
THE DYNAMICS OF LOCUST NON-SPIKING LOCAL
INTERNEURONS
Responses to Imposed Limb Movements
Oliver P. Dewhirst, Natalia Angarita-Jaimes, David M. Simpson, Robert Allen
Institute of Sound and Vibration Research, University of Southampton, Southampton, SO17 1BJ, U.K.
Philip L. Newland
School of Biological Sciences, Building 85, University of Southampton, Highfield Campus, Southampton, SO17 1BJ, U.K.
Keywords: Reflex Dynamics, Nonlinear System Identification, Wiener Laguerre.
Abstract: A key feature of the locusts hind leg control system is a reflex loop that uses a stretch sensor, the Femoral
Chordotonal organ, to monitor the position and movements of the tibia relative to the femur. A population
of non-spiking local interneurons in the metathoracic ganglia receive synaptic inputs from the sensory
neurons in the chordotonal organ and indirect inputs from other interneurons. They function to integrate
these signals and generate the motor pattern required for coordinated limb movement. Nonlinear Volterra
models combined with Gaussian white noise stimulation have, for the first time, been used to characterise
the dynamics of this population of interneurons. The results show that the interneurons can be clustered into
three groups, those which are position, position/velocity and velocity sensitive.
1 INTRODUCTION
Reflexes are a critical part of vertebrate and
arthropod motor control systems allowing posture
and movement to be adapted to changes in the
external environment. Greater understanding of the
reflex control of limb movement should allow the
features of such systems to be exploited to improve
the design of engineering control systems (bio-
inspired design) (Bar-Cohen, 2006). Arthropods
provide an opportunity to develop new investigative
techniques and gain insight into a relatively simple
and accessible neuromuscular reflex control system.
Three ganglia in the locusts thorax contain neurons
responsible for controlling movements of the legs
(Figure1). A key feature of its hind limb reflex
control system is a stretch sensor called the Femoro-
tibial Chordotonal Organ (FeCO) (Burrows, 1996).
This sensor monitors the movement of the tibia
about the femoro-tibial joint (Figure 1). Movements
of the tibia are converted into action potentials by
sensory neurons located in the FeCO (~90 cells).
These signals are processed by spiking local
interneurons and then integrated with information
from other sensors by the non-spiking local
interneurons. The non-spiking local interneurons
transmit this information using graded potentials to
the leg motor neurons which activate muscle
contraction (Burrows, 1996). It is believed that the
non-spiking interneurons have the ability to
modulate the reflex response in one limb given
information integrated from both local sensors and
those on other limbs and hence play a crucial role in
the production of coordinated limb movement
(Burrows, 1996). Previous work has described the
connections between the non-spiking local
interneurons and the motor neurons in the hind leg
of the locust (Laurent and Burrows, 1989). Little is
known, however, of the range of inputs received by
these interneurons. In this paper a nonlinear Volterra
model combined with a Gaussian White Noise
(GWN) stimulation signal has been used to
characterise the input sensitivity of a population of
non-spiking local interneurons to imposed
movements of the locust hind leg femoro-tibial joint.
A similar technique has been used to model a
population of spiking local interneurons (Vidal-
Gadea et al., 2009).
That study, however, used the Wiener series and
a cross correlation parameter estimation method
270
Dewhirst O., Angarita-Jaimes N., M. Simpson D., Allen R. and L. Newland P..
THE DYNAMICS OF LOCUST NON-SPIKING LOCAL INTERNEURONS - Responses to Imposed Limb Movements.
DOI: 10.5220/0003168002700275
In Proceedings of the International Conference on Bio-inspired Systems and Signal Processing (BIOSIGNALS-2011), pages 270-275
