
COLLABORATIVE IT PLATFORM FOR RARE DISEASES
Dragan Jankovic, Tatjana Stankovic
University of Nis, Faculty of Electronic Engineering, Aleksandra Medvedeva 14, 18000 Nis, Serbia
Branimir Todorovic
University of Nis, Faculty of Science and Mathematics, Visegradska 33, 18000 Nis, Serbia
Keywords: Rare diseases, Collaborative IT platform, Web services, Data mining, OLAP, Medical data analyses,
Electronic healthcare record.
Abstract: For a long time the rare diseases have not been in the "focus" of pharmaceutical companies and research
because of potentially lower wages and the fact that very few institutions have a representative set of data
necessary for quality research. Unfortunately, patients suffering from rare diseases are left in the margins of
many societies, their drugs are usually not on the “positive lists” of insurance organisations and their price is
extremely high. The number of rare diseases is between 6000 and 8000 and the estimated number of cases is
about 5%, i.e. about 250 million. This paper presents Collaborative IT platform model for rare diseases by
reviewing four important aspects: creating a national register of people suffering from rare diseases that can
potentially grow into an international; establishment of a central repository for rare diseases with a
collections of medical data characteristic for rare diseases, with modern data analysis tools in order to create
better conditions for scientific research in the field of rare diseases, where some tools would be oriented to
help doctors to more easily and with less cost came to proper diagnosis; improving living conditions and
treatment of patients by forming a set of virtual patient’s associations to exchange experiences and find
useful information; to create conditions for better education of medical workers and patients. The proposed
platform is the subject of the project that we apply to the call for proposals of the Ministry of Science and
Technological Development (MSc&TD) in the Republic of Serbia for a period 2011 - 2014.
1 INTRODUCTION
EHR (Electronic Healthcare Records) has had great
public attention lately all over the world (Kukafka,
2007). As the amount of collected electronic medical
data increases everywhere, the health-care services
and supporting industry are making efforts to
identify better ways to use this data for patients care
(Ford, Menachemi, Phillips, 2006). Ideally, data is
collected in a real time, can support point-of-care
clinical decisions, and, by providing instantaneous
quality metrics, can create the opportunities to
improve clinical practice as the patient is being
cared for (Michael, Holl, Badawi, Riker, Silfen,
2010).
However, all segments of the health-care
industry are plagued by many challenges that have
made it a latecomer to business intelligence and data
mining technology (Wickramasinghe, Schaffer,
2006). For example, the adoption of electronic
medical records is delayed in many countries,
integration between different medical information
systems (MIS) is poor, and there is a lack of uniform
technical standards. There is a poor interoperability
between complex medical devices and MIS. Until
basic technical infrastructure and well designed
clinical applications are implemented through the
health-care system, data aggregation and
interpretation cannot effectively progress
(DesRoches, 2008).
Unfortunately, all these facts affect the area of
rare diseases tracking even more than other
healthcare related processes. Rare diseases occur
with significantly less frequency than common
diseases. But, their frequency should not reduce the
professional attention that is given both to diseases
and to patients suffering from them. Unfortunately,
practice often shows just the opposite: patients are
309
Jankovic D., Stankovic T. and Todorovic B..
COLLABORATIVE IT PLATFORM FOR RARE DISEASES.
DOI: 10.5220/0003171003090314
In Proceedings of the International Conference on Health Informatics (HEALTHINF-2011), pages 309-314
