
A MEDICAL DEVICE INFORMATION SYSTEM
AND ITS ARCHITECTURE
Daniela Luzi and Fabrizio Pecoraro
Institute for Research on Population and Social Policies (IRPPS), National Research Council, Rome, Italy
Keywords: Clinical investigation, Medical device, National competent authority’s information system, System
architecture, Document management.
Abstract: The paper describes a Medical Device Information System (MEDIS) that supports applicants in the
submission of a new clinical investigation (CIV) proposal as well as National Competent Authority in the
evaluation and monitoring CIVs carried out at national level. An overview of system design is provided in
the description of its conceptual model and architecture as well as providing an example of the interface of
the system developed.
1 INTRODUCTION
Progress in clinical research depends largely on the
results of clinical investigations (CIV). CIVs are
complex processes encompassing different steps,
from specification and planning to execution and
final result analysis. There are an increasing number
of applications that support CIV data management,
project management and data quality control,
contributing to reduce time and costs and most
importantly to improve research quality (Oliveira
and Salgado, 2006).
However, not much attention has been put on
information systems that support applicants in the
regulatory submission of CIV proposals as well as
National Competent Authorities (NCA) in the
process of evaluating proposals and monitor CIV
execution. The adoption od these systems contribute
to reduce time for CIV start, enhance transparency
of evaluation criteria and improve the monitoring of
ongoing and concluded CIVs at national level.
The paper describes a Medical Device
Information System (MEDIS) developed by the
National Research Council within a project
supported by the Italian Ministry of Health. MEDIS
plays the role of both a registry of clinical
investigation data and a content repository of
documents submitted by manufacturers to the NCA
to obtain the approval for the clinical investigation
start. In particular, MEDIS supports manufacturers
in the documentation submission process as well as
NCA evaluators in assessing the regulatory
documentation received. It also manages the
communication among the different stakeholders
and collects the data produced during the whole
lifecycle of clinical investigations. A high level
description of the CIV business process is described
in details in previous works (Luzi et al., 2009,
Pecoraro et al., 2010, Luzi et al., 2010). The design
of the MEDIS system was based on the analysis of
the domain of CIV on MDs in close collaboration
with the office in charge for the evaluation of CIV
proposals, according to national laws, European
Directives (EU, 2007) and ISO technical norms
(ISO, 2008). Moreover, MEDIS design has been
based on the application of Health Level 7 (HL7) v.3
standards to develop a flexible and interoperable
system. This paper presents the results of the
MEDIS development describing in particular its
architecture and giving an example of its interface.
2 MEDIS ARCHITECTURE
From an architectural point of view, MEDIS is a
client-server three-tiered system. The high level
description of the MEDIS system architecture is
depicted in figure 1 using the UML Component
Diagram notation. All the components of the
MEDIS system reside at the application logic layer,
using the Tapestry framework based on Java
technologies such as JSP and Servlet. The MEDIS
presentation layer is composed by two web clients
providing specific interfaces for both applicants and
547
Luzi D. and Pecoraro F..
A MEDICAL DEVICE INFORMATION SYSTEM AND ITS ARCHITECTURE .
DOI: 10.5220/0003153005470550
In Proceedings of the International Conference on Health Informatics (HEALTHINF-2011), pages 547-550
ISBN: 978-989-8425-34-8
Copyright
c
2011 SCITEPRESS (Science and Technology Publications, Lda.)

NCA evaluators. The web pages are transmitted to
the web client using the protocol HTTP. Moreover,
the HTTPS protocol is used to guarantee both
reserved transfers and secure channels over the web.
The application logic layer consists of a set of
modules in charge of the following functionalities:
1) The authentication of internal users is managed
by MEDIS, while external users access the system
through a single sign on portal which controls their
access to multiple and independent systems. The
authorization of both external and internal users
depends on their profiles. Each external user can
access his/her portion of MEDIS system containing
the list of submitted notifications together with those
that are in the process of submission. Once a
notification is submitted no change is allowed. The
authorization of internal users depends on the role
played in the evaluation of the CIV proposal
(supervisor, medical/technical evaluator,
administrative secretary). For instance each
evaluator can access and modify only the evaluation
report he/she is in charge of, while the supervisor
can access the evaluation report only when the
evaluator allows him/her to do it and use it to make a
final evaluation report.
2) The enterprise data manager supports
information related to external users, the role they
are allowed to play in the notification submission
(manufacturer, authorized representative) and the
data related to organizations responsible for the
notification or delegated to do it. The administrative
secretary accesses this component and gives the
users the right to initialize a new notification.
3) The DAM (Domain Analysis Model) component
manages MEDIS conceptual model based on HL7
RIM. This component supports: a) the rules that
validate the association between DAM objects; b)
the rules that manages the workflow of the CIV
lifecycle. The design of MEDIS DAM and related
data model are described in details in previous
works (Luzi et al., 2009). This component specifies
the underlying workflow determining the allowed
steps depending on the state of the CIV lifecycle
enabling specific functionalities according to the
CIV actual state as well as to the user profile.
