
INFORMATION AND COMMUNICATION TECHNOLOGIES
(ICTS) FOR BIOBANKING AND ONCOLOGY RESEARCH
Analysis, Support Scenarios and a Case Study
Elena Sini
1
, Michele Torresani
1
, Silvia Veneroni
2
1
ICT,
2
Dept.of Experimental Oncology and Molecular Medicine
Fondazione IRCCS, Istituto Nazionale dei Tumori, Via Venezian 1, Milan, Italy
Paolo Locatelli, Nicola Restifo
Fondazione Politecnico di Milano, Piazza Leonardo da Vinci 32, Milan, Italy
Keywords: Tissue bank, Traceability, Radio frequency identification, Oncology research, Process reengineering.
Abstract: Human Tissue Banks are key for Oncological research and practice. Biobanking processes cross many care
departments and have a number of stakeholders, often carrying different objectives: quality assurance and
process efficiency are hard to garrison. Key issues in a biobanking project are: dedicated organization,
process control, completeness of clinical information on samples, integrated Information and
Communication Technologies. Fondazione IRCCS Istituto Nazionale dei Tumori is an oncologic research
and treatment institution in Milan (Italy). Our project started in October 2007 aiming at revising the whole
tissue collection process (from Surgery to Anatomical pathology assessment, to analysis and storage in the
Biobank), developing a clinical biobank management system collecting structured data on cases, and
designing an RFId-based system able to track the time- and temperature-sensitive specimens’ flow. Now
that go-live has begun, technological and - above all - organizational challenges of the project can be
discussed in detail. We hope other organizations will appreciate our efforts and are willing to apply a
biobanking network as soon as possible.
1 INTRODUCTION
Founded in 1925, the Fondazione IRCCS Istituto
Nazionale dei Tumori in Milan (henceforth: INT) is
recognized as a Scientific Research and Treatment
Institution in Oncology. Over 176 research projects
are currently under way, publishing nearly 400
scientific papers each year (IF 2272.32). In 2009
INT cared for about 14,000 inpatients, 10,000 day-
hospital admissions, 1 million outpatient treatments,
11,500 surgical operations (including 28 liver
transplants). It also inspired the Lombardy Oncology
Pathology Network (ROL).
This paper will describe our experience as
regards the development of a organizational and ICT
solutions for quality assurance, traceability and
operation support to biobanking of surgical samples
for experimental oncology research, sharing
challenges and results of our efforts.
2 EXPECTATIONS
AND CHALLENGES
IN BIOBANKING
Biosamples – e.g. pathologic and normal tissues,
blood, serum, nucleic acids – can play a vital role in
research that seeks to find new means of preventing,
diagnosing or treating cancer, mainly feeding in
vitro studies at in vivo conditions (e.g. on
biomarkers, molecular targets, biomolecular
characterization, genomics and proteomics),
minimizing research costs for future studies. Quality
assurance of sampling and processing activities
always worries researchers, e.g. because of possible
unknown biases on gene expression after tissue
devascularization (Spruessel, 2004). In order to
produce clinically valid and comparable results,
adequate case records, high-tech machinery,
standard sampling and testing procedures (SOPs) are
274
Sini E., Torresani M., Veneroni S., Locatelli P. and Restifo N..
INFORMATION AND COMMUNICATION TECHNOLOGIES (ICTS) FOR BIOBANKING AND ONCOLOGY RESEARCH - Analysis, Support Scenarios
and a Case Study.
DOI: 10.5220/0003161802740279
In Proceedings of the International Conference on Health Informatics (HEALTHINF-2011), pages 274-279
ISBN: 978-989-8425-34-8
Copyright
c
2011 SCITEPRESS (Science and Technology Publications, Lda.)

paramount (Teodorovic, 2003, Bloom 2003).
We started analyzing the Italian and international
biobanking scenario (in Oncology above all), trying
to find organizational models and ICT solutions to
inspire our project. In general, we observed that
biobanks in other countries seem to be more
recognized as a valuable asset and institutionalized
within organizations. Biobanks are often connected
to research centers or universities, e.g. the IARC in
Lyon – France (www.iarc.fr), the Emory University
Hospital’s Winship Cancer Institute in Atlanta –
GA, USA (http://winshipcancer.emory.edu).
Moreover, in the majority of Italian cases,
biobanking is not an institutionalized process within
organizations and conceived as non-core by clinical
units. Sample collection is not systematic, most are
pathology-oriented, and often considered as own
property by physicians.