ISBN: 978-989-8425-34-8
Copyright
c
2011 SCITEPRESS (Science and Technology Publications, Lda.)
usually marginalized by the society. The number of
medications used for rare diseases treatment is
relatively small, many of them are not in the list of
medicine that government pays for, and their price is
often even ten times higher than the price of
medications for common illnesses. This situation
may be justified by the fact that the interest of
researchers and pharmaceutical companies
especially in these diseases and these drugs was
minor, when compared to the interest in common
diseases. Rare diseases are too specific to study, to
find the appropriate causes of disease (most of rare
diseases are genetic), treatment methods, and the
appropriate preparations. Researching difficulties are
certainly the result of a small amount of relevant
data in this area, in research centres separately. The
only goal of this paper is to emphasize the problem
of patients suffering from rare diseases, and to
propose one way to help those patients, their
doctors, and the researchers interested in the area of
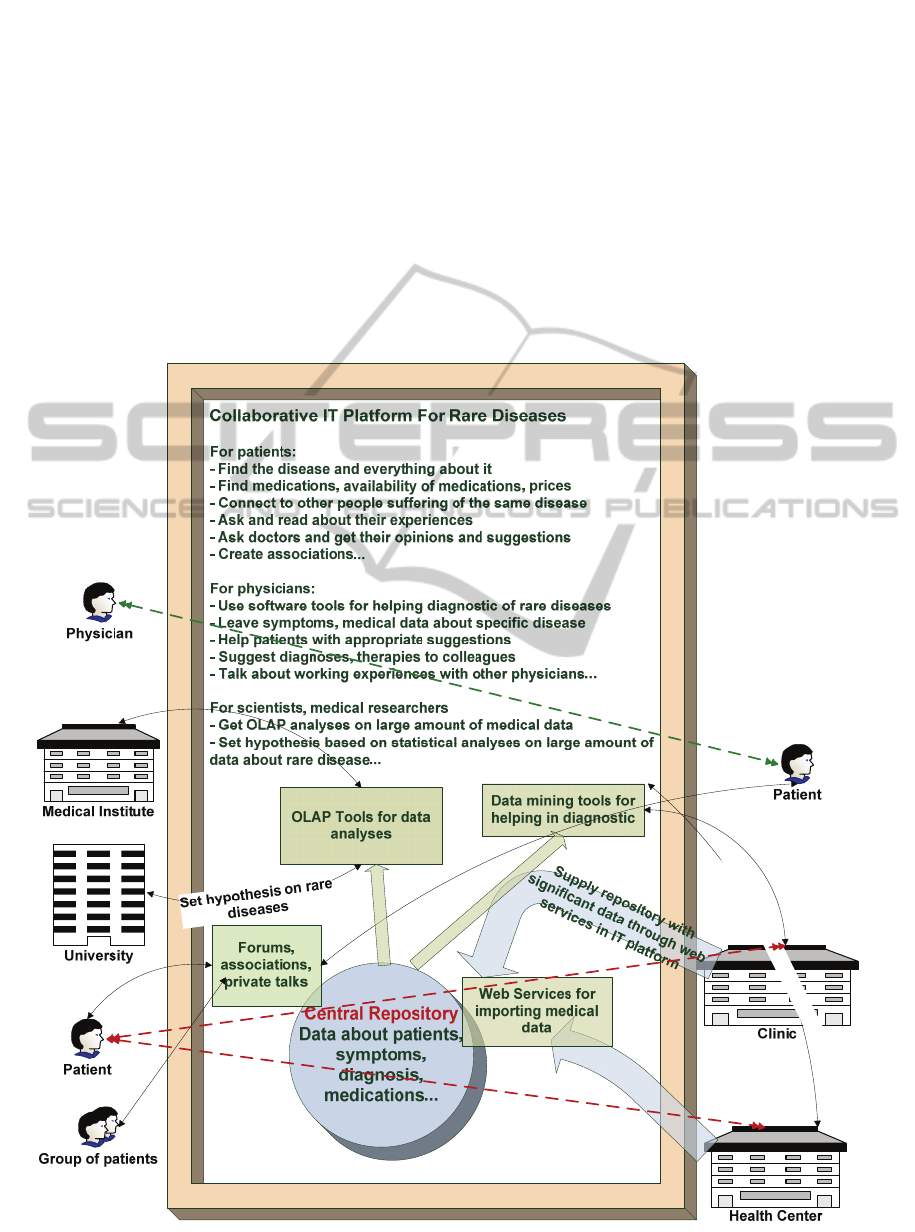
rare diseases. Our suggested solution introduces one
model of Collaborative IT (CIT) platform for rare
diseases (Fig. 1).
