and which as A in each of the individual plots. The x-axis
shows time in minutes, where t=0 denotes the point at which the anaesthetic agent was administered. Patient was awake for
t<0, and asleep for t>0. For example, plot (c) denotes the GC between Left Frontal (A) and Right Posterior (B). Thus, the
solid line corresponds to
riorRightPostelLeftFronta
GC
→
, the dashed line to
lLeftFrontariorRightPoste
GC
→
and the dotted line to
riorRightPostelLeftFrontalLeftFrontariorRightPoste
GCGCD
→→
−=
. In this case, a strong unidirectional coupling from the left
frontal area to the right posterior area is observed after anaesthetic induction. Note the different y-axis scales.
3 RESULTS AND DISCUSSION
To assess the interactions between different brain
areas, five electrode grids were first defined (see
figure 1). Each electrode grid represents the gross
activity from each of the following brain areas: left
and right frontal, left and right posterior, and midline
area. The activity of each of these areas is estimated
as the average activity from the electrodes contained
within the specified electrode grid. For some
subjects not all electrodes were available.
Specifically, the following electrodes were
unavailable: (1) S1: P4, T6, Pz; (2) S2: C3, O1; (3)
S3: P3, P4, T6, O2, Cz; (4) S4: T5; and (5) S5: Cz.
The EEG segments extracted were of 8-minute
duration, where 3mins are prior to and 5mins are
after induction, based on the event markers
USING GRANGER CAUSALITY TO CHARACTERISE BIDIRECTIONAL INTERACTIONS IN THE HUMAN
BRAIN DURING INDUCTION OF ANAESTHESIA
191
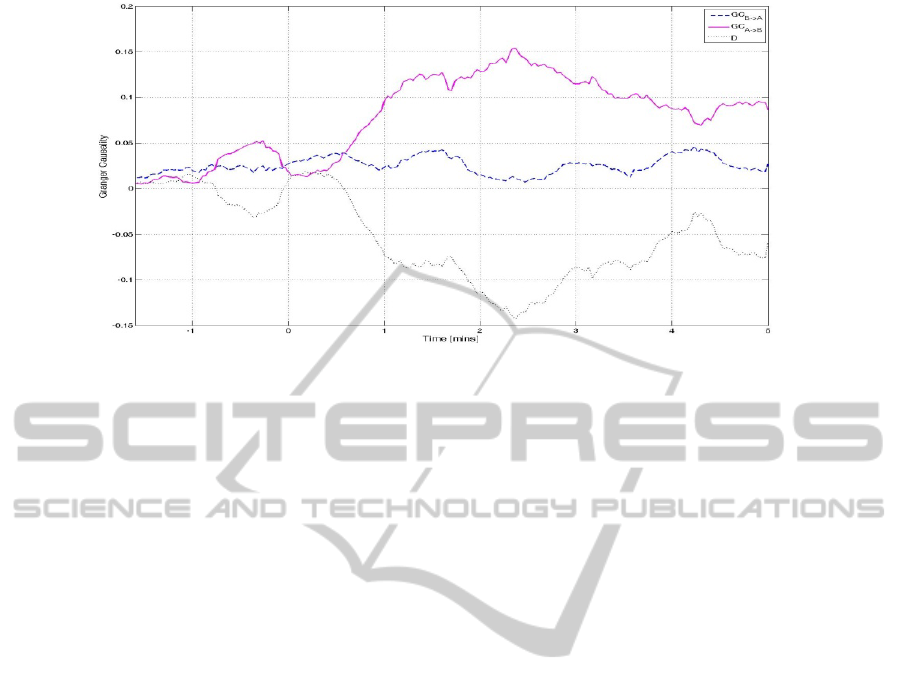
Figure 3: Granger causality between left frontal (A) and right posterior (B) for a single subject, and their difference, D, prior
and after induction of anaesthesia (at time t=0). Prior to anaesthetic induction GC indicates weak interactions between the
two areas. However, administration of anaesthesia induces strong unidirectional interaction from the left frontal to the right
posterior area. This is reflected as a change in the sign of D.
indicating administration of anaesthesia in each
patient record. Each 8-minute segment is then split
into 2-second non-overlapping windows and
Granger causality is estimated for each window. No
artefact removal has been performed as averaging
removes the effect of some artefacts present in the
data. In addition, after anaesthetic induction there is
minimal presence of artefacts as the patient is not
moving and surgery has not yet commenced.
Figure 2 shows the interactions between the
different brain areas as characterised by Granger
causality and the difference, D (equation 6). The
results shown are averaged over all subjects and a
moving average of order 20 has been applied for
better visualisation. Changes in the sign of D
indicate a change in the direction of interaction.
Whether D is positive or negative depends on which
brain area is taken as B and which area as A. Thus,
the actual sign of D is not important, but the change
in the sign is: negative values indicate a stronger
interaction from A to B, and vice versa for positive
values.