Further on, we looked for biobank information
systems and traceability technologies. The first thing
we found, is that DNA, semen, or blood banks, for
example, are more used in implementing
technologies for sample identification and biobank
management than tissue biobanks are. This gap is
even more relevant in private-run institutions.
Relevant cases of tissue banks show dedicated
management systems, integrated with local
laboratory equipment, which cover not only filing,
but also clinical information of patients and
specimens. For example, the biobank management
system developed by IBM at Karolinska Institutet in
Solna – Sweden (http://ki.se) is an excellence case
among those studied, also as regards the
implementation of syntactic and semantic standards
to gather data on clinical cases from the hospital
information system to the biobank. In Italy, we
found mainly local biobanks, where some cancer
centers run their own collections and poor support
systems (standalone software, or FileMaker®
archive..), which generally handle only storage
positions and basic clinical data. As regards
traceability, we found that, apart from hand-written
information on tubes, there is a basic use of barcode
labeling and only few interesting cases of Radio
Frequency Identification (RFId). In fact, RFId is
being recognized also in the healthcare sector as a
useful means to improve process safety and control.
Despite the growing number of implementation in
patient identification, internal logistics, clinical
operations (e.g. transfusion/drug administration
traceability – Vilamovska, 2009; School of
Management of the Politecnico di Milano 2005-
2009), there are still few implementations as regards
biobanking: on one hand this process requires quite
complex functionalities, on the other hand, a local
use of this technology only in the biobank may not
prove sustainable compared to barcoding. Major
issues are seamless integration within the sampling
process from surgery to anatomical pathology and to
the biobank, as well as operations at very low
temperatures. Istituto Ortopedico Rizzoli in Bologna
- Italy (www.btm.ior.it) traces bone tissues from the
biobank to the operatory theatre, where they get
updated with information on implanted patients.
Moreover, Paoli-Calmettes Institut in Marseilles –
France (www.institutpaolicalmettes.fr) and Mayo
Clinic in Rochester – MN, USA
(http://cancercenter.mayo.edu/mayo/research/bioba
nk) experimented in pilots that the use of high tech
RFId tags for freeze reading is still a capability to be
further developed.
Networking is another main issue, on one hand
to support the development of biobanks in smaller
institutions, on the other hand to exploit the value of
local biological assets joining international research
pipelines. In Italy focus is still on the setting up of
providers’ awareness, while foreign initiatives are
getting extensive: Tubafrost (www.tubafrost.org),
the Spanish National Tumor Bank Network
(www.cnio.es), the Wales Cancer Bank
(www.walescancerbank.com), EuroBioBank
(www.eurobiobank.org), the American NCI CaBIG
project (https://cabig.nci.nih.gov) and the Canadian
Tumor Repository Network (https://www.ctrnet.ca).
These projects follow different models, from the
creation of virtual collections to the centralization of
biobanking facilities.
Summing up, five key challenges can be
identified for present and future biobanking projects:
Organization: institutionalization of the
biobanking process within organizations process-
driven view, internal communication and
commitment.
Quality assurance and process monitoring
SOPs and traceability technologies. In particular,
RFId has innovative features like dynamic memory,
distance accessibility, bulk identification, embedded
sensors.
Completeness of information on case profiles
merging data from clinical subsystems data
coding and system integration implementing
semantic and syntactic standards (SNOMED, ICD9-
CM, ICD-10, EHR HL7, XML).
Time-lasting preservation and stability
adequate investments in laboratory instrumentation
and storage sites.
Contributing to research sample use protocols,
INFORMATION AND COMMUNICATION TECHNOLOGIES (ICTS) FOR BIOBANKING AND ONCOLOGY
RESEARCH - Analysis, Support Scenarios and a Case Study
275

syntactic/semantics, biobanking/pathology networks.
Web Rich Internet Applications (RIA) enable
sophisticated interfaces as in client/server systems,
but with a natural capability of supporting
networking and cooperation with external
organizations.
These subjects will now be addressed presenting the
real case of the Oncologic Tissue Bank at INT.