4) The proposal data manager supports the
acquisition of the notification, guiding applicants in
the collection and submission of regulatory data and
documents as well as verifying the completeness and
consistency of data and documents submitted.
5) The CIV data manager supports the evaluation
team to write an evaluation report focused on crucial
aspects such as MD characteristics, risk analysis and
procedures planned for patient safety. This
component makes it also possible to share the report
among the evaluation team as well as to edit the
official communication of CIV approval or deny.
Moreover, it supports the communication between
applicants and NCA during the CIV performance,
allowing applicants to notify important steps reached
by an ongoing CIV and register clinical protocol
amendments and/or serious adverse events. This
component also manages the acquisition of the
report of CIV final result.
6) The Dossier Manager supports the storage and
retrieval of documents uploaded as well as those
created during the CIV lifecycle (i.e. NCA internal
documents and communications). Through the XML
component filled electronic forms are transformed in
XML documents and then in PDF (Report builder)
in order to produce a digital signed document.
7) The vocabulary/classification manager connects
MEDIS to external systems to retrieve data such as
vocabularies, classifications, and nomenclatures (i.e.
MESH, MD repertoires, etc.).
Functionalities used in the above-described
components that manage the CIV lifecycle are:
a) The communication exchange that supports the
information flow between applicants and the NCA
evaluation team (e.g. request for data and/or
document integration; reports of amendments and/or
serious adverse events), and also takes track of the
communication exchange.
b) The control of the completeness and consistency
of the data and documents uploaded in a single form
or in a correlated set of forms.
c) The dynamic generation of the electronic forms
depending on the user, the MD under investigation,
and the state reached in the workflow, so that certain
types of communication is allowed only in a specific
phase of the CIV lifecycle.
d) The legal authentication of the documents and
data submitted through a digital signature.
Finally, the persistence layer is divided into:
1) A relational database containing information on
applicants and their belonging organization, as well
as the organization that delegates them to submit
CIV proposals.
2) A relational database that contains data
describing MD and CIV instances, data tracking the
CIV lifecycle workflow and metadata related to
documents stored in the content repository.
3) A content repository in charge of archiving
documents uploaded as attachments of the
HEALTHINF 2011 - International Conference on Health Informatics
548
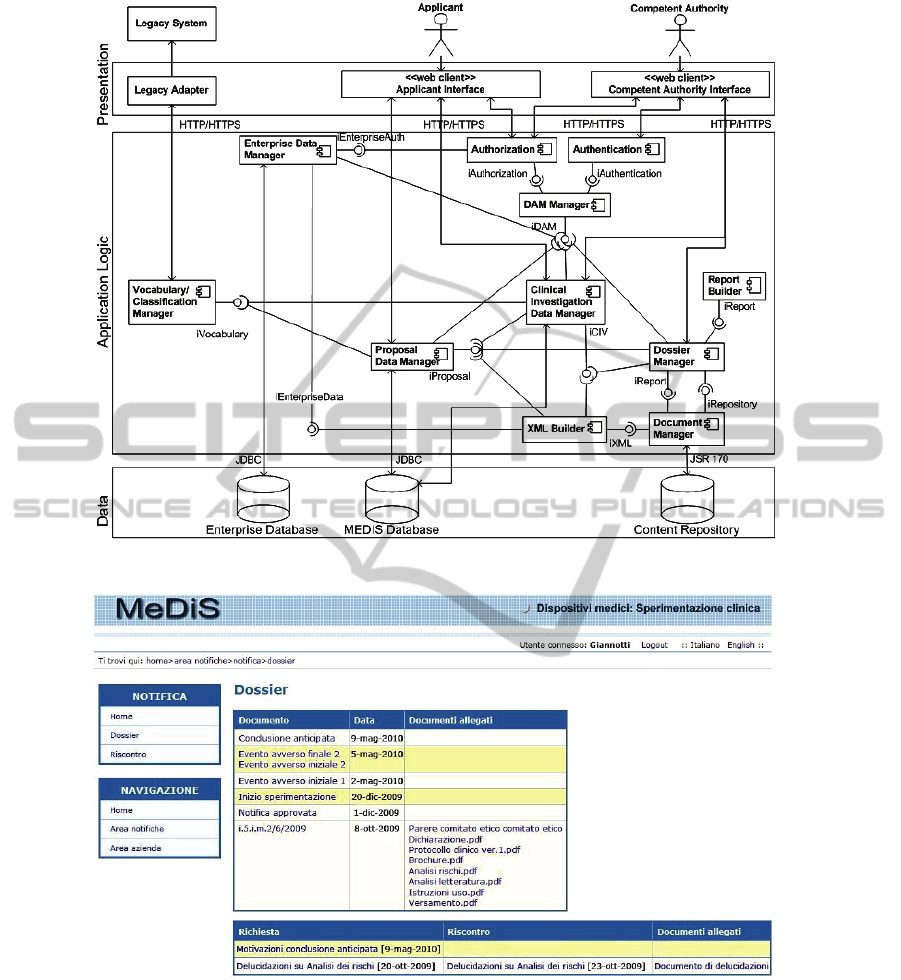
Figure 1: Architecture of the MEDIS system.