A clear change in the direction of interactions
before and after induction of anaesthesia is
observed. All changes in the GC values at pre- and
post-induction are statistically significant (ANOVA
F-test, α=0.05, p=0; except for GC from LP to RP,
where p=0.03). While the patients are awake, weak
interactions between all brain areas can be observed,
resulting into values of D around zero. The
administration of the anaesthetic agent increases the
strength of interactions in all directions, except of
interactions from posterior to all other areas, which
remain at the same level as prior to anaesthetic
induction. The most striking change related to
anaesthetic induction is the strong unidirectional
inter- and intra-hemispheric interactions from frontal
to posterior areas (fig.2 (b), (c), (e), (f)). These
interactions start occurring approximately 20-30
seconds after induction, thus there is strong reason to
hypothesize that they indicate the point of loss of
consciousness. The strongest interactions are
observed from the left and right frontal areas to the
left posterior area. Figure 3 shows the interaction
between left frontal and right posterior area for a
single subject, whereby this switch of the direction
of interaction induced by administration of the
anaesthetic agent is clear. An increase in the strength
of interaction from midline to all other areas is also
observed, indicated by the positive D values (fig.2
(d), (g), (i), (j)). Anaesthetic induction also induces
strong bidirectional intra-hemispheric frontal
interactions (fig.2 (a)), which are not mirrored in the
posterior areas (fig.2 (h)). In general, administration
of anaesthesia appears to increase information flow
from frontal to posterior areas and from midline to
all other areas.
The lack of strong unidirectional interactions
while the patient is awake is a direct reflection of the
lack of generalised ‘synchrony’, as each brain area is
involved in performing individual tasks. However,
induction of anaesthesia induces strong
unidirectional interactions. This indicates that the
brain has now entered a ‘synchronised’ state, with
frontal and midline areas in the focus. This is in
agreement with observations that anaesthetic drug
administration causes frontal predominance by
BIOSIGNALS 2011 - International Conference on Bio-inspired Systems and Signal Processing
192

increasing frontal cortical activity (Jameson and
Sloan, 2006).
Cortical sensory integration is considered as a
common mechanism of anaesthetic suppression of
conscious experience. It now seems more and more
likely that unconsciousness during anaesthesia is a
result of the brain’s inability to integrate information
(Hudetz, 2008, John and Prichep, 2005). One
possibility is that anaesthesia induces
unconsciousness through degradation of information
integration by disconnecting communication
between cortical networks. Another possibility is
that anaesthesia disrupts consciousness by putting
cortical networks in a synchronised state such that
they are no longer able to integrate incoming
information. Indeed, anaesthesia and other
consciousness-depressing mechanisms are
associated with increased cortical synchrony
(Rampil, 1998). Our observations here suggest that
Granger causality has indeed managed to capture
this shift of the brain activity to a more synchronised
state, and with decreased communication from
posterior to all other areas. Thus, Granger causality
can capture the physiological changes in the EEG
activity, which are associated with administration of
anaesthetic agents.
This work raises some additional considerations.
Firstly, an interesting observation is that the strength
of interaction appears to decrease towards baseline
some minutes after induction of anaesthesia for
interactions between the left frontal areas and other
areas, whereas the strength of interaction remains at
the same level for interactions between the right
frontal and other areas. It would be interesting to
observe longer periods after induction of anaesthesia
in order to investigate the role of each frontal
hemisphere in synchronisation during maintenance
of anaesthesia, whether this is disrupted by strong
stimuli, such as tracheal intubation, and whether the
same patterns of interactions are observed again at
the end point of anaesthesia, but in the reverse
direction. Secondly, the effect of neuromuscular
blocking agents on the EEG is still not fully
understood. Thus, it would be useful to investigate
whether the observed patterns of interaction are
similar when neuromuscular blocking agents are not
administered. Thirdly, analysis with increased
spatial resolution would allow us to identify a more
exact location of the areas that are acting as
synchronisation pacemakers. For this, Granger
causality should be estimated for smaller electrode
grids, and even for individual electrodes. However,
these are beyond the scope of this work and remain
the subject for future investigations.
Taking these additional considerations in mind,
if changes in the bidirectional interactions identified
by Granger causality could be expressed in the form
of a single number from 0-100, then it might be
possible in the future to utilise this measure to alert
the anaesthetist in cases of impending awareness
during surgery.
4 CONCLUSIONS
We have shown that Granger causality can be used
to extract information reflecting the physiological
interactions between different brain areas during
induction of general anaesthesia. A measure that can
extract the deeper interactions within the brain
through the observed EEG activity would be useful
not only for studying the physiological mechanisms
of anaesthesia, but also in a monitor of anaesthetic
depth to provide objective assessment of the state of
hypnosis of the patient.
ACKNOWLEDGEMENTS
The authors would like to thank the staff at Nicosia
General Hospital and the anonymous volunteers.
This work is part of the Cyprus Research Promotion
Foundation’s Framework Programme for Research,
Technological Development and Innovation 2008
(DESMI 2008), co-funded by the Republic of
Cyprus and the European Regional Development
Fund.