ISBN: 978-989-8425-35-5
Copyright
c
2011 SCITEPRESS (Science and Technology Publications, Lda.)
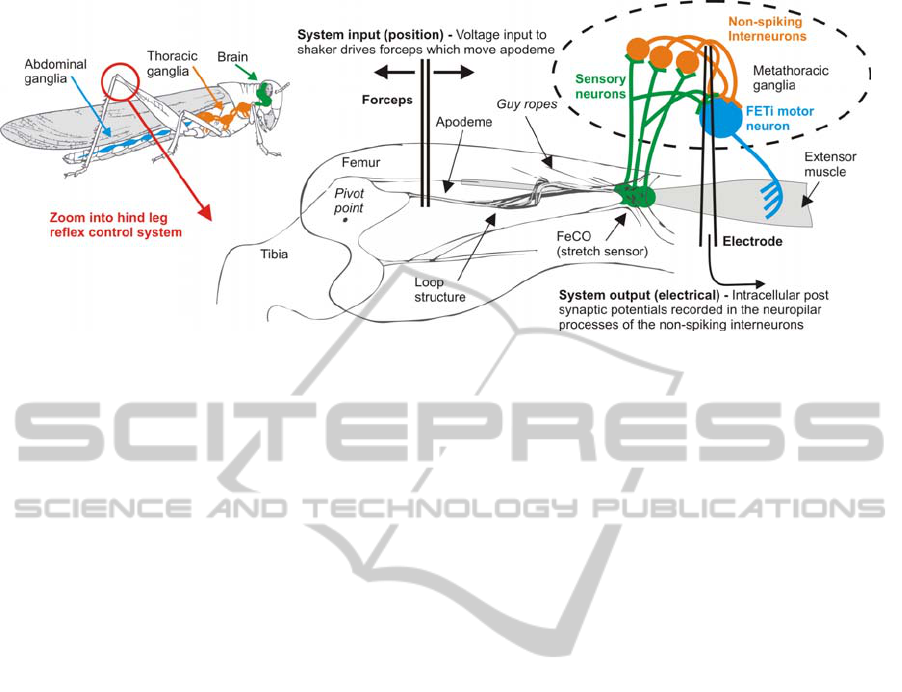
Figure 1: The locust hind leg control system and the non-spiking local interneurons modelled in this study.
(Schetzen, 1981). Whilst the cross correlation
method is relatively computationally efficient, its
accuracy relies on the properties of the input signal
(Westwick et al., 1998). Parameter estimation
accuracy is improved in the current study by
estimating the parameters of a Volterra model using
a Least Squares technique. Model complexity is
significantly reduced using Laguerre basis functions
(Marmarelis, 1993).
2 METHODS
2.1 Experimental Methods
Experiments were performed on 11 adult male and
female desert locusts, Schistocerca gregaria
(Forskål) at room temperature (21.5±°C, relative
humidity 35.7±3.7%). Locusts were mounted ventral
side uppermost in modelling clay. The apodeme of
the FeCO (Figure 1) was exposed and attached by
forceps to a shaker (Ling Altec 101). The FeCO was
stimulated by applying a 27Hz low pass filtered
GWN signal to the shaker (CG-742, NF Circuit
Design Block). Intracellular recordings were made
using a glass microelectrode which was inserted into
the neuropillar processes of the interneurons in the
metathoracic ganglia. The synaptic potentials were
amplified using an Axoclamp 2A amplifier (Axon
Instruments). Signals were stored on magnetic tape
(digital format) using a PCM-DAT recorder (RD-
101T, TEAC) operating at a sampling rate of
24KHz. The data were transferred to a computer
using a PCMCIA interface card and QuikVu
software (TEAC) and analysis was carried out using
MATLAB (Mathworks, Cambridge UK).
2.2 Signal Processing: Theory
The second order Volterra series is written as
1
12
1
011 1
0
11
212 1 2
00
() ( )( )
( , )( )( )
L
LL
yn h h un
hunun
τ
ττ
ττ
τ
τττ
−
=
−−
==
=+ − +
−−
∑
∑∑
(1)
where
)(nu
is the input;
0
h
,
11
()h
τ
and
),(
212
τ
τ
h
are the zero, first and second order
kernels and L is the number of lags. To facilitate
parameter estimation, Wiener (1958) expressed the
series in terms of a set of orthogonal functions:
1
12
1
011
0
11
212 12
00
() ( ) ()
(, )
J
j
j
JJ
jj
jj
y
nc c
j
n
cjj
ϑ
ϑϑ
−
=
−−
==
=
++
∑
∑∑
(2)
where J is the number of functions in the
decomposition and c are the coefficients of the
Wiener kernels. The orthogonal basis functions
)(n
j
ϑ
are obtained using
∑
=
−=
L
m
jj
mnumLn
0
)()()(
ϑ
(3)
()
()
() ( )
1/2
/2
0
() 1
11
nj
j
j
kk
jk
k
Ln
nj
kk
αα
α
α
−
−
=
=−
⎛⎞⎛⎞
−−
⎜⎟⎜⎟
⎝⎠⎝⎠
∑
(4)
where
j
L
is the
th
j
order Laguerre function and
α
is the “decay parameter” controlling the damping of
the Laguerre function. A lag of 100ms,
α
=0.5 and J
THE DYNAMICS OF LOCUST NON-SPIKING LOCAL INTERNEURONS - Responses to Imposed Limb Movements
271
= 6 were required to capture the dynamics of the
interneurons. The outputs of the series can be
calculated recursively using
() () ( )
11)(
11
−−+−=
−−
nnnn
jjjj
ϑϑαϑαϑ
(5)
with
)(
0
n
ϑ
defined as:
()
)(11)(
00
nuTnn
αϑαϑ
−+−=
(6)
where T is the sampling interval. The
coefficients
)( jc
(Equation 2) were calculated from
the basis functions using the Least Squares method.