The proposed platform is the subject of the
project we apply to the call for proposals of the
MSc&TD in Serbia. Another purpose of this paper is
to find potential partners for an international project
with the same subject and goals and a wider set of
users from different countries.
S
u
p
p
l
y
r
e
p
o
s
i
t
o
r
y
w
i
t
h
s
i
g
n
i
f
i
c
a
n
t
d
a
t
a
t
h
r
o
u
g
h
w
e
b
s
e
r
v
i
c
e
s
i
n
I
T
p
l
a
tf
o
r
m
G
e
t
s
u
g
g
e
s
t
i
o
n
s
f
o
r
d
y
a
g
n
o
s
t
i
c
Figure 1: CIT platform for rare diseases illustration.
HEALTHINF 2011 - International Conference on Health Informatics
310

The existence of a project DILS (Delivery of
Improved Local Services) is very important for the
success of our project (http://www.dils.gov.rs).
DILS main objectives are: to increase the capacity of
institutional actors and beneficiaries in order to
improve access and the efficiency, equity and
quality of local delivery of health, education and
social protection services (see
http://web.worldbank.org/external/projects/main?pa
gePK=64312881&piPK=64302848&theSitePK=409
41&Projectid=P096823). One of the DILS
components is establishment of the information
systems that connect local service providers (health
centres, schools, centres for social work and non-
governmental organizations) with the relevant
ministry and allow efficient and transparent
financing of services, service delivery and inter-
agency information sharing. 157 health centres in
Serbia will be provided by computer equipment and
relevant MIS. Therefore, our platform for rare
diseases will have sufficient IT base for successful
work.
2 RESEARCH SIGNIFICANCE
This research will represent continuation of our
previous work (http://medisc.elfak.edu.rs, Rajković,
Janković, Tošić, 2009, Rajković, Janković,
Stanković, 2009) on development of different MIS.
Working on both ambulatory and clinical MIS, we
have offered many data retrieving forms to medical
professionals as well as connection to laboratory
devices, different reporting and data analyzing tools.
Our systems are primarily oriented to fit the needs of
Serbian public healthcare, but designed configurable
so they could be applied anywhere. Mentioned
solutions are in process of adoption within medical
facilities in Southern and Eastern Serbia. Population
of this region is about 1.7 million people, which
ensures representative population for data retrieval.
Since we have realized that rare diseases had more
complex medication process we have decided to
start research in this area, having in mind wider
sociological significance.
Research and activities that took place during the
modelling of the platform are important from several
aspects: scientific research, national, social, and
educational.
2.1 Scientific Research Aspect
Our first goal is to establish central repository for
rare diseases that would be, in the beginning, used in
the Republic of Serbia. The establishment and
operation of a central repository will provide, in a
relatively short time, critical amount of data
necessary for quality research to a number of
researchers and research centres dealing in the area
of rare diseases. Without the existence of a central
repository, with variety of relevant medical
information about patients suffering from the rare
diseases, research is performed on the small amount
of data from real patients, in separated centres,
which can potentially reduce the quality and
effectiveness of research. Initially, central repository
will import data retrieved by previously developed
medical information systems that are in active use in
Nis region. Next step will be defining data
interchange standards (under the authority of the
Serbian Ministry of Health and National health
insurance) to allow other medical information
systems to share data with central repository. Also,
legal point of view must be included to ensure that
data exchange process will not intrude patients’
privacy. As a side repository effect, the complete list
of patients suffering from rare diseases (that does
not exist at the moment in Serbia) will be formed.
A very important point of the research aspect is a
novelty in the approach to data analyses.
Specifically, power software tools will be developed
for analysis of data entered by patients themselves.
Similar approach is mentioned in (Michael, Holl,
Badawi, Riker, Silfen, 2010), but not against the
wide spectrum of medical data entered by patients.
2.2 National Aspects
By establishing a rare diseases central repository, we
will create a suitable location that offers different
kinds and pieces of information useful for patients.