REFERENCES
Barr, G., Jakobsson, J. G., Owall, A. & Anderson, R. E.
(1999) Nitrous oxide does not alter bispectral index:
study with nitrous oxide as sole agent and as an
adjunct to i.v. anaesthesia. British Journal of
Anaesthesia, 82, 827-830.
Bennett, C., Voss, L. J., Barnard, J. P. M. & Sleigh, J. W.
(2009) Practical use of the raw electroencephalogram
waveform during general anesthesia: the art and
science. Neurosurgical Anesthesiology and
Neuroscience, 109, 539-550.
Dahaba, A. A. (2005) Different Conditions That Could
Result in the Bispectral Index Indicating an Incorrect
Hypnotic State. Anesth Analg, 101, 765-773.
Granger, C. W. J. (1969) Investigating causal relations by
econometric models and cross-spectral methods.
Econometrica, 37, 424-438.
USING GRANGER CAUSALITY TO CHARACTERISE BIDIRECTIONAL INTERACTIONS IN THE HUMAN
BRAIN DURING INDUCTION OF ANAESTHESIA
193

Hammeroff, S. R. (2006) The entwined mysteries of
anaesthesia and consciousness. Anesthesiology, 105,
400-412.
Hudetz, A. G. (2008) Are we unconscious during general
anesthesia? Intl Anesth Clinics, 46, 25-42.
Jameson, L. C. & Sloan, T. B. (2006) Using EEG to
monitor anesthesia drug effects during surgery. J. of
Clinical Monitoring and Computing, 20, 445-472.
John, E. R. & Prichep, L. S. (2005) The Anesthetic
Cascade: A Theory of How Anesthesia Suppresses
Consciousness. Anesthesiology, 102, 447-471.
Messner, M., Beese, U., Romstock, J. & AL., E. (2003)
The bispectral index declines during neuromuscular
block in fully awake persons. Anesth Analg, 97, 488-
491.
Mychaskiw, G., Horowitz, M., Sachdev, V. & Heath, B. J.
(2001) Explicit intraoperative recall at a Bispectral
index of 47. Anesth Analg, 92, 808-809.
Myles, P. S., Symons, J. A. & Leslie, K. (2003)
Anaesthetists' attitudes towards awareness and depth-
of-anaesthesia monitoring. Anaesthesia, 58, 11-16.
Pereda, E., Quian-Quiroga, R. & Bhattacharya, J. (2005)
Nonlinear multivariate analysis of neurophysiological
signals. Progress in Neurobiology, 77, 1-37.
Rampersad, S. E. & Mulroy, M. F. (2005) A case of
awareness despite an "adequate depth of anaesthesia"
as indicated by a Bispectral Index monitor. Anesth
Analg, 100, 1363-1364.
Rampil, I. J. (1998) A primer for EEG signal processing in
anesthesia. Anesthesiology, 89, 980-1002.
Ranta, S. (2002) Awareness with recall during general
anesthesia. Dept of Anaesthesia and Intensive Care
Medicine. Helsinki, University of Helsinki, Finland.
Russell, I. F. (2006) The Narcotrend "depth of
anaesthesia" monitor cannot reliably detect
consciousness during general anaesthesia: an
investigation using the isolated forearm technique.
British Journal of Anaesthesia, 96, 346-352.
Sigl, J. C. & Chamoun, N. G. (1994) An introduction to
bispectral analysis of the electroencephalogram.
Journal of Clinical Monitoring and Computing, 10,
392-404.
Sleigh, J. W., Andrzejowski, J., Steyn-Ross, A. & Steyn-
Ross, M. (1999) The Bispectral Index: a measure of
depth of sleep? Anesth Analg, 88, 659-661.
Tseng, S.-Y., Chen, R.-C., Chong, F.-C. & KUO, T.-S.
(1995) Evaluation of parametric methods in EEG
signal analysis. Med Eng Phys, 17, 71-78.
Vaz, F., De Oliveira, P. G. & Principe, J. C. (1987) A
study on the best order for Autoregressive EEG
modelling. Int. J. Bio-Medical Computing, 20, 41-50.
Viertiö-Oja, H., Maja, V., Särkelä, M., Talja, P.,
Tenkanen, N., Tolvanen-Laakso, H., Paloheimo, M.,
Vakkuri, A., Yli-Hankala, A. & Meriläinen, P. (2004)
Description of the Entropy Algorithm as applied in the
Datex-Ohmeda S/5 Entropy Module. Acta
Anaesthesiol Scand, 48, 154-161.
Voss, L. J. & Sleigh, J. W. (2007) Monitoring
consciousness: the current status of EEG-based depth
of anaesthesia monitors. Best Practice & Research
Clinical Anaesthesiology, 21, 313-325.
Wiener, N. (1956) The theory of prediction. In
Beckenbach, E. F. (Ed.) Modern Mathematics for
Engineers. New York, McGraw-Hill.
BIOSIGNALS 2011 - International Conference on Bio-inspired Systems and Signal Processing
194