3 TISSUE BIOBANKING AT INT:
KEY ISSUES AND RESULTS
The project at the Italian National Cancer Institute in
Milan started in 2007 when the Experimental
Oncology Dept. discussed with the Chief
Information Officer (CIO) the opportunities of ICT
support for the local tissue biobank. The ICT Unit
and partner Fondazione Politecnico di Milano
started investigating processes, staff organization
and existing tools, highlighting critical points and
needs. Analysis highlighted a situation coherent with
the scenario discussed in Section 2: highly
fragmented way of working with poor support for
activities and low overall effectiveness (uncertain
quality assurance, high number of uncollected
cases…). Thus, a new targeted project was set up in
order to:
Establish a real biobanking infrastructure;
Investigate quality control procedures for tissue
processing and storing and review the organization
implementing a clearly structured biobanking
process;
Ensure the exact identification of specimens;
Guarantee traceability of operations and
transport lead times, monitoring specimens’
environmental conditions;
Automate information flows between Depts.;
Develop a shared scientific data-base integrated
with the hospital information system, where to
gather significant clinical information on patients
and data on specimens, to support diagnosis and
research.
A first help was in 2009 the opening of the
Amadeo-Lab, a new seat to concentrate research
units and labs, centralizing storage in a dedicated
infrastructure. Before, freezers were dispersed
among the 9 floors of the INT main building.
The solution we designed is mainly based on a
new Tissue Bank information system and the
extension of the INT RFId platform to support the
entire biobanking workflow (from surgery to freeze).
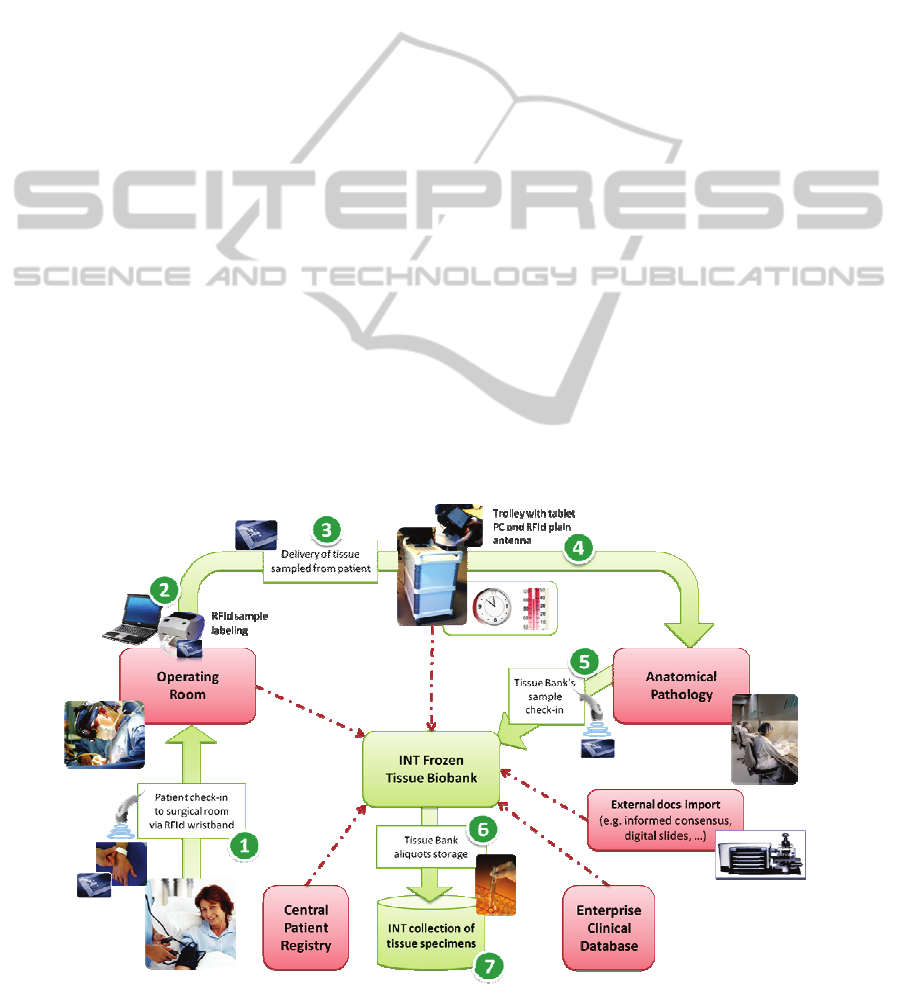
This is represented in Figure 1 (see numbers in
brackets). Inpatients at INT are always assigned an
RFId wristband at admission, where an
accompanying nurse stores information for its
unique identification [ref. 1]. This as INT is running
an own RFId traceability platform developed for
patient/staff identification, access management,
treatments safety and so on (Locatelli, 2010).