The kernels were then obtained using
1
12
1
11 1 1
0
11
212 212 1 22
00
() () ()
(, ) (, ) () ()
J
j
j
JJ
jj
jj
hcjL
hcjjLL
ττ
τ
τττ
−
=
−−
==
=⋅
=⋅⋅
∑
∑∑
(7)
2.3 Signal Processing: Application
Our analysis is based on that used in similar studies
(Newland and Kondoh, 1997a and Vidal-Gadea et
al., 2009) on different neurons. Models were fitted
between the first 20 seconds of steady state adapted
response (Figure 2B s3) and the corresponding
samples of input signal. Validation was carried out
using the last 4s of the recording by calculating the
fitness function (Figure 2B s4).
()
ˆ
100 1 ( ) ( ) / ( ))
f
it yt yt yt=×− −
(8)
where
•
is the Euclidean norm (mean square
value),
()yt is the measured output and
ˆ
()yt
is the
predicted output. In order to study patterns in the
interneuron’s responses kernels were clustered using
the K-means algorithm (Hartigan and Wong, 1979).
In order to focus on their sensitivity to position,
velocity and acceleration (of primary interest to
neurophysiologists), the gradient m of a linear
function y=mx+c fitted to the frequency response
(magnitude only) calculated from the first order
kernel between 2 and 15Hz was used as the feature
for the k-means algorithm. The interpretation of the
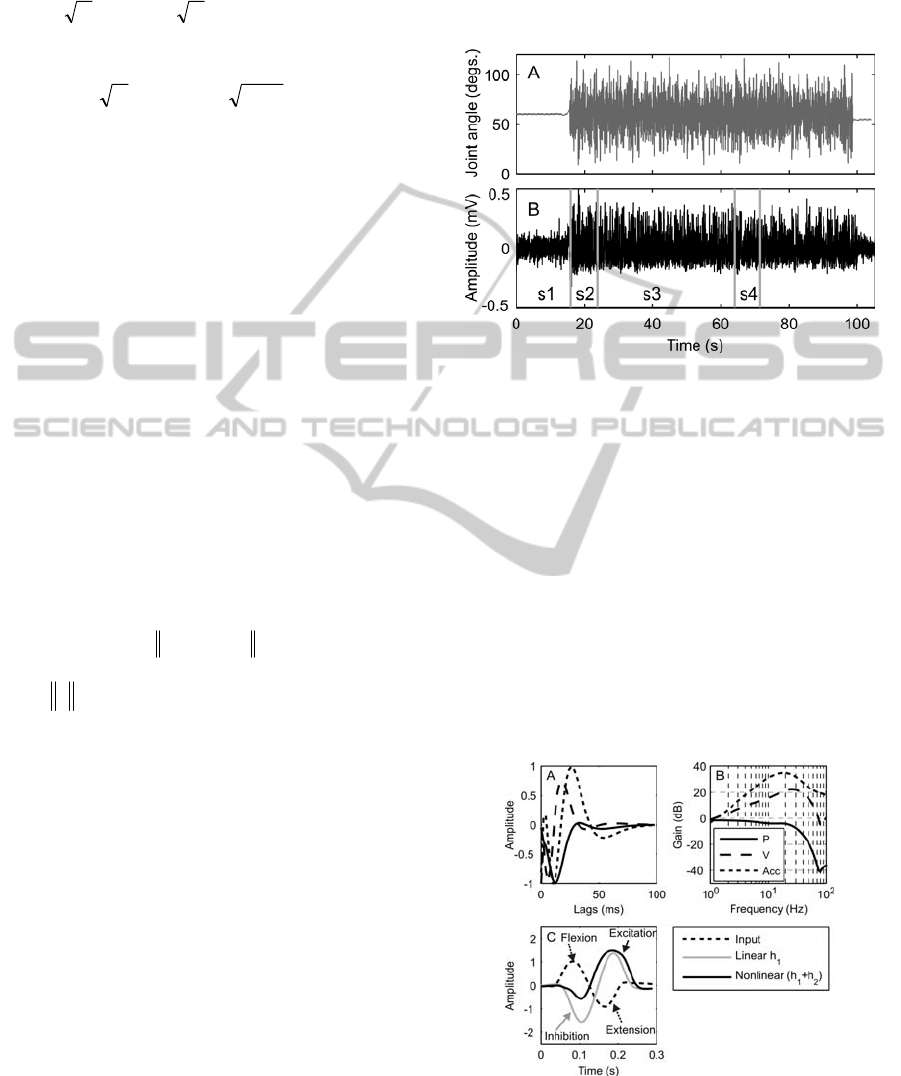
linear kernels is illustrated in Figure 3A and B. A
position sensitive model has a kernel with a
monophasic impulse response and flat frequency
response. A velocity sensitive model has a biphasic
kernel and a frequency response with a linear
increase (20dB/decade, Figure 3B). A triphasic
impulse response indicates an acceleration sensitive
interneuron (40dB/decade, Figure 3B). Whilst the
first order kernel provides a means to describe the
linear dynamic sensitivity of the interneurons, the
majority of the interneurons have a nonlinear
response, such as being primarily excitatory or
inhibitory, or more sensitive to extension or flexion.
Figure 2: The band limited (0-27Hz) GWN input signal