Data such is common symptoms of diseases, specific
symptoms, medical and general advices, lists of
medicaments, trends in medical practice,
geographical distribution, and the success rates of
different treatment under different circumstances
will be maintained by applications based on the
repository. This will bring national (even
international) importance to a repository. Such a
repository should provide access to all researchers in
this field, as well as clinicians who participate in the
project, to store the data they have on their clinics.
One goal is to enable all doctors to store the relevant
data in the central repository, whenever they detect
rare diagnosis.
Another crucial national aspect is bringing
systemised set of data directly to the patients, in
their own language. There are many different
COLLABORATIVE IT PLATFORM FOR RARE DISEASES
311

sources about rare diseases on the Internet, but there
are just few South Slavic languages sites, having
pure information about rare diseases.
The existence of repository will allow
appropriate planning of the activity, primarily in the
Ministry of Health and National health insurance
organisation, and then in the medical institutions.
That way rare diseases data repository should
become a significant referent centre, which should
be recognizable in the region, and we hope even in
Europe.
2.3 Social Aspects
Serbian government has made significant efforts
during the last decade to educate people in the health
issues area, but those suffering of rare diseases are
still treated, unfortunately, as marginalized group of
citizens. Having CIT Platform for rare diseases,
patients would be able to actively participate in their
own treatment in different ways. They will have
better status in society along with better ability to
independently organize themselves, and the
motivation to do additional efforts to decrease the
consequences of their illness and healing where
possible. They will be able to contact and share
experiences with other people, find information
about the disease, medications, therapies, medical
institutions that deal with their disease, research
centres, pharmaceutical companies, the latest
scientific developments and other useful links.
In addition to the project significance (which will
over time get more and more important) goes the
fact that the estimation of the number of rare
diseases patients (about 5%) is very significant and
it grows. Only in Serbia the number of those patients
reaches 400000, about 30 million in Europe, i.e. 250
million around the world! As science progresses, the
number of rare diseases will probably increase, even
though it has not been small yet.
Because of these figures, pharmaceutical
companies nowadays increase attention directed to
this segment. Their income from this area becomes
more important. We can expect that their further
engagement will grow, and then, medical data
collected and stored in repositories can be essential
for them, both for planning and for the research
itself, in the area of new medications. The problem
of rare diseases is known and marked as extremely
important all over the world. The number of
projects, international associations, organizations
and sites dealing with this stuff grows every day
(www.rarediseases.org, www.raredisease.org.uk,
www.eurordis.org, http://www.crdnetwork.org, etc.).
In Serbia, some medications for rare diseases
lately became a part of government’s so-called
“positive list”, which means that patients can get
them free of charge. Unfortunately, only few of
them are on positive list currently.
3 CENTRAL REPOSITORY
The number of patients suffering from rare diseases
is not significant in health-care facilities; there are
diseases with one or zero patients per facility. The
problem with rare diseases is that critical amount of
data needed for research and education is spread all
over the world. Also, due to the procedural,
administrative and other problems those data cannot
be gathered easily from different clinics located in
different countries which are pretty interesting from
researchers’ point of view. For this reason we are
commencing development of this platform, having
leading idea to easy gather medical data about rare
diseases as much as possible at one place.
The first step in implementing CIT platform for
rare diseases would be the classification of diseases
and gathering significant medical information about
every entity (name, type, common and specific
symptoms, possible treatments, and many more).
That would be much easier part of the research. The
harder one is gathering medical data about patients
suffering from these diseases. The idea how to
gather significant medical data about particular cases
is described later. This process must be performed in
order not to harm patients’ privacy and make their
lives even more complex.
3.1 Collecting Rare Diseases Data
We will try to define several use cases how to
collect data in proper way, both from medical and
legal point of view. Our intention is, again, to collect
more relevant medical data without making patient’s
personal data directly connected and exposed in
public. Possible cases are:
Physicians directly involved in rare disease
medical treatments will be able to enter medical and
demographic patients data (with the respect of
patients privacy) when they discover new case of
rare disease (through the corresponding web
application). They will leave symptoms, used
medicaments, results of laboratory analyses,
recommended therapies, results of therapeutic
treatments, etc.