Operating Rooms daily schedule and real time status
are broadcasted via HL7 messages, so that
technicians in the biobank can plan activities and
signal surgeons which cases have a higher relevance.
Staff in the O.R. [1] checks-in the patient, verifying
its wristband vs. the room planning and their sheets
in the clinical system. During surgery [2], samples
of tumor or sane tissue are taken for diagnosis. Staff
can register and RFId-label sampled specimens on
the surgery system (e.g. site of sampling,
information for pathologists, notes for the Tissue
Bank..) while filling the digital surgery report on the
O.R. laptop. Samples are now ready to be sent to the
Anatomical Pathology labs: a clerk takes them out
and checks them in on the “Tissue Express” [3, 4], a
trolley with a plain RFId antenna and tablet PC
running a touch-screen software, where to declare
which new samples loaded and by whom (each staff
member has an RFId badge to log in to systems).
Dedicated staff deliver the trolley to the pathology
labs and check them in, while it tracks lead times
and surrounding temperatures thanks to a RFId
semi-active tag installed in the box containing the
samples. The software on the tablet PC collects its
transmission and joins them with data on carried
samples. These are checked in by pathologists
reading their labels on their laboratory system [5].
Once they have assessed a sample (macro-, micro-,
diagnosis), parts of it are given to a Tissue Bank
technician, who identifies it again reading the
original RFId label printed in the O.R [5]. The
received sample is recorded into the new Tissue
Bank information system. Alerts are shown in case
processing times or temperatures crossed preset
thresholds. The technician proceeds subdividing the
sample into aliquots and stocking them into vials,
each one labeled with a unique identifier and key
data printed into a Datamatrix 2D barcode. After
examinations and documentation on the system,
aliquots are frozen in the laboratory and periodically
transferred to the new biolab [6, 7].
The Tissue Bank information system routinely
collects relevant process and clinical data via HL7
and Web Services integration from the O.R. system,
the Anatomical Pathology, the slides digitalizer, the
HEALTHINF 2011 - International Conference on Health Informatics
276
RFId platform, the Enterprise Patient Registry, the
Enterprise Clinical and Reports Repository, and
from external documentation. All relevant data is
linked to the tissue collection and organized
enabling the user to browse collections per sample-,
patient-, or clinical data. The system manages
storage and sample retrieval, offering research and
browsing capabilities through rooms, freezers and
boxes containing samples, supporting technicians in
withdrawing cases and aliquots for research.
In our context, as probably in many other
research institutions, the biggest challenge we had to
face was to standardize sample-related data collected
in two heterogeneous historical databases: an
experimental stand-alone application for breast and
ovarian cancer cases and a MSExcel® archive for
other kind of samples. Basic data on samples was
written down on paper during handling and then
entered manually. No integrated system could ever
have been implemented with such a data structure.
The review took almost a year and required a tight
collaboration between the ICT Unit and clinical
staff. As results we obtained a common data
structure to be implemented, thus recovering all
historical collections. The new database was
designed to be more flexible and general as possible,
thus being able in the future to extend the
institutional biobank to other types of biological
material (e.g. blood, RNA, tissue microarray), and
also to join networks. The Tissue Bank information
system has been designed as a web-based Rich
Internet Application, allowing a very complete and
friendly user interface. Moreover, the three-tier
application architecture will enable us to easily scale
the system, simplifying maintenance and evolution
(data and application server are centralized, while
users access the latest version of the application via
web browser).
4 DISCUSSION:
TECHNOLOGY-RELATED
AND ORGANIZATIONAL OPEN
ISSUES
The solution has been available to users in
Experimental Oncology Labs since May 2010 for
user testing. Go live was October 2010, starting
from the Urology and Melanoma-Sarcoma
Operating Room, reaching full coverage of 10 ORs
at INT by Spring 2011.
To ease organizational change, we involved key
actors and final users in analysis and design phases:
since the beginning we engaged Operating Theater,
Anatomical Pathology and Tissue Bank referees in
order to approach the process from a global point of
view. Decision makers and operative referees were
committed to analyze processes, IT support,
documentation and information flows, critical
aspects, considering all points of view. Once As-Is
analyses were shared in focused meetings, discus-
Figure 1: Schema of the process and ICT solution supporting the new Tissue Bank at INT.