(A). The typical response of an interneuron (B) with
spontaneous activity (s1), transient adapting (s2), steady
state adapted response (s3) and validation section (s4).
This is illustrated in Figure 3C where the response of
a model of an interneuron to sinusoidal movement of
the tibia is shown. It should be noted that the linear
model gives equal sensitivity to both flexion and
extension, with inhibition during flexion and
excitation during extension. The response of the
nonlinear model, however, shows how this
interneuron is only weakly inhibited during flexion
of the leg but has a strong excitatory input when the
leg is extended. The response to such a sinusoid will
be used to illustrate the overall (linear and nonlinear)
response of the neuron.
Figure 3: Illustrative 1
st
order kernels (A) and gain curves
(B) showing position (P), velocity (V) and acceleration
(Acc) sensitive models. Typical model response to a 6Hz
sinusoidal input signal is shown in (C).
BIOSIGNALS 2011 - International Conference on Bio-inspired Systems and Signal Processing
272
3 RESULTS
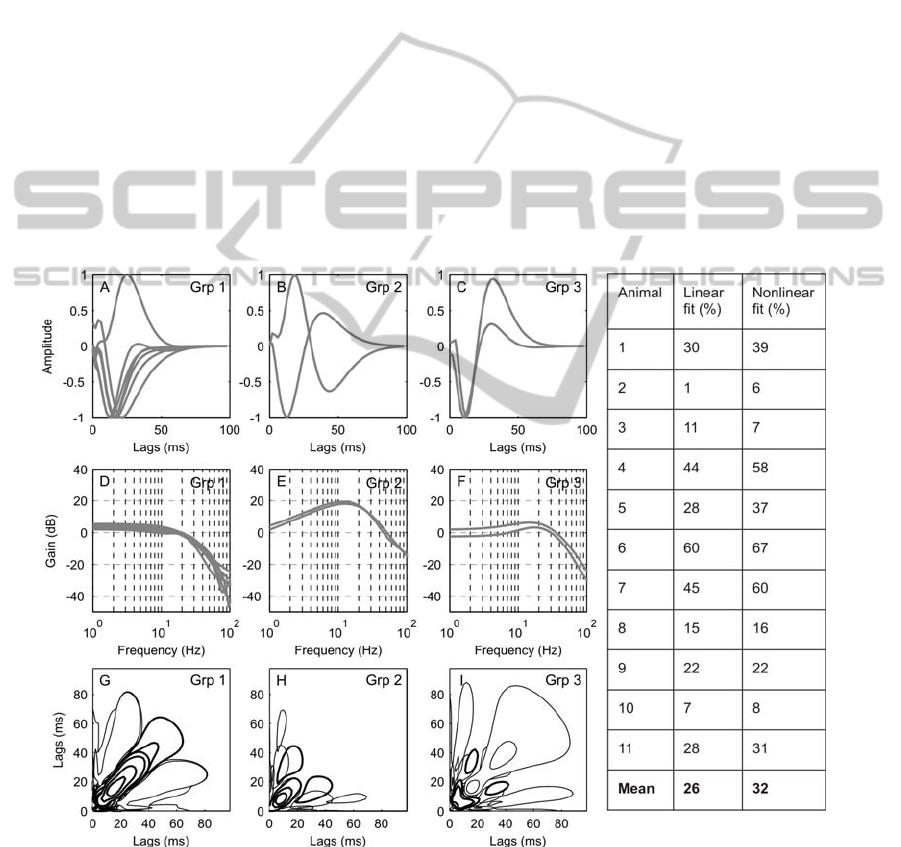
The linear dynamics of the 11 interneurons clustered
into three groups according to the sensitivity of their
responses to position, velocity or acceleration is
shown in Figure 4. Clearly the idealized patterns in
Figure 3B are not observed, but consistent patterns
that indicate a range of sensitivities are evident in
Figure 4D-F. The models of the interneurons in
group 1 (Figure 4A, D) show a monophasic 1
st
order
kernel. They have a flat frequency response in the
range 0-20Hz and a decrease of -30dB/dec > 20 Hz
indicating that interneurons in this group are
primarily position sensitive. The models in groups 2
and 3 show a biphasic first-order kernel and a
positive slope in their frequency response between
0-20Hz indicating that they mainly responded to the
rate of change of movement of the stimulus. The two
groups, however, differ in their response at lower