Web services that could collaborate with differ-
HEALTHINF 2011 - International Conference on Health Informatics
312

rent medical information systems will be developed
as a part of IT platform, to help clinics partners
provide electronic data from theirs EHR. This step is
very difficult to project, because of databases
heterogeneity in different MIS. To avoid bad data,
beside data recognition, human factor will have to
take place in this way of collecting data.
Patients themselves will be able to leave their
data. This kind of data will be marked as ‘patient
left’, so it could be included or excluded from
research on researcher’s request.
3.2 Data Evaluation
All gathered data should be evaluated in order to
avoid bad or missing data for further researching.
Curtain procedures will be developed to check data,
and to filter or delete bad data. In some cases
procedures will add references to provide missing
referential data integrity. The set of procedures that
will be performed on gathered relevant data will
present “Data evaluation tool”.
Relevant data, in this context, is any medical
information relevant to the disease. It is possible to
develop various software tools for analytics, faster
diagnosis, conclusion based on data-mining
algorithms, and so on.
4 SOFTWARE TOOLS
Three kinds of software tools will be developed
inside of the platform: communication tools,
business intelligence (BI) tools and data mining
tools as described further.
4.1 Communication Tools
Communication tools are of importance both for
physicians and for patients themselves. Generally,
there is a possibility to use different tools for patient
to patient communication, patient to doctor, patient
to institution, and patient to patients association.
Communication tools will provide technical ability
to the patients to create "virtual associations for
certain types of rare diseases" through CIT platform.
If implemented in international level, the platform
would be localized to different languages.
The idea is every patient to have account, every
association to have account and responsible person,
and every institution or facility to have account and
responsible person. The relation between entities
will be precisely defined.
4.2 Business Intelligence Tools
BI in medicine can be referred to as clinical
intelligence (Wickramasinghe, Schaffer, 2006). In
our plan, this kind of tools represents the set of
software tools for analyzing data repositories that
can trigger periodically or on demand, and are
designed to find appropriate templates related to
specific diseases. Beside analysis tools, there can be
software tools for setting hypotheses about the
relationship of appropriate data and factors that
could potentially be of use to researchers dealing
with rare diseases.
These tools would be available to any medical
institution or university and its researchers who want
to participate in the project. Researchers should also
share their experiences, opinions, suggestions and
results between them on platform’s forums.
4.3 Data Mining Tools
Each patient record consists of medical history
symptoms, medical conditions, and various tests and
lab results. In order to categorize those symptoms,
and to recognize the existence of rare disease, we
will apply machine learning classifiers: decision
tree, support vector machine (Vapnik, 1998) and
Bayesian sparse trained logistic regression (Tipping,
2001).
Basically, there are two main tasks which will be
pursued during the building of these classifiers. First
task will be of course, to obtain as accurate as
possible classifiers which will be able to recognize
(based on symptoms and lab results) the presence of
rare disease. The second one, also important will be
to recognize which symptoms and lab (test) results
are relevant features for obtaining good recognition
accuracy. By selecting relevant test results which are
sufficient for accurate categorization (recognition) of
the disease we are able to cut the cost of the
potentially expensive tests and save the patients
from unnecessary physical exertion.
5 PLANNED ACTIVITIES
Brief overview of CIT platform planned activities
can be presented in two parts, as follows. Activities
related to data repository:
a. classification of diseases and data structure
modelling;
b. definition of information relevant to certain types
of diseases;
COLLABORATIVE IT PLATFORM FOR RARE DISEASES
313

c. central repository database designing;
d. design and creation of web application for
manual data entry by physicians;
e. design and creation of web services for
automatic data extraction;
f. definition of tools for data analyzing and
reporting (Data Mining and BI);
g. definition of tools for publishing the results of
analysis;
Activities related to collaborative platform:
h. portal design and implementation;
i. setting up an initial rare diseases data;
j. Portal promotion in national and international
level.