INFORMATION AND COMMUNICATION TECHNOLOGIES (ICTS) FOR BIOBANKING AND ONCOLOGY
RESEARCH - Analysis, Support Scenarios and a Case Study
277

sion followed on hypothesis about new ICT
solutions and process reengineering. Several
meetings were necessary to address issues like: what
type and how to use RFId, how to integrate the new
Tissue Bank system to the different Hospital
Information System (HIS) modules involved in the
biobanking process, which would be the tracking
steps in delivering samples, how to modify surgical
workflow in taking and recording tissue samples,
and so on. After defining specifications, we had to
coordinate all technology partners, one for each
system involved in the biobanking process. This
required a huge effort, to reconcile views and
garrison system integration.
Change management and implementation
activities have been running for almost a year, while
consensus building efforts accompanied the project
from the beginning. In fact, change management
issues were challenging, because of some
peculiarities common to many healthcare
projects._First of all, a process like biobanking
crosses at least three different care departments and
has a number of other stakeholders (Anesthetists,
Auxiliary Personnel, the Scientific Directorate, the
Ethics Office...), often carrying different priorities
and views of the process. Common to contexts like
public institutions were a certain resistance to
change while introducing a process-driven way of
thinking (instead of focusing on own clinical areas),
and a general low computer literacy. Internal project
management at INT was undertaken by the CIO (as
usually happens here for ICT projects, the ICT
Office takes leadership), with strong internal
commitment by INT top management. The CIO was
supported by a direct delegate and colleagues from
Fondazione Politecnico di Milano. Strong support
was required from the clinical area, so that clinical-
scientific issues could be taken into consideration
during design. The existence of previous successful
projects (e.g. RFId transfusion traceability, new
Surgery management system...) involving the same
roles and their referees helped to boost cooperation.
4.1 RFId Maturity – Can Healthcare
Organizations Face this Alone?
Positive experiences on using HF13,56 MHz RFId
technology for patient, operator and item
identification with near field applications (e.g. in the
transfusion chain) led us to extend the use of this
technology also to biobanking. We searched the
market for RFId solutions ready for biobanking, but
the only one meeting our needs was focused on vials
identification, too expensive, and from outside Italy
(with potential difficulties in customization and
integration activities). So, we evaluated how to
develop the extension on our own.
First of all, we learned RFId is not at all an “on-
the-shelf” technology. Even being supported by a
high-profile partner, many solutions had to be found
in an experimental way. Variety in implementation
of interoperability and communication standards by
producers of tags and devices, hardness to find
mature RFId handheld readers, unexpected
behaviour of devices and drivers instability, are
some of the main challenges to be faced. In fact, also
because of low experienced suppliers, we had to
work by a trial-and-error approach, often re-
designing integration components. This slowed up
system developments substantially.
Two key examples will help understanding this
issue. First, the trolley had to be designed and
produced with craftsmanship, while unexpected
interactions of the electromagnetic field with
samples required many modifications to obtain a
field with required characteristics such as shape and
strength. The second example comes from a request
by researchers to prove that RFId would not damage
samples. Once we started assessing literature
(among which: ICNIRP, 1998; Ahlbom, 2004;
Jauchem, 2008), we discovered that RFId
technology isn’t supported by a consolidated
environment due to lack of specific laws and
implementation guidelines for the healthcare sector:
studies on long-term consequences of biological
interactions connected to HF RFId fields have not
led to conclusive result yet. What we concluded after
scrutinizing a large number of papers, is that, given
the physical characteristics of tissues and the type of
RFId emissions used (short impulses, frequency,
power of few dozen mW), both short- and long-term
effects on tissues can be negligible. Besides, we
verified through several tests that the use of RFId
would not interfere with ordinary clinical activities
(Radiology, Radiotherapy..) and medical equipment.
Only tests done on infusion pumps led to a 5-10 cm
minimum distance requirement in RFId operations,
due to slight alteration in measured volumes in case
of repeated read/write activities.
Another issue was transport temperature
monitoring via semi-active or active tags: scouting
to find the right device with proper reliability and
battery duration was hard.
But the main critical point in using RFId for
biobanking is represented by extreme low storage
temperatures. Starting from -80°C of mechanic
freezers, tissues can be stocked in liquid nitrogen at -
196°C: standard RFId tags are not readable under -
30/-40°C. Specific extremely expensive RFId
solutions (vials with little ad-hoc button-size tags)
can improve reliability when sample is defrosted,
HEALTHINF 2011 - International Conference on Health Informatics
278

but with no significant enhancements for readability.
This were the main reasons why we decided – for
now- to switch to barcode for supporting sample
identification at storage and retrieval, relying on 2D
Datamatrix labels to code more data on each vial.