frequencies. Whilst the models in group 2 show a
constant slope in their frequency response (~20dB
/dec), in group 3 the responses are flatter at lower
frequencies (0-3Hz) followed by a positive slope
from ~3-20Hz (6dB/dec). No clearly acceleration
sensitive interneuron models were found in this
study. The second order kernels are shown in Figure
4G to I. The majority of interneurons in group 1
(Figure 4G) have a long positive excitatory peak
along the diagonal line of their 2
nd
order kernel
(Figure 4G) and a dominant negative peak in their
first order kernel (Figure 4A).
Interneurons in the 2
nd
and 3
rd
groups have
second-order kernels with a main inhibitory area (or
excitatory depending on the direction of the
dominant peak of the linear response) that are
smaller compared to those in group 1 (Figure 4H, I).
Also, these dominant areas peak closer to the origin
reinforcing the hypothesis that these interneurons
respond faster to stimulus changes.
An initial positive peak of the first order kernel
Figure 4: The impulse responses of the 1
st
order Volterra kernels of 11 interneurons separated into three groups using the K
means clustering algorithm (A-C). Impulse responses have been normalised to unit peak value. The monophasic impulse
response (A) and flat frequency response (D) indicate that group 1 is position sensitive. The biphasic impulse response (B
and C) and positive slopes in the frequency response indicate that groups 2 and 3 are more velocity sensitive. The 2
nd
order
Volterra kernels (G-I), positive values are represented by a thick line, negative values by a thin line. The kernels from group
1 are shown in G and have a dominant elongated peak along the diagonal. The kernels from groups 2 and 3 are shown in H
and I respectively. They have dominant deflections closer to the origin than those in group 1 and are therefore faster to
respond to stimulus changes. The predictive accuracy (fit) of the linear and nonlinear models is shown in the table.