The most complicated phase in the CIT platform
project development is expected to be the phase e.,
because it implies collecting and importing data
from heterogeneous medical data sources.
Differences in medical standards between countries
will create special difficulties, which could be partly
overcome by involving international medical centers
in the very beginning of the project.
6 CONCLUDING REMARKS
Due to the project specificity and its national
(international) importance, precise analysis of the
evaluation plan for the return of investment is
relatively difficult. The very nature of the platform is
such that its’ result is significant at the national level
and in a broader perspective. For these reasons we
made a project application and funding request that
has been sent to Serbian government. Since we are
supported in this work partly by Nis Clinical Centre
and partly from National health insurance
organization we expect that our efforts will be
recognized as national interest.
At the same time there is a benefit immeasurable
financially, reflected in the satisfaction of patients,
and their restored sense that society cares about
them, and kind of returning them from the social
care margins. The other useful effect can be
achieved by including the pharmaceutical companies
in the platform through advertising their
manufactures, through the use of data collected and
payment for the service. A special aspect is the
possibility of forming a Balkan or even European
data centre to collect the data on rare diseases.
Certainly, the perspective is that after the project
achieves the planned results, it can be spread in
some international projects (for example within so-
me of FP7 calls).
There is also a factor directly immeasurable: the
patients themselves can access useful information to
reduce their cost of treatment and personal
problems. There is a great probability that the costs
of rare diseases diagnostic can be significantly
reduced, if we successfully develop such a platform.
If we involve medical doctors employed in the
public health in education and use of such CIT
platform, they can reduce the number of expensive
medical analyses for diagnostic in rare diseases.
Software tools can greatly assist rapid diagnosis
of rare diseases, of course after a period of data
collection in the repository, in order to create a
sufficient quantity of data to perform the conclusions
based on the knowledge base.
And at the end, software tools that will be
developed to analyze the data stored in the
repository can be used for many similar and
commercial databases. Also designed model of CIT
platform can be used in other social and public needs
(justice, sport, investment, etc.).
REFERENCES
Michael, M., Holl, R., Badawi, O., Riker, R.R., Silfen, E.,
2010. A Collaboration Between Industry, Health-Care
Providers, and Academia, IEEE Engineering in
Medicine and Biology, Volume 29, Number 2, pp. 18-
25.
Wickramasinghe, N., Schaffer, J. L., 2006, Creating
knowledge-driven healthcare processes with the
Intelligence Continuum, International Journal of
Electronic Healthcare, Vol. 2, No. 2, pp. 164-174.
Rajkovic, P., Jankovic, D., Tosic, V., 2009, A software
solution for ambulatory healthcare facilities in the
Republic of Serbia, in Proc. of HealthCom2009,
Sydney, Australia, IEEE, pp. 161-168
Rajkovic, P., Jankovic, D., Stankovic, T. N., 2009, An e-
health solution for ambulatory facilities, in Proc. of
ITAB 2009, Larnaca, Cyprus, IEEE, pp. 1-4
Kukafka, R., August 2007, Redesigning electronic health
record systems to support public health, Journal of
Biomedical Informatics Volume 40, Issue 4, Pages
398-409.
Ford, E.W., Menachemi, N., Phillips, M. T., 2006,
Predicting the adoption of electronic health records by
physicians: when will health care be paperless?, J Am
Med Inform Assoc 13, pp. 106–112.
DesRoches, C. M., at all, Electronic Health records in
Ambulatory Care – Survey of Physicians, The New
England Journal of Medicine, 2008, 359: 50-60.
Tipping, E. M., 2001, Sparse Bayesian Learning and the
Relevance Vector Machine, Journal of Machine
Learning Research, pp. 211-244.
Vapnik, V. N., Statistical Learning Theory, Wiley, 1998.
HEALTHINF 2011 - International Conference on Health Informatics
314