4.2 Expected Improvements
Technicians experience a remarkable rise in number
and quality of surgical specimens provided by the
operating theatre to the bank: before the bank was
able to collect specimens from less than 40% of
surgeries, often not knowing certain data about how
they have been processed before. State-of-the-art
facilities and tools will allow a more accurate
screening of incoming tissue samples, better support
high level research activities, and even to offer its
services (both storage space and sample provision)
to external organizations.
5 CONCLUSIONS
In 2007-2010 INT redesigned biobanking processes
from the operating theatre to storage, developing a
new Biobank Management System conceived as a
collector of all information flows about patients and
samples coming from clinical subsystems (Surgery,
Anatomical pathology, Laboratory...). This was also
integrated with the enterprise RFId platform to
identify samples, register process and transport lead
times, and to monitor specimens’ processing thanks
to readers at key steps of the process and trays
tracing environmental conditions on samples leaving
the operatory theatre. Systems are supporting
information flows all over processes, creating links
between applications and units that were not
cooperating at best and enabling process control and
quality, with an expected impact also on research.
The biobank management system was designed as a
flexible structure, and a web-based application able
to manage different biobanks and collections into a
virtual repository, the main feature of a network. A
first initiative of shared infrastructure has just started
with partner hospitals from a Regional research
group on Colon-Recto Cancer. Moreover, INT is
now trying to develop an extranet module and align
its systems to international guidelines in order to
apply international biobank networks (BBMRI...)
and offer its services to other institutions.
Challenges to be faced are very high. Innovation
in Public Healthcare meets a lot of difficulties in
being adopted due to strong organizational habits
and rules, as well as low computer literacy; rising
staff commitment towards a project/process
involving many different departments is a daily
commitment. Thus, change management issues and
accurate process redesign activities are key to
success. Introduction of innovation in such contexts
must be gradual (pilot projects), proceeding by
further refining and upgrades of systems. Challenges
were also technology-related. We realized that RFId
technology is not at all an “on-the-shelf” technology
and its implementation is not supported by a mature
environment due to lack of specific laws and
guidelines for the healthcare sector; this required
experimenting solutions and developing technology
skills on our own together with partners.
REFERENCES
Ahlbom A., Green A., Kheifets A., Savitz D., Swerdlow
A., 2004. ICNIRP - International Commission on
Non-Ionizing Radiation Protection, Standing
Committee on Epidemiology. Epidemiology of health
effects of radio frequency exposure, Environ. Health
Perspect, 2004; 112:1741-54.
Bloom G., Brower J., Clancy N., Eiseman E., Olmsted S.,
2003. Case studies of existing human tissue
repositories: Best practices for a biospecimen
resource for the genomic and postgenomic era. Santa
Monica, CA. RAND Corp.; www.Rand.Org/
publications/mg/mg120
ICNIRP - International Commission on Non-Ionizing
Radiation Protection, 1998. Guidelines for limiting
exposure to time-varying electric, magnetic and
electromagnetic fields (up to 300GHz), Health
Physics; 1998; 74(4).
Locatelli P., Restifo N., Facchini R., Sini E., Torresani M.,
July 2010. Strategic Planning of Identification
Technologies in Healthcare Processes: a Model and a
Real Case, regular research paper and presentation at
WORLDCOMP 2010, Intl. Conference on
Bioinformatics and Computational Biology, Las
Vegas, USA.
School of Management of the Politecnico di Milano,
Osservatorio RFID / Osservatorio Mobile&Wireless
Business. Reports 2005-2009. Politecnico di Milano,
Dipartimento di Ingegneria Gestionale, Milano, Italy.
Spruessel A, Steimann G, Jung M et al., 2004. Tissue
ischemia time affects gene and protein expression
patterns within minutes following surgical tumor
excision. BioTechniques. 2004;36:1030-7.
Teodorovic I, Therasse P, Spatz A, Isabelle M, Oosterhuis
W, 2003. Human tissue research: EORTC
recommendations on its practical consequences. Eur J
Cancer. 2003; 39:2256-2263.
Vilamovska AM, Hatziandreu E, Schindler HR, Van
Oranje-Nassau C, De Vries H, Krapels J, 2009. Study
on the requirements and options for RFID application
in healthcare, RAND Corporation.
INFORMATION AND COMMUNICATION TECHNOLOGIES (ICTS) FOR BIOBANKING AND ONCOLOGY
RESEARCH - Analysis, Support Scenarios and a Case Study
279