THE DYNAMICS OF LOCUST NON-SPIKING LOCAL INTERNEURONS - Responses to Imposed Limb Movements
273
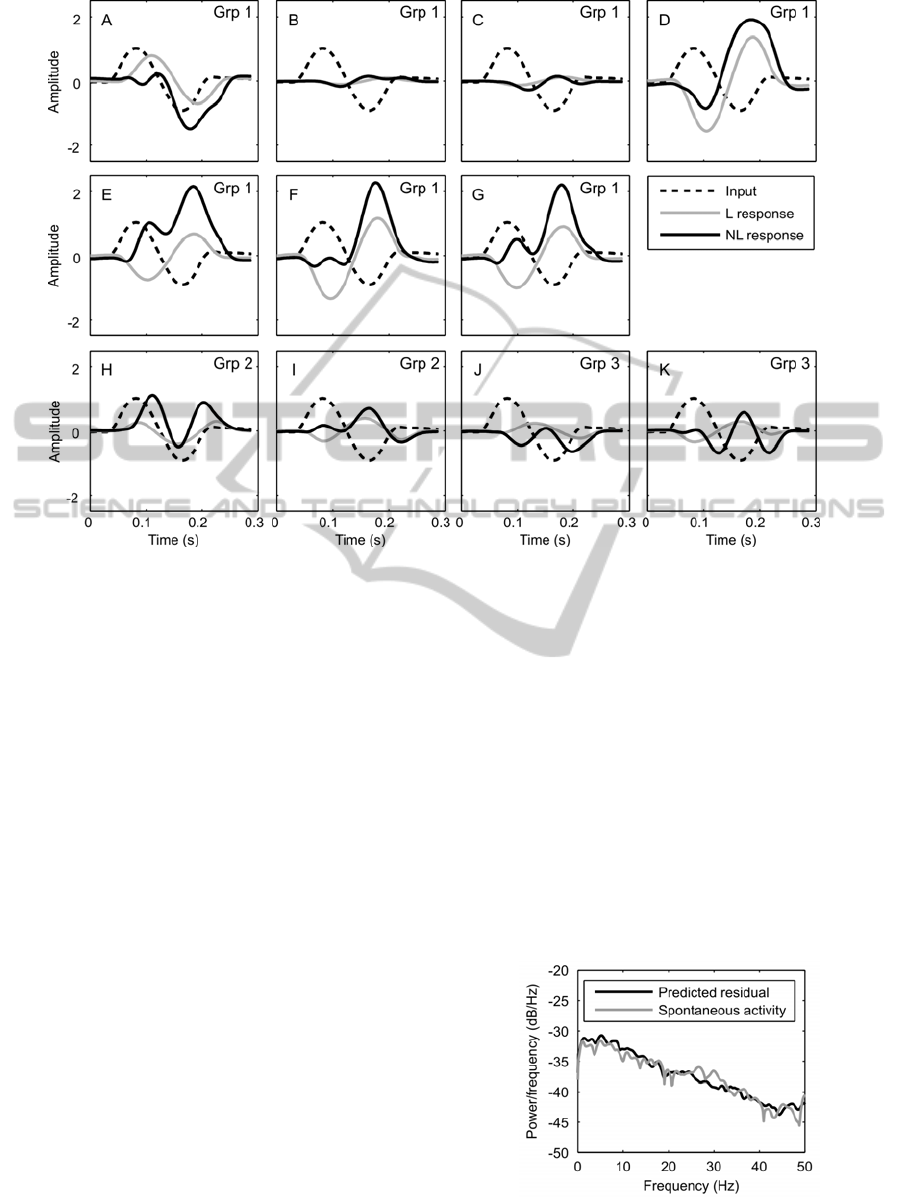
Figure 5: The response of the linear (L, first order kernel) of the nonlinear model and the nonlinear response of the model
(NL, combined response of the first and second order kernels) to one period of a 6Hz sinusoidal input signal.
indicates that a neuron is excited during flexion and
inhibited during extension. An initial negative phase
would indicate excitation during extension and
inhibition during flexion. In order to get the
complete picture of the neurons characteristic,
however, the response of the nonlinear model must
be added. The effect that the first (linear) and the
combined first and second order (nonlinear) kernels
from group 1 have on a 6Hz sinusoidal input is
shown in Figure 5A-G. The linear component gives
equal sensitivity to both flexion and extension with
inhibition during flexion and excitation during
extension in 6 out of 7 cases (Figure 5A is the
exception). When combined (linear + nonlinear)
responses are taken, however, the interneurons in
Figure 5E, F and G no longer respond with strong
inhibition during flexion, and those in Figure 5D, E,
F and G are strongly excitatory during extension.
There would appear to be less consistency in the
responses of the non-spiking local interneurons in
groups 2 and 3.
The performance of the Volterra models was
evaluated by comparing the predicted response
given by the models and the response (synaptic
potential) recorded from the non-spiking local
interneurons. Model fit was calculated using
validation data. The fit of the linear (first order) and
the nonlinear (first + second order) models for the 11
interneurons is shown in Figure 4. In some cases
(especially for animals 2, 3 and 10) the fit is very
poor, and here kernel estimates are probably not
very reliable. It should be noted that these cases also
show the lowest amplitude responses to sinusoidal
input (Figure 5B,C and J). In the remaining cases,
the NL model fit was better (or equal for animal 9)
than for the linear model. The average model fit was
26% and 32% for liner and non-linear models,
respectively, and thus rather poor. The recordings
prior to the start of stimulation when the input is
held constant (Figure 2B s1), however, show high
levels of spontaneous activity.