Figure 2: MEDIS interface for accessing the dossier documents.
notification or generated by the system starting from
the data filled in the electronic forms.
3 DOSSIER MANAGEMENT
One of MEDIS features is represented by the content
management component. Data and documents
exchanged between NCA and applicants are closely
related to the different phases of the CIV lifecycle.
Given the amount of data and documents exchanged,
their legal value that includes also the management
of different document versions, a Dossier manager
component was developed (see § 2).
An example of the MEDIS interface showing a
representation of the Dossier is depicted in Figure 2
showing the entry point to retrieve detailed
information of each CIV. Each Dossier has it own
A MEDICAL DEVICE INFORMATION SYSTEM AND ITS ARCHITECTURE
549

unique identification code that identifies the set of
the different types of related documents (technical
and administrative) collected during CIV process.
On the left side MEDIS provides two menus, the
upper one is dynamically created according to the
state of the notification. In this case it allows an
applicant to access the dossier as well as to reply to a
received request of further information (Riscontro).
The lower menu allows the applicant a) to access the
notification area (Area notifiche) where the user can
view its CIV proposals and/or make a new one, b) to
access the Organization area (Area Azienda) to view
and update information on the manufacturer and/or
authorized representative. On the right side, the
upper table shows the list of documents exchanged
during the CIV lifecycle, note that each document
type is linked to the attached documents (Documenti
allegati). In this case the notification identified by
the code i.5.i.m.2/6/2009 (meaning the 6
th
notification of pre-market CIV received in the year
2009) gathers all regulatory documents submitted
for the CIV approval. The lower table shows the list
of requests (Richiesta) and eventually the related
response (Riscontro) given by the applicant. In both
tables applicants can access the full text of the
document selected clicking on each item of the list.
4 CONCLUSIONS
MEDIS is a NCA’s information system developed to
support both CIV applicants to correctly submit trial
proposals and NCA to evaluate them as well as
monitor CIVs carried out at national level.
Moreover, MEDIS was designed on the basis of
HL7 v.3 methodology and standards in order to
make the system interoperable with other National
registries and in particular with the European
Databank on Medical Device (EUDAMED) that is
also developing an European system comprising
information on CIVs. At the moment the MEDIS
system has to be validated by real users in order to
test system performance and functionalities.
The role played by the content management
component has been described in this paper
considering it one of the main feature of NCA’s
information systems. The Dossier management has
the function of storing and retrieving documents and
data exchanged in the different phases of the CIV
lifecycle.
Moreover, clinical protocols describing CIV on
MDs as well as investigator’s brochures have been
analysed both in their structure and content
following the recommendation of Good Clinical
Practice (ISO, 2008) that guide applicants to
correctly write these technical documents. On the
basis of these features, our future intension is to
adopt HL7 CDA standard to further specify the
structure and semantic of CIV documents, taking
also into account BRIDG conceptual model
(Fridsma et al., 2007). This is in line with the
MEDIS design and development based on HL7 RIM
and it would further improve document exchange as
well as information retrieval of meaningful parts of
these documents.
ACKNOWLEDGEMENTS
This study was supported by the Italian Ministry of
Health through the MEDIS project (MdS-CNR
collaboration contract n° 1037/2007).
REFERENCES
European Parliament, Directive 2007/47/EC of the
European Parliament and of the Council of 5
September 2007, Official Journal of the European
Union, L 247/21, 21.9.2007.
Fridsma, B. D., Evans, J. Hastak, S., mead, C. N. (2007).
The BRIDG project: a technical report. JAMIA 15, pp.
130-137.
Health Level Seven, Inc. HL7 Reference Information
Model. Ann Arbor, MI: Health Level Seven, Inc.
Available at: www.hl7.org.
International Organisation for Standardization (2008).
Clinical investigation of medical devices for human
subjects – Good clinical practice. draft international
Standard ISO/DIS 15155, ISO TC 194 (revision of
ISO 14155-1:2003 and ISO 14155-2:2003), ISO 2008.
Luzi, D., Contenti, M., Pecoraro, F. (2010). The HL7 RIM
in the design and Implementation of an Information
System for Clinical Investigations on Medical
Devices. In: First IMIA/IFIP Joint Symposium,
Brisbane, 2010. Advances in Information and
Communication Technology 335, Berlin, Springer, pp.
5-14.
Luzi, D., Pecoraro, F., Mercurio, G., Ricci F. L. (2009). A
medical device domain analysis model based on HL7
Reference Information Model. In: Medical Informatics
in a United and Healthy Europe. Proceeding of MIE
2009, IOS Press, 162-166.
Oliveira, G. A., Salgado, N. C. (2006). Design aspects of a
distributed clinical trials information system. Clinical
Trials, 3, 385-396.
Pecoraro, F., Contenti, M., Luzi, D. (2010). Clinical
Investigation on Medical Device: A data model based
on HL7 reference information model. Proceedings of
IADIS 2010, Freiburg, 26-31 July 2010.
HEALTHINF 2011 - International Conference on Health Informatics
550