Figure 6: The power spectrum of the mean residual signal
is compared with that of the spontaneous activity.
BIOSIGNALS 2011 - International Conference on Bio-inspired Systems and Signal Processing
274

Analysis has shown that the spectrum of the
residual signal (the difference between the model
and the measured output signal) is very similar
(Figure 6). This suggests that model fit is probably
as good as might reasonably be expected, given that
the model cannot predict spontaneous background
activity (Marmarelis, 2004).
4 DISCUSSION
Previous work which characterised the dynamics of
sensory, motor and spiking local interneurons in the
locusts hind leg reflex control system has been
extended to a group of non-spiking local
interneurons. The models of the interneurons were
classified into three groups using the k-means
algorithm and the frequency response of the first
order kernels. We found that 7 out of the 11
interneurons might be considered position sensitive;
two were position/velocity sensitive and two were
strongly velocity sensitive. The position sensitive
interneurons were strongly sensitive to extension,
with all but one having an excitatory input with
extension. This was contrary to the results found by
Vidal-Gadea et al. (2009) for the spiking local
interneurons where extension caused inhibition. In
general, the position/velocity and velocity sensitive
interneurons received an excitatory input with
movement of the tibia into extension. As was found
by Vidal-Gadea et al. (2009) the non-spiking local
interneurons were either sensitive to extension or to
both extension and flexion. The current study found
no evidence of a non-spiking local interneuron
which responded solely to flexion.
While the members of the groups identified
show common features, there are a range of
responses included in each cluster. Further
experimental work and analysis may identify
additional clusters, or indicate that responses are
graded rather than clustered, or can be separated into
distinct clusters based on higher order features. The
approach taken, using Gaussian white noise
stimulation and system identification, has provided
new insights into the operation of the neuronal
network controlling reflex movements in the hind
leg of the locust. In the continuation of this study we
will probe the significance of these features during
functional movements.
ACKNOWLEDGEMENTS
The authors would like to thank the BBSRC and the
EPSRC for their financial support.
REFERENCES
Bar-Cohen, Y., 2006. Biomimetics-using nature to inspire
human innovation. Bioinsp. Biomim. 1:1-12.
Burrows, M., 1996. The neurobiology of the insect brain.
Oxford University Press. Oxford, 1
st
edition.
Hartigan, J. A., and Wong, M. A., 1979. A k-means
clustering algorithm. Appl Stats, 28:100-108.
Laurent, G. and Burrows, M., 1989. Intersegmental
interneurons can control the gain of reflexes in
adjacent segments of the locust by their action on non-
spiking local interneurons. J. Neuroscience,
9(9):3030-3039.
Marmarelis, V. Z., 2004. Nonlinear dynamic modelling of
physiological systems. Wiley IEEE press, first edition.
Marmarelis, V. Z., 1993. Identification of nonlinear
biological systems using Laguerre expansions of
kernels. Ann Biomed Eng, 21(6):573-80.
Newland, P. L., and Kondoh, Y., 1997a. Dynamics of
neurons controlling movements of a locust hind leg II.
Flexor tibiae motor neurons. J. Neurophysiol,
77:1731-1746.
Newland, P. L., and Kondoh, Y., 1997b. Dynamics of
neurons controlling movements of a locust hind leg
III. Extensor tibiae motor neurons. J. Neurophysiol,
77:3297-3310.
Shetzen, M., 1981. Nonlinear system modelling based on
the wiener theory. Proc IEEE, 69(12): 1557-1573.
Vidal-Gadea, A .G., Jing, X. J., Simpson, D., Dewhirst,
O. P., Kondoh, Y., Allen, R. and Newland, P. L.,
2009. Coding characteristics of spiking local
interneurons during imposed limb movements in the
locust. J. Neurophysiol, 103:603-615.
Wiener, N., 1958. Nonlinear problems in random theory.
The MIT press, first edition.
Westwick, D. T., Suki, B. and Lutchen, K. R, 1998.
Sensitivity analysis of kernel estimates: implications
in nonlinear physiological system identification.
Annals of Biomedical Engineering, 26:488-501.
THE DYNAMICS OF LOCUST NON-SPIKING LOCAL INTERNEURONS - Responses to Imposed Limb Movements
275
